-
PDF
- Split View
-
Views
-
Cite
Cite
O.B. Christiansen, B. Pedersen, H.S. Nielsen, A.-M. Nybo Andersen, Impact of the sex of first child on the prognosis in secondary recurrent miscarriage, Human Reproduction, Volume 19, Issue 12, 1 December 2004, Pages 2946–2951, https://doi.org/10.1093/humrep/deh516
Close - Share Icon Share
Abstract
BACKGROUND: The carriage of a male fetus often initiates maternal immunological reactions against male-specific minor histocompatibility (HY) antigens, which, in theory, could result in subsequent recurrent miscarriage (RM). METHODS: Information about subsequent pregnancy outcome was procured among 182 women with RM after a birth (secondary RM) referred since 1986 using questionnaires, telephone interviews and registers. RESULTS: Significantly more of the women had had a male first-born as compared with a female first-born (110 versus 72; P<0.02). By January 2002, 58% of those who had a male first-born had given birth to a second live infant compared with 76% of those who previously had had a female first-born (P=0.01). Women in the former group had a significantly lower chance of having a second child than those in the latter (adjusted hazard ratio 0.59; 95% confidence interval 0.41–0.86). The number of miscarriages after admission and the risk of secondary infertility were significantly greater in women with a male first-born than among those with a female first-born (P<0.001 and P=0.02; respectively). CONCLUSIONS: A male first-born seems to be associated with a less favourable reproductive potential among women with secondary RM. Maternal immunization against HY antigens may be responsible for these findings.
Introduction
During reproductive life, ∼0.5% of all women will experience recurrent miscarriage (RM), characterized by the diagnostic criteria of three or more consecutive miscarriages. An accumulation of several markers of immune dysfunction (Unander et al., 1987; Xu et al., 1990; Hill et al., 1992; Aoki et al., 1995; Kruse et al., 2002) or particular human leukocyte antigen (HLA) alleles (Pfeiffer et al., 2001; Kruse et al., 2004) can be found in these women, pointing towards the involvement of the immune system in many cases of RM. There are some observations to support the theory that immunological factors play a greater role in women with a series of miscarriages after a birth (secondary RM) than in women with RM who had never had a successful pregnancy (primary RM). The HLA-DR3 allele found to be associated with RM displays a much stronger association to secondary than to primary RM (Kruse et al., 2004), and some evidence from immunotherapy studies suggests that a possible therapeutic effect of intravenous immunoglobulin (IvIg) seems to be restricted to women with secondary RM (Christiansen et al., 2002). Furthermore, an important non-immunological risk factor for RM, the factor V Leiden mutation, seems not to be associated with RM in women with secondary RM (Wramsby et al., 2000).
There are indications that a maternal immune reaction against male-specific minor histocompatibility antigens (HY antigens) may play a role in pregnancy outcome in RM women. Reactions against HY antigens play an important role in graft rejection in transplantation immunology (Spierings et al., 2003), and can be initiated by the carriage of a pregnancy (Verdijk et al., 2004). In two placebo-controlled trials of intravenous immunoglobulin treatment in RM (Christiansen et al., 2002) where inclusions were randomly undertaken (in the sequence the patients became pregnant), we noticed that among patients with secondary RM, 35 (74.5%) had given birth to a boy before the series of miscarriages compared with only 12 (25.5%) who had had a girl (P=0.015). This could indicate that the birth of a boy in the first pregnancy predisposes to subsequent RM, and supports the theory of immunization against HY antigens as being important.
The aim of the present study was to investigate the future reproductive outcome in terms of live birth rate and birth weight in a large cohort comprising all patients with secondary RM seen for the first time in our clinic from 1986 to 2000, according to the sex of the first-born child. We wished to explore the theory that a previous male birth may negatively affect the outcome of subsequent pregnancies.
Subjects and methods
Participants
From June 1, 1986 to January 1, 2000, a total of 204 women with secondary RM were admitted to the Danish RM Clinic located at the Department of Obstetrics and Gynaecology, Aalborg Hospital, where a complete record of all previous pregnancies was taken. RM was defined as a history of at least three consecutive losses of intrauterine pregnancies before the 28th gestational week. Patients with at least one pregnancy that had progressed to at least 20th gestational week prior to a series of pregnancy losses were classified as secondary RM patients in accordance with previous definitions (Coulam, 1992; Stephenson, 1996). Thirty-six of the women had given birth to two or more live-born or stillborn children before the series of miscarriages (Table I). In 22 of these women, the children had different sexes and they were excluded from further analysis as they were uninformative in this study.
All reported pregnancies were confirmed by a positive pregnancy test, ultrasonic examination and/or histology of aspirated tissue from the uterus, documented in the hospital's or practitioner's records. Among the live-born children from first pregnancies, two had chromosome abnormalities and one was born with a cleft palate.
All couples had normal karyotypes as evaluated by the G-banding technique on peripheral blood cells, and the women had normal uterine anatomy as evaluated by hysterosalpingraphy or hysteroscopy, had regular periods and were negative for the lupus anticoagulant (Christiansen et al., 2002).
Twenty-one of the patients received intravenous transfusions of third party leucocytes before one pregnancy and 39 patients received IvIg during one pregnancy after admission. These treatments were almost exclusively given in placebo-controlled trials and the allocation to the treatments was, of course, independent of the sex of the first-born.
Collection of information
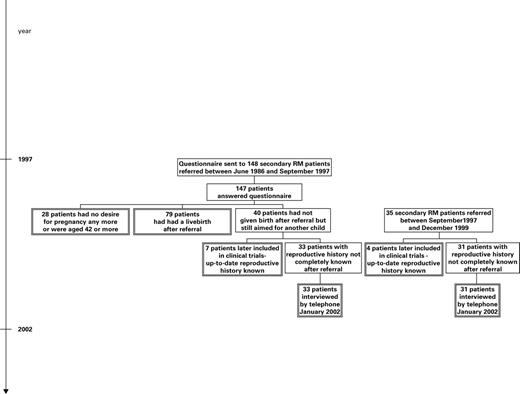
In the cohort of 182 secondary RM patients who had given birth to one child or to children of the same sex, we traced information on subsequent pregnancy outcome as follows (Figure 1).
Step 1: in September 1997, we mailed questionnaires to all 148 secondary RM patients who had consulted the RM clinic since 1986, asking whether they had experienced further pregnancies after visiting the clinic and asking for information about the outcome of these. We also asked for permission to collect further information about the pregnancies from the hospitals or the practitioners, and subsequently this information was collected. If the questionnaire was not returned, a new one was mailed after 3 months, and if there was still no response, we interviewed the women by telephone. If the women had had no further pregnancies we asked them whether they had abandoned further pregnancy attempts. We succeeded in obtaining data from all but one of these women.
Step 2: in January 2002, a number of 33 secondary RM women who, according to the previous questionnaires, had been unsuccessful in achieving a live birth despite wishing to do so, were re-contacted by telephone. We did not re-contact women aged ≥42 years or women who had denied further pregnancy attempts in 1997 (28 women). At the same time, 31 women with secondary RM seen for the first time in the RM clinic between September 1997 and January 2000 were also interviewed by telephone. In four patients referred after 1997, complete reproductive history was already known due to participation in treatment trials.
Step 3: to validate information and ensure complete follow-up, a register-linkage of the cohort was made with the Danish Civil Registration System in 2003. All Danish citizens, including live-born children, are registered in the Civil Registration System and the system has a link between mother and child. We identified all live-born offspring from the cohort and compared this information with information obtained in steps 1 and 2. Among the 28 women who had not been contacted since 1997, four were registered as having become the parent of a child after 1997. We contacted these women: in two cases the children had been adopted but in two cases the women had indeed given birth. All children, traced by step 1 or 2, were found in the records from the Civil Registration System.
Ethics
Ethical approval for the study was obtained from the Ethics Committee of the Counties of Viborg and Nordjylland. The Danish Data Protection Agency approved the register linkage. All patients gave written informed consent to their participation.
Statistical analyses
All data were entered into a Microsoft Access 97 database, differences in baseline and categorical data were determined by the χ2-test or the two-sample Mann–Whitney test, and medians and their 25 and 75 percentiles were calculated for continuous variables.
The hazard ratio for having a birth after the secondary RM diagnosis was estimated according to sex of the first-born using Cox's proportional hazards regression. Women entered the cohort at the date of referral to the clinic and follow-up ended at date of the first live birth or the December 31, 2001, whichever came first. We entered maternal age at referral into the model in three groups (<30, 30–35, 35+ years), as these categories seem to define separate risk populations (Nybo Andersen et al., 2000). The number of miscarriages prior to admission were entered in three groups (three, four and five or more miscarriages).
In cases of subsequent live birth, we analysed the association between sex of the first-born and birth weight of singleton children born after RM. We estimated the effect of the sex of the first-born on birth weight of the child born after the RM diagnosis with logistic regression, taking the sex of the second child into account.
Statistical analysis was done in SAS version 8 using the PROC PHREG and the PROC GENMOD procedures.
Results
In the following, the word ‘first-born’ is used to describe the child prior to a series of miscarriages. To fulfil the definition, the child should be born after 20th gestational week, irrespective of whether it was live-born or stillborn. In the 14 cases where the women had given birth to two or more children of the same sex before RM, these are jointly designated ‘first-born’.
Table I shows that women with a male first-born constituted the majority of the cohort. Compared with women with a female first-born before the secondary RM diagnosis, the male:female ratio was 110:72. This ratio was significantly different from the observed ratio of 51.8% boys and 48.2% girls among newborns in Denmark (P<0.02, χ2-test).
In the 182 women studied prospectively, the observation period ranged from 2 to 16 years. We found no significant differences between women with a male or female first-born in a series of background variables of potential importance for miscarriage risk and birth weight (Table II).
By January 2002, 119 of the women had given birth to at least one live child after having received the secondary RM diagnosis. Among these, 116 were singleton births and three were twin deliveries, one of them with discordant sexes of the infants. Only 58% of women with a male first-born had a live birth during follow-up, compared with 76% of the women with a female first-born (P=0.01). The crude hazard ratio for having a subsequent live born child in patients with a male first-born was 0.58 [95% confidence interval (CI) 0.40–0.83] relative to patients with a female first-born. The corresponding hazard ratio adjusted for maternal age and number of miscarriages before referral was 0.59 (95% CI 0.41–0.86) in patients with a male first-born.
The number of miscarriages (confirmed intrauterine pregnancy losses) between the referral date and the first subsequent live birth or December 31, 2001, whichever date came first, was significantly higher (P<0.001) in women with a male first-born compared with a female first-born (Table III). In addition, more women in the former group did not, after admission, achieve a subsequent intrauterine pregnancy compared with women in the latter group (21% versus 8%; P=0.02).
Among patients who received allogenic leucocyte immunization, three of 12 (25%) of those with a male first-born had a live birth in the treated pregnancy, compared with five of nine (56%) of those with a female first-born. Among patients who received IvIg, 16 of 32 (50%) of those with a male first-born had a live birth in the IvIg-treated pregnancy, compared with six of seven (86%) of those with a female first-born.
Excluding the one delivery with twins of discordant sexes, there was a boy:girl ratio of 0.84 (54:64) among the children born after RM (P=0.42, not significant).
Adjusted for sex of the second child, the mean birth weight of the first offspring born after the RM diagnosis among women who had a male first-born was 3334 g, compared with 3506 g among those who had a female first-born (P=0.25, not significant).
Discussion
As far as we know, the present study comprises the largest cohort of women with RM whose reproductive outcome has been closely monitored for up to 16 years.
The study indicated that a male first-born before secondary RM may be a prognostically poor sign, since the chance of achieving a subsequent live birth is only 59% of that of women with a female first-born. The smaller chance of a subsequent live birth in the group with a male first-born can partly be explained by a higher number of subsequent miscarriages and a higher secondary infertility rate (Table III). The higher infertility rate may reflect a higher frequency of repeated early subclinical miscarriages being registered by the women as failure to conceive. Furthermore, we found that among women who sought medical care for secondary RM, significantly more women have had a male first-born as compared with a female first-born (Table I).
The collection of data about subsequent reproduction in the cohort study was based on mailed questionnaires, telephone interviews and register links. This strategy ensured that the ascertainment of the women's live births after their first admission to our clinic was complete. Twenty-four women were not contacted again after 1997 due to high age or no desire for further pregnancy attemps (Figure 1), and if they did experience further miscarriages after 1997, these were not ascertained. However, this did not have any influence on the results for subsequent live births, and it will exhibit only minimal influence on the data concerning subsequent miscarriages and infertility (Table III) since very few subsequent pregnancies are expected in this group.
The response rate was very high, probably because all women had previously had several personal consultations with one of the authors (O.B.C. and B.P.), and all could be traced easily through the Civil Registration System.
In this study, we focused on women with secondary RM mainly due to the suggestion that the possibility of maternal immunization against fetally or trophoblast-expressed antigens is high because the immune system has been challenged with a large volume of fetal/trophoblast tissue for a long time during an ongoing pregnancy. In early miscarriages, the potential of an immune reaction against fetally expressed antigens is much less (Coulam, 1992), and furthermore, the sex of the aborted fetus is rarely known.
The increased risk of future pregnancy loss after the delivery of a boy or male fetus cannot, in our view, be explained by simple Mendelian autosomal or sex chromosome-linked inheritance. In societies where boys are the preferred offspring, cultural circumstances might explain the higher frequency of subsequent births in secondary RM women with first-born girls if couples make more eager attempts to have a second child than couples who have had a boy. However, this is not likely to be the case in a Scandinavian setting. On the contrary, a large population study has shown that in Danish families with one or two previous boys there was a small but significant excess of couples attempting to have an additional child (in the hope of having a girl) compared with families with one or two previous girls (Jacobsen et al., 1999).
Our observations can, in our view, best be explained by assuming that immunological reactions against male-specific minor histocompatibility antigens, the so-called HY antigens, expressed on the feto-placental unit play an important role in the pathogenesis of secondary RM. The HY proteins display a ubiquitous tissue expression (de Bueger et al., 1992; Warren et al., 2000), and expression of one of the three known HY genes, UTY, has specifically been demonstrated on human placenta tissue and fetal heart, kidney, liver and thymus by RNA dot-blot analysis (Warren et al., 2000). It is still unknown how early in pregnancy HY proteins are expressed on the trophoblast and fetal tissue; it will certainly be of interest to determine whether HY antigens are targets for the initiation and exertion of a harmful immunological reaction already in the first trimester. HY-derived peptides may be presented to maternal immune cells by non-classical HLA-G molecules on the trophoblast, since the latter molecules can be predicted to bind a peptide with the amino acid sequence LPHNHTDL, which can be derived from one of the HY antigens (Marsh et al., 2000; Warren et al., 2000). Alternatively, the HY peptides may be presented to the maternal cells by classical HLA class I molecules on cells from the fetal blood, which very often traffic through the trophoblast already from the second trimester (Lo et al., 1996)
HY antigens are considered to be important factors in graft-versus-host disease reactions. Male recipients of HLA-identical female bone marrow seem to be at an increased risk of developing graft-versus-host disease compared with other donor/recipient gender combinations (Atkinson et al., 1986; Eisner and August, 1995; Gratwohl et al., 2001). The risk is especially high if the female donor is parous (Atkinson et al., 1986), suggesting that term pregnancy can induce long lasting immunity against HY antigens (Verdijk et al., 2004).
If the increased risk of miscarriage after a male first-born is due to immunity to HY antigens, we would expect that male conceptions were preferentially miscarried. In accordance with this, in inbreed mice hyperimmunized to produce anti-HY antibodies, a significantly reduced male:female sex ratio was reported (Singh and Verma, 1987). In a large trial of immunotherapy in women with RM, a male:female ratio of ∼0.84 was reported among the children that were born (Ober et al., 1999). This is exactly the same ratio as found in the present study, and could be interpreted that some RM patients selectively miscarry male fetuses. However, the relatively small excess of girls being born after secondary RM in the present study indicates that if anti-HY immunity developed during the first ongoing pregnancy, it might also affect subsequent pregnancies with girls, although to a lesser degree than pregnancies with boys. It may be assumed that the originally specific initial reaction against HY antigens loses specificity after some time and becomes directed towards non-gender-specific antigens that have achieved immunogeneity due to the inflammatory processes initiated by the anti-HY reaction. This determinant spreading process is a recognized and common feature in the pathogenesis of a series of T-lymphocyte-mediated autoimmune diseases (Lehmann et al., 1993) such as type 1 diabetes (Ott et al., 2004). We admit that neither we nor others so far have laboratory data to support that this theorectical spreading of the maternal immune reaction to non-gender-specific antigens actually happens in secondary RM patients, but we cannot see why cellular immunity against the fetus or trophoblast should behave differently from that directed against other organs.
The mean birth weights tended to be lower in mothers with secondary RM with a first-born male. This may indicate that anti-HY immunity negatively affects placental or fetal growth. An association between the delivery of a boy and a series of obstetrical complications such as placental abruptions, pre-eclampsia, stillbirth and neonatal morbidity has often been reported (Bracero et al., 1996; Kramer et al., 1997; Basso and Olsen, 2001; Haddad et al., 2001).
Thirty-three percent (60 of 182) of the included patients received immunotherapy with allogeneic leucocytes or IvIg in one pregnancy after admission, but the frequency of successful outcomes in these patients was still 31–36% higher in those with a female first-born compared with those with a male first-born; a difference that is slightly larger than in the whole study population.
Women with uterine anomalies and menstrual irregularities and couples with chromosomal aberrations were excluded, but we did not exclude women with potential risk factors for RM such as autoantibodies and haemophilic factors, except for the lupus anticoagulant. In most cases of RM, several risk factors can be found; however, except for the lupus anticoagulant, the majority of these display weak or undetermined prognostic impact. Furthermore, there is no reason to believe that these potential risk factors are found more often in patients with a male first-born compared with a female first-born, and inclusion of patients with such factors will thus tend to ‘dilute’, and not bias, the findings of this study. The impact of a previous birth of a boy is only expected to be strengthened if rigorous exclusions of women with real risk factors were undertaken.
We believe this study demonstrates that sex of the first-born is associated with future reproductive capability in women with secondary RM. Maternal immunization against HY antigens on the fetus or trophoblast may be responsible for these findings. So far, the evidence that HY antigens play a role in secondary RM is observational. Future studies could attempt to investigate whether the immunological response against HY antigens is greater in women with RM with a male first-born than in controls, and whether this response exhibits a prognostic impact.
Flow chart showing the procedure of collection of information about subsequent pregnancy outcome in 182 women with secondary RM with first-borns of the same sex. The double-framed boxes contain the 182 patients included in the study. See text for details.
Outcome of the first births and sex of the first-born in 204 women referred to the clinic with unexplained secondary RM
| Outcome . | Sex of first-born . | . | . | |||
|---|---|---|---|---|---|---|
| . | Male . | Female . | Mixed . | |||
| One birth | ||||||
| Live births | 80 | 66 | 0 | |||
| Stillbirths | 19 | 3 | 0 | |||
| Two or more births | ||||||
| Live births | 8 | 2 | 14 | |||
| Stillbirths | 1 | 1 | 1 | |||
| Live+stillbirthsa | 2 | 0 | 7 | |||
| Total | 110* | 72* | 22 | |||
| Outcome . | Sex of first-born . | . | . | |||
|---|---|---|---|---|---|---|
| . | Male . | Female . | Mixed . | |||
| One birth | ||||||
| Live births | 80 | 66 | 0 | |||
| Stillbirths | 19 | 3 | 0 | |||
| Two or more births | ||||||
| Live births | 8 | 2 | 14 | |||
| Stillbirths | 1 | 1 | 1 | |||
| Live+stillbirthsa | 2 | 0 | 7 | |||
| Total | 110* | 72* | 22 | |||
Refers to twins and triplets.
P<0.02 (χ2-test) for the deviation of the male and female distribution form the expected boy:girl ratio among newborns in Denmark, excluding patients with mixed oucomes regarding sex of offspring.
Outcome of the first births and sex of the first-born in 204 women referred to the clinic with unexplained secondary RM
| Outcome . | Sex of first-born . | . | . | |||
|---|---|---|---|---|---|---|
| . | Male . | Female . | Mixed . | |||
| One birth | ||||||
| Live births | 80 | 66 | 0 | |||
| Stillbirths | 19 | 3 | 0 | |||
| Two or more births | ||||||
| Live births | 8 | 2 | 14 | |||
| Stillbirths | 1 | 1 | 1 | |||
| Live+stillbirthsa | 2 | 0 | 7 | |||
| Total | 110* | 72* | 22 | |||
| Outcome . | Sex of first-born . | . | . | |||
|---|---|---|---|---|---|---|
| . | Male . | Female . | Mixed . | |||
| One birth | ||||||
| Live births | 80 | 66 | 0 | |||
| Stillbirths | 19 | 3 | 0 | |||
| Two or more births | ||||||
| Live births | 8 | 2 | 14 | |||
| Stillbirths | 1 | 1 | 1 | |||
| Live+stillbirthsa | 2 | 0 | 7 | |||
| Total | 110* | 72* | 22 | |||
Refers to twins and triplets.
P<0.02 (χ2-test) for the deviation of the male and female distribution form the expected boy:girl ratio among newborns in Denmark, excluding patients with mixed oucomes regarding sex of offspring.
Characteristics of women with secondary RM included in the study according to the sex of the first-born
| . | Sex of first-born . | . | . | ||
|---|---|---|---|---|---|
| . | Male (n=110) . | Female (n=72) . | P . | ||
| Maternal age at referral [median (interquartile range)] | 31.8 (28.6–35.0) | 31.8 (29.2–34.4) | 0.69 | ||
| No. of miscarriages at referral [median (interquartile range)] | 4.0 (3.3–4.3) | 4.0 (3.2–4.2) | 0.37 | ||
| New partnera (%) | 22/110 (20) | 12/72 (17) | 0.57 | ||
| . | Sex of first-born . | . | . | ||
|---|---|---|---|---|---|
| . | Male (n=110) . | Female (n=72) . | P . | ||
| Maternal age at referral [median (interquartile range)] | 31.8 (28.6–35.0) | 31.8 (29.2–34.4) | 0.69 | ||
| No. of miscarriages at referral [median (interquartile range)] | 4.0 (3.3–4.3) | 4.0 (3.2–4.2) | 0.37 | ||
| New partnera (%) | 22/110 (20) | 12/72 (17) | 0.57 | ||
A different partner than the one fathering the last delivery before the diagnosis of secondary RM.
Characteristics of women with secondary RM included in the study according to the sex of the first-born
| . | Sex of first-born . | . | . | ||
|---|---|---|---|---|---|
| . | Male (n=110) . | Female (n=72) . | P . | ||
| Maternal age at referral [median (interquartile range)] | 31.8 (28.6–35.0) | 31.8 (29.2–34.4) | 0.69 | ||
| No. of miscarriages at referral [median (interquartile range)] | 4.0 (3.3–4.3) | 4.0 (3.2–4.2) | 0.37 | ||
| New partnera (%) | 22/110 (20) | 12/72 (17) | 0.57 | ||
| . | Sex of first-born . | . | . | ||
|---|---|---|---|---|---|
| . | Male (n=110) . | Female (n=72) . | P . | ||
| Maternal age at referral [median (interquartile range)] | 31.8 (28.6–35.0) | 31.8 (29.2–34.4) | 0.69 | ||
| No. of miscarriages at referral [median (interquartile range)] | 4.0 (3.3–4.3) | 4.0 (3.2–4.2) | 0.37 | ||
| New partnera (%) | 22/110 (20) | 12/72 (17) | 0.57 | ||
A different partner than the one fathering the last delivery before the diagnosis of secondary RM.
Recorded pregnancies in the follow-up period of 182 women with secondary RM according to sex of the first-born
| . | Sex of first-born . | . | . | |||
|---|---|---|---|---|---|---|
| . | Male (n=110) [n (%)] . | Female (n=72) [n (%)] . | P . | |||
| No subsequent pregnancya | 23 (21) | 6 (8) | 0.02 | |||
| Subsequent pregnancya | ||||||
| No. of miscarriagesb | <0.001c | |||||
| 0 | 43 (39) | 48 (67) | ||||
| 1 | 17 (16) | 13 (18) | ||||
| 2 | 12 (11) | 3 (4) | ||||
| 3 | 10 (9) | 2 (3) | ||||
| ≥4 | 5 (5) | 0 (0) | ||||
| . | Sex of first-born . | . | . | |||
|---|---|---|---|---|---|---|
| . | Male (n=110) [n (%)] . | Female (n=72) [n (%)] . | P . | |||
| No subsequent pregnancya | 23 (21) | 6 (8) | 0.02 | |||
| Subsequent pregnancya | ||||||
| No. of miscarriagesb | <0.001c | |||||
| 0 | 43 (39) | 48 (67) | ||||
| 1 | 17 (16) | 13 (18) | ||||
| 2 | 12 (11) | 3 (4) | ||||
| 3 | 10 (9) | 2 (3) | ||||
| ≥4 | 5 (5) | 0 (0) | ||||
Pregnancies after date of admission.
Confirmed ectopic pregnancies excluded.
Mann–Whitney test for the different distribution of miscarriages.
Recorded pregnancies in the follow-up period of 182 women with secondary RM according to sex of the first-born
| . | Sex of first-born . | . | . | |||
|---|---|---|---|---|---|---|
| . | Male (n=110) [n (%)] . | Female (n=72) [n (%)] . | P . | |||
| No subsequent pregnancya | 23 (21) | 6 (8) | 0.02 | |||
| Subsequent pregnancya | ||||||
| No. of miscarriagesb | <0.001c | |||||
| 0 | 43 (39) | 48 (67) | ||||
| 1 | 17 (16) | 13 (18) | ||||
| 2 | 12 (11) | 3 (4) | ||||
| 3 | 10 (9) | 2 (3) | ||||
| ≥4 | 5 (5) | 0 (0) | ||||
| . | Sex of first-born . | . | . | |||
|---|---|---|---|---|---|---|
| . | Male (n=110) [n (%)] . | Female (n=72) [n (%)] . | P . | |||
| No subsequent pregnancya | 23 (21) | 6 (8) | 0.02 | |||
| Subsequent pregnancya | ||||||
| No. of miscarriagesb | <0.001c | |||||
| 0 | 43 (39) | 48 (67) | ||||
| 1 | 17 (16) | 13 (18) | ||||
| 2 | 12 (11) | 3 (4) | ||||
| 3 | 10 (9) | 2 (3) | ||||
| ≥4 | 5 (5) | 0 (0) | ||||
Pregnancies after date of admission.
Confirmed ectopic pregnancies excluded.
Mann–Whitney test for the different distribution of miscarriages.
References
Aoki K, Kajiura S, Matsumoto Y, Ogasawara M, Okada S, Yagami Y and Gleicher N (
Atkinson K, Farrell C, Chapman G, Downs K, Penny R and Biggs J (
Basso O and Olsen J (
Bracero LA, Cassidy S and Byrne DW (
Christiansen OB, Pedersen B, Rosgaard A and Husth M (
Coulam CB (
de Bueger M, Bakker A, van Rood JJ, van der Woude F and Goulmy E (
Eisner MD and August CS (
Gratwohl A, Hermans J, Niederwieser D, van Biezen A, van Houwelingen HC and Apperley J (
Haddad B, Mercer BM, Livingston JC and Sibai BM (
Hill JA, Polgar K, Harlow BL and Anderson DJ (
Jacobsen R, Moeller H and Engholm G (
Kramer MS, Usher RM, Pollack R, Boyd M and Usher S (
Kruse C, Rosgaard A, Steffensen R, Varming K, Jensenius JC and Christiansen OB (
Kruse C, Steffensen R, Varming K and Christianen OB (
Lehmann PV, Sercarz EE, Forsthuber T, Dayan CM and Gammon G (
Lo YM, Lo ES, Watson N, Noakes L, Sargent IL, Thilagagathan B and Wainscoat JS (
Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J and Melbye M (
Ober C, Karrison T, Odem RR, Barnes RB, Branch DW, Stephenson MD, Baron B, Walker MA, Scott JR and Schreiber JR (
Ott PA, Bittrich MT, Herzog BA, Guerkov R, Gottlieb PA, Putnam AL, Durinovic-Bello I, Boehm BO, Tary-Lehmann M and Lehmann PV (
Pfeiffer KA, Fimmers R, Engels G, van der Ven H and van der Ven K (
Singh J and Verma IC (
Spierings E, Vermeulen CJ, Vogt MH, Doerner LEE, Falkenburg JHF, Mutis T and Goulmy E (
Stephenson MD (
Unander AM, Norberg R, Hahn L and Årfors L (
Verdijk RM, Kloosterman A, Pool J, van de Keur M, Naipal AMIH, van Halteren AGS, Brand A, Mutis T and Goulmy E (
Warren EH, Gavin MA, Simpson E, Chandler P, Page DC, Disteche C, Stankey KA, Greenberg PD and Ridddell SR (
Wramsby ML, Sten-Linder M and Bremme K (
Author notes
1Fertility Clinic 4071, Rigshospitalet, Copenhagen University Hospital, Blegdamsvej 9, DK-2100 Copenhagen, 2Department of Obstetrics and Gynaecology, Aalborg University Hospital, Aalborg and 3Department of Social Medicine, Institute of Public Health, University of Copenhagen, Denmark