-
PDF
- Split View
-
Views
-
Cite
Cite
H.G.M. Lukassen, D. D.Braat, Alex M.M. Wetzels, Gerhard A. Zielhuis, Eddy M.M. Adang, Eduard Scheenjes, Jan A.M. Kremer, Two cycles with single embryo transfer versus one cycle with double embryo transfer: a randomized controlled trial, Human Reproduction, Volume 20, Issue 3, 1 March 2005, Pages 702–708, https://doi.org/10.1093/humrep/deh672
Close - Share Icon Share
Abstract
BACKGROUND: With the aim of reducing the number of multiple pregnancies after IVF we investigated the effectiveness of two cycles with single embryo transfer (SET) and one cycle with double embryo transfer (DET) after IVF and calculated the cost-effectiveness of both strategies. Methods: A randomized controlled trial was performed in 107 women, aged <35 years, in their first IVF cycle, with at least one good quality embryo. They were randomized to the SET (n=54) or DET (n=53) group using a computer-generated random block number table, stratified for primary or secondary infertility. RESULTS: The cumulative live birth rates per woman randomized of two consecutive cycles of SET [41%; 95% confidence interval (CI) 27–54] versus one cycle of DET (36%; 95% CI 23–49) were comparable, whereas the multiple pregnancy rate was significantly higher: 37% (95% CI 15–59) in the DET and 0% in the in the SET group (P=0.002). Combining the medical costs of the IVF treatments (where 1.5 more SET cycles were required to achieve each live birth) and of pregnancies up to 6 weeks after delivery, the total medical costs of DET per live birth were €13 680 and €13 438 for SET. CONCLUSIONS: Two cycles with SET were equally effective as one cycle with DET, and the medical costs per live birth up to 6 weeks after delivery were the same. However, if lifetime costs for severe handicaps are included, more than €7000 per live birth will be saved after implementing SET. Because of the high probability of multiple pregnancies in this group of IVF patients, only SET should be performed.
Introduction
Today, multiple pregnancies are considered to be the most serious complication of IVF treatment for both mother and child. In The Netherlands a maximum of two embryos are routinely transferred per cycle to prevent higher-order multiple pregnancies. The risk of a twin pregnancy with this regime, however, is still 20–35% (Coetsier et al., 2001), which is a 15-fold increase relative to the risk of 1.6% after natural conception (Crosignani and Rubin, 2000; Coetsier et al., 2001).
There is a need to convince both health-care workers and infertile couples that multiple pregnancies are not a desirable outcome of an IVF treatment. The maternal mortality in Europe is twice as high for multiple pregnancies as compared with singleton pregnancies (Senat et al., 1998). Multiple pregnancies are associated with higher risks of hypertensive disorders, anaemia and haemorrhage during pregnancy (Sebire et al., 2001). The risk of neonatal death in twins is seven times that of singletons (Scher et al., 2002). In infants from multiple pregnancies many perinatal complications are attributable to the fact that they are more likely to be born prematurely and with a lower birth weight than babies from singleton pregnancies (Martin et al., 2002). There is an increased risk of long-term medical and developmental problems, in particular neurological impairment, in children from multiple pregnancies. Therefore, there is a higher risk of severe handicap in twins (Luke and Keith, 1992). Multiple pregnancies impose also a steep burden on government expenses and health services (Callahan et al., 1994; Wolner-Hanssen and Rydhstroem, 1998; De Sutter et al., 2002; Gerris et al., 2004). Retrospective studies identified age, number of embryos available and quality of embryos as the most important predictors for multiple birth (Vilska et al., 1999; Strandell et al., 2000).
The only solution to minimize twin pregnancies after IVF is to transfer one embryo per cycle. Up to now only four randomized controlled trials comparing single embryo transfer (SET) and double embryo transfer (DET) have been published (Gerris et al., 1999; Martikainen et al., 2001; Gardner et al., 2004; Thurin et al., 2004). The study by Gerris et al. was performed in 53 patients <34 years of age who had at least two top quality embryos available. They found an ongoing pregnancy rate of 38.5% in the SET group and 74.1% in the DET group (Gerris et al., 1999). The study by Martikainen et al. was performed in 144 patients who had at least four good quality embryos. There was no statistical difference in cumulative live birth rate (fresh and frozen cycles) between the SET group (39%) and the DET group (51%) (Martikainen et al., 2001). Gardner et al. randomized 48 IVF patients with at least 10 follicles >12 mm on day of HCG administration to transfer of either one blastocyst or two blastocysts on day 5. There was no significant difference in ongoing pregnancy rate between single blastocyst transfer (61%) and double blastocyst transfer (76%) (Gardner et al., 2004). Finally, Thurin et al. performed a multicentre randomized trial in 661 patients <36 years of age who had at least two good quality embryos available. The cumulative ongoing pregnancy rate in the SET group (one fresh SET and one frozen SET cycle) was 40% versus 44% in the DET group (without a frozen DET cycle) (P=0.344) (Thurin et al., 2004). This study has only been published in abstract form. While these randomized controlled trials make an important contribution to the SET discussion, the number of subjects are relatively small, except for the study by Thurin et al. (2004), and the studies were not combined with a cost analysis to determine the cost-effectiveness of the two strategies.
Therefore, we conducted an additional randomized controlled trial, in which we compared the live birth rate after two consecutive SET cycles with the live birth rate after one DET cycle. Freeze–thaw cycles were not included in this study. We hypothesized that two SET cycles might be needed to compensate for the possibly lower pregnancy rate as compared with DET. This study was performed among patients with a high risk for multiples (women <35 years of age, first IVF treatment cycle and at least two embryos, of which one is of excellent or good quality). Additionally, we calculated the cost-effectiveness of the two strategies to determine the best transfer strategy for efficiency. The SET strategy could be more expensive due to the extra SET cycles needed to achieve an equal live birth rate, while the DET strategy could generate more costs because of the complications related to twin pregnancies. The cost analysis was done by implementing the results of our previous retrospective study (Lukassen et al., 2004), showing that the medical costs of twin pregnancies were more than five times higher than the costs of singleton pregnancies after IVF.
Materials and methods
Study design
The protocol was approved by the Medical Ethical Review Committee of the University Medical Centre Nijmegen (UMCN) and all couples participating in the study signed a written informed consent after they had been thoroughly informed regarding the strict study design. The study objective was to investigate the live birth rate of SET after two consecutive treatment cycles with the live birth rate of DET after one treatment cycle, excluding freeze–thaw cycles. Cumulative live birth rate was the primary outcome measure. Multiple births, live birth rates after only one treatment cycle and clinical pregnancy rates (number of abortions and ectopic pregnancies) were secondary outcome measures.
Participants
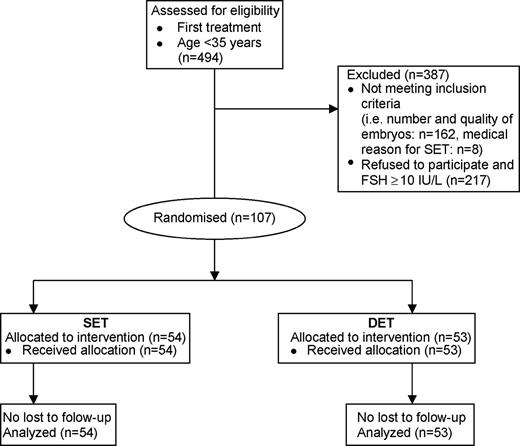
Only patients undergoing their first IVF/ICSI cycle ever or the first cycle after a successful treatment were included. The age of the women had to be <35 years (at the time of ET) with a basal FSH level <10 IU/l (Vilska et al., 1999; Strandell et al., 2000). Patients with a medical reason for elective SET were excluded, e.g. uterine malformation or history of cervical incompetence. At least two embryos, with one excellent (grade 4) or one good (grade 3) quality embryo, had to be available for transfer on day 3 after oocyte retrieval during the first cycle, according to Steer et al. (1992): grade 4=no fragmentation; grade 3=<10% fragmentation; grade 2=10–50% fragmentation; grade 1=>50% of the embryo is fragmented. The number of blastomeres was not an inclusion criterion. A total of 494 IVF patients from the department of Obstetrics and Gynecology at the UMCN and Gelderse Vallei Hospital in Ede underwent oocyte retrieval and embryo transfer in the UMCN and were <35 years old (Figure 1). The pregnant patients were followed up until delivery. Of the 494 IVF patients, 217 did not agree to participate or were excluded because of the following reasons: request for DET in the supposition that they would obtain the highest chance of pregnancy after the first cycle, request for SET to avoid multiple pregnancy, having concerns about randomization as such and basal FSH level ≥10 IU/l. From 162 patients the number or quality of the available embryos did not meet the inclusion criteria and eight patients had a medical reason for SET. The characteristics of the patients and their treatment cycles are shown in Table I.
Clinical follow-up
A total of 107 patients were randomized to the SET (n=54) or DET group (n=53) from January 2001 to February 2003. All pregnant patients were followed up until delivery. The characteristics of the randomized patients and their first treatment cycle were similar between the SET and DET group, as well as between the participants and non-participants. ICSI was performed in 28/54 cycles [52%; 95% confidence interval (CI) 38–66%] of the SET, 21/53 (40%; 95% CI 26–53%) of the DET and 117/217 (54%; 95% CI 47–61%) of the cycles of the non-participants. These percentages were not statistically different. The number of patients with at least one good quality embryo available for transfer was not different between the SET and DET group (Table I).
All patients completed the first treatment cycle. One patient in the SET group experienced total fertilization failure in the second treatment cycle. Four patients in the SET group did not (yet) undergo their second treatment cycle. One patient became pregnant spontaneously and one patient got divorced after the first treatment cycle. Two patients have yet to undergo the second cycle. These patients were analysed as if no pregnancy had occurred. In the SET group two patients elected to receive two instead of one embryo at transfer in the second cycle. One of these patients became pregnant with a singleton. Three patients received two embryos during the second SET cycle by mistake. Violation of the protocol after randomization occurred in two cases: in the SET group, in one patient no embryo transfer took place because of severe ovarian hyperstimulation syndrome. The embryos of this cycle were cryopreserved and one good quality embryo was transferred after thawing, resulting in an abortion. In the DET group one patient was included despite the fact that this patient had already undergone a previous IVF treatment cycle with transfer of only one moderate quality embryo. No pregnancy occurred. In the current cycle two good quality embryos were transferred resulting in a singleton pregnancy. Because of the intention-to-treat principle, all patients mentioned above were included in the analyses.
Assignment
Randomization to the SET or DET group was performed using a computer-generated random block number table, stratified for primary or secondary infertility, executed by an independent statistician. Allocation to the randomized group by an opaque, sealed envelope took place just before embryo transfer by the laboratory personnel to maintain concealment to the last moment. Patients and physicians were not blinded to treatment group.
IVF/ICSI procedure
Pituitary desensitization (long protocol) was achieved using triptoreline (Decapeptyl®; Ferring, The Netherlands). Ovarian stimulation was accomplished using recombinant FSH (Puregon®; Organon, Oss, The Netherlands). Thirty-six hours after the injection of 10 000 IU of HCG (Pregnyl®; Organon) we performed transvaginal oocyte retrieval. Embryo transfer was performed 3 days after oocyte retrieval.
Luteal support was given through progestogen intravaginal capsules of 100 mg, at a daily dose of 600 mg (Progestan®; Organon). On day 15 after embryo transfer a pregnancy test was performed, using a commercial urinary kit. Five weeks after embryo transfer clinical pregnancy was confirmed by ultrasonic evidence of an intrauterine gestational sac and a positive heart beat.
After the retrieval, oocyte–cumulus complexes were prepared, washed and incubated (37°C, 5% CO2 in air) in IVF medium consisting of human tubal fluid medium (Quinn et al., 1985) (Bio Whittaker, Verviers, Belgium) supplemented with 10% pasteurized plasma solution (Central Laboratory of Blood Transfusion, Amsterdam, The Netherlands). Freshly ejaculated semen was mixed with 5 ml IVF medium, layered onto an 80% Pure-Sperm (Nidacon International, Gothenberg, Sweden) gradient, centrifuged at 600 g for 20 min and washed twice with IVF medium. Insemination was carried out in Falcon dishes by adding ∼200 000 motile spermatozoa to the oocytes in 2 ml of IVF medium. If ICSI was performed, the oocytes were treated with hyaluronidase solution (Medi-cult, Jyllinge, Denmark) and denuded with a capillary pipette before injection was performed. ICSI was performed according to the method described by Van Steirteghem et al. (1995). Only morphologically normal (at 200×magnification), motile spermatozoa were injected. After injection or insemination, the oocytes were transferred to 50 ml drops of culture mineral oil (one oocyte/droplet), and were judged for the presence of 0, 1, 2 or ≥3 pronuclei. On day 3 after oocyte retrieval, the embryos were scored for cell number and embryo quality score on a scale from 1 to 4 according to Steer et al. (1992).
Excess embryos of good morphological quality (<10% fragmentation and at least seven blastomeres) were cryopreserved using the standard protocol with the cryoprotectant 1,2-propanediol (Testart et al., 1986).
Cost-effectiveness analysis of singleton versus twin pregnancies after IVF
The cost-effectiveness analyses of both embryo transfer strategies were performed using the results of a previous study of our group (Lukassen et al., 2004) and by using the direct medical costs of an IVF cycle in The Netherlands [Dutch National Health Tariffs Authority (CTG)].
In our previous study we determined the medical costs from pregnancy after IVF up to 6 weeks after delivery, using the following cost-drivers: mode of antenatal care, mode of delivery, and maternal and neonatal hospital admission days (Lukassen et al., 2004). Estimations of volumes were based on data from a representative sample of twin (n=172) and singleton (n=168) pregnancies from a database containing all couples with a live born singleton or at least one live born twin after IVF treatment at the UMCN between 1995 and 2001 (n=963 pregnancies, 24% twins). The medical costs from the induction of IVF pregnancy up to 6 weeks after delivery were €13 469 for twin pregnancies and €2550 for singleton pregnancies (P<0.001) (Lukassen et al., 2004).
Furthermore, the direct medical costs of an IVF treatment in The Netherlands, including hospital charges and medication costs of a long protocol and a mean starting dose of 200 IU recombinant FSH, have been researched using 2003 rates (CTG). The medical costs of freeze–thaw cycles were not included. This cost analysis was combined with the randomized controlled trial data using the primary outcome measure ‘live birth’ for effectiveness of treatment. This results in a cost-effectiveness ratio using a medical perspective of costs per live birth rate.
Statistical analysis
The analyses were based on the intention-to-treat principle. Cumulative live births were analysed as primary outcome (first plus second fresh cycle in SET group). Multiple births, live birth rate after just one IVF cycle in the SET group, ongoing pregnancy rate and number of abortions and extra-uterine pregnancies were analysed as secondary outcome. Categorical data were analysed by the χ2-test. The Student's t-test for independent samples was used to test continuous variables between the SET and DET.
Power analysis indicated a requirement of 52 patients in each group to show a reduction in live birth rate of 20% in the SET group after one cycle (assuming a live birth rate of 50% in the DET group and 30% in the SET group after one cycle {[1−(1−0.3)2]×100=51% after two cycles} with an α error of 0.10 and a β error of 0.20, one-sided.
P-values ≤0.05 were considered significant. All analyses were two-sided, performed by SPSS (Statistics Package for Social Sciences).
Results
Clinical results
The primary outcome, the cumulative live birth rate, was in the same order of magnitude for two cycles of SET (22/54; 41%, 95% CI 27–54%) and in the DET group (19/53; 36%, 95% CI 23–49%) (Table II). Therefore, the difference in live birth rate between two fresh cycles of SET and one fresh cycle of DET is 5% (95% CI −10.5–20.5%). The number of twins, as secondary outcome, in the DET group was seven (six twins and one dizygotic triplet) out of 19 live births (37%; 95% CI 15–59%) versus 0 out of 21 in the SET group (P=0.002). One fetus of a twin pregnancy was stillborn at 36 weeks of gestation, cause unknown. The cumulative incidence of miscarriages and ectopic pregnancies was also similar in the SET and DET groups: 8/54 (15%; 95% CI 5–25%) and 6/53 (11%; 95% CI 3–20%), respectively.
The number of live children born in the DET group was 26, including one dizygotic triplet and one stillborn child. The number of preterm born babies was not significantly higher in the DET group than in the SET group: 5/25 (20%; 95% CI 4–36%) and 2/22 (9%; 95% CI −3–21%), respectively. Nine out of 10 babies born before 37 weeks of gestation were from twin pregnancies. The number of babies with a low birth weight (<2500 g) was higher in the DET group, entirely attributable to the twins, as compared with the SET group: 10/26 (38%; 95% CI 19–58%) and 1/22 (5%; 95% CI −4–13%), respectively (P=0.002).
Even if we compared just one cycle of SET with DET, the live birth rate was not significantly different between the two groups: 14/54 (26%; 95% CI 14–38%) and 19/53 (36%; 95% CI 23–49%), respectively (Table II). The difference in live birth rate between one cycle of SET and DET is −10% (95% CI −24.7–4.7%). The percentages of miscarriages and ectopic pregnancies were similar in both groups, 6/54 (11%; 95% CI 3–20%) after one cycle in the SET and 6/53 (11%; 95% CI 3–20%) in the DET group.
Health economic results
The cost-effectiveness starts with the calculation of the costs of the IVF treatments per live birth. The calculated costs are all expressed per live birth, although the outcome of a live birth can be one healthy or sick child, or two, even three, healthy and/or sick children; so the mean costs per live birth are calculated. The medical costs of an IVF treatment in The Netherlands are €2532, including the medication (CTG 2003).
The mean number of IVF cycles performed per live birth was 4.3 in the SET group [(54+40 cycles)/22 live births] and 2.8 in the DET group (53 cycles/19 live births). Therefore, 1.5 more SET cycles were needed per live birth (4.3/2.8). The medical costs of the IVF treatment per live birth in the SET group are €10 888 (4.3×€2532) and €7090 in the DET group (2.8×€2532). This results in extra treatment costs per live birth after SET of €3798 (€10 888–€7090). The percentage of live born twins was 0% in the SET group and 37% in the DET group. Based on the results of a previous study (Lukassen et al., 2004) the average medical costs of an IVF pregnancy up to 6 weeks after delivery were €13 469 for twin pregnancies and €2550 for singleton pregnancies. For the DET group this resulted in €6590 per live birth (37%×€13 469+63%×€2550), compared with €2550 for SET (100%×€2550). Medical costs of the pregnancies up to 6 weeks after delivery after SET were €4040 (€6590–€2550) lower than after DET.
Combining the medical costs of the IVF treatments and pregnancies up to 6 weeks after delivery, the total medical costs of SET per live birth are €13 438 (€10 888+€2550) and €13 680 (€7090+€6590) for DET.
Discussion
In order to diminish the number of twin pregnancies after IVF substantially, SET is considered a serious option for daily IVF practice. Before such protocols can be implemented, the effectiveness of SET had to be determined. This randomized controlled trial assessed the live birth rate after two consecutive IVF treatment cycles utilizing SET compared with one cycle with DET, in a population with a high risk for multiple births. The cumulative live birth rate in the SET group (22/54; 41%, 95% CI 27–54%) was remarkably similar to the live birth rate in the DET group (19/53; 36%, 95% CI 23–49%). The difference in live birth rate between two cycles of SET and DET is therefore 5% (95% CI −10.5–20.5%). In contrast, six twins and one triplet were born out of 19 live births in the DET group (37%; 95% CI 15–59%) versus zero twins out of 22 live births in the SET group (P=0.002). Even after one treatment cycle the live birth rate in the SET group (14/54; 26%, 95% CI 14–38%) was already close to, and not significantly different from, the rate in the DET group (19/53; 36%, 95% CI 23–49%). The difference in live birth rate between one cycle of SET and DET is −10% (95% CI −24.7–4.7%).
The characteristics of the patients and first treatment cycle were not different between the SET and DET group (Table I), indicating that the randomization was successful. As the pregnancy rate in the non-response group (103/276; 37%) was similar to the rate in the DET group (19/53; 36%), selection bias has probably not occurred.
To date, four randomized controlled trials comparing one versus two embryo transfer have been performed (Gerris et al., 1999; Martikainen et al., 2001; Gardner et al., 2004; Thurin et al., 2004). Gerris et al. performed their study in a highly selected population. Patients included had at least two top quality embryos (four or five blastomeres on day 2, at least seven blastomeres on day 3, <20% fragments and the absence of multinucleated blastomeres). This may explain the high pregnancy rates in their SET (38.5%) and DET (74.1%) groups. At the same time, the number of twin pregnancies in the non-eligible population was also remarkably high (35.8%). So, it can be expected that the effect of SET in this highly selected group will have little effect on the overall multiple pregnancy rate. However, Gerris and colleagues studied, in a subsequent retrospective cohort analysis over a 4-year period, the effect of elective SET in a larger group of patients (n=1559). They showed that at least one top embryo with the embryo criteria described above could be transferred in ∼70% of all cycles. Over these 4 years SET increased from 13% to 31%, whereas the ongoing pregnancy rate per retrieval did not change (35.9% to 31.0%), but the multiple pregnancy rate decreased by almost 50% (33.6% to 18.6%) (Gerris et al., 2002). Martikainen and colleagues used the inclusion criteria for embryo quality of at least four good quality embryos available (even sized blastomeres and <20% fragmentation on day 2), but remarkably almost half of the patients fulfilled the inclusion criteria, so SET was applicable to a large group of patients (Martikainen et al., 2001). Gardner and colleagues evaluated all blastocysts using a previously described scoring system (Gardner and Schoolcraft, 1999), but they did not describe inclusion criteria for blastocyst quality in their randomized controlled trial. Thurin and colleagues included only patients with at least two good quality embryos available, but the definition for good quality was not described in their abstract (Thurin et al., 2004). In our study only one good quality embryo out of a total of at least two embryos had to be available, with <10% fragmentation, irrespective of the number of blastomeres, so SET could be performed in a large group of patients. The present study makes a contribution to properly designed randomized controlled trials evaluating the effectiveness of SET in a selected IVF population. In the future, performing a meta-analysis could raise the level of evidence with respect to the effectiveness of SET. This study is distinguished from the other studies by the fact that we performed two consecutive fresh cycles with SET versus one cycle with DET to determine how many extra cycles were needed to obtain a similar live birth rate per strategy.
Another notable difference with the other randomized controlled trials is that we also performed a cost-effectiveness analysis. We used the medical costs from the induction of IVF pregnancy up to 6 weeks after delivery for singleton and twin pregnancies, determined in a previous published retrospective study by our group (Lukassen et al., 2004). The total costs per live birth of the two strategies in the present study, including IVF treatments and pregnancy up to 6 weeks after delivery, did not differ significantly (SET: €13 438; DET: €13 680). Gerris and colleagues recently showed, in a real-life prospective health economic study, that in Belgium the cost of SET was €7126 and the cost of DET was €11 039 (Gerris et al., 2004). All medical costs from IVF treatment, pregnancy and neonatal period up to 3 months after delivery were prospectively analysed in patients <38 years of age. One cycle of SET with one high quality embryo resulting in a live birth rate of 37.4% was compared with one cycle of DET resulting in a live birth rate of 36.6%. In the DET group, embryo quality was not a selection criterion; therefore, the two groups were not comparable with respect to chance of pregnancy. This was not a randomized controlled trial, but based upon patient choice for SET or DET, whereas SET was exclusively performed if a high quality embryo was available (Gerris et al., 2004).
In our study, however, the long-term outcome of the DET strategy may be much more expensive when adding lifetime costs for handicaps. A French study conducted in 1982 showed that the risk of serious handicap was 6.9-fold higher for a twin child as compared with a singleton child (Papiernik, 1983). Based on these figures, Wolner-Hanssen calculated that the estimated average cost for care of severely handicapped children was €20 477 for twins and €1489 for singletons (average Euro value of SEK in 2004=9.219) (Wolner-Hanssen and Rydhstroem, 1998). So, in the DET group the lifetime costs for handicaps will be €8514 (37%×€20 477+63%×€1489). In the SET group this amount will be €1489 (100%×€1489). Finally, combining the costs of IVF treatments and (singleton and twin) IVF pregnancies up to 6 weeks after delivery with the costs for severely handicapped (singleton and twin) children, DET live births will cost €22 194 (€13 680+€8514) and SET live births €14 927 (€13 438+€1489). So, hypothetically, when handicaps are included, the DET strategy could be more than €7000 more expensive per live birth than the SET strategy.
An additional advantage of SET is the higher number of cryopreserved embryos, which can contribute to the pregnancy rate per cycle. The mean number of cryopreserved embryos per first treatment cycle was 1.8 (SD 2.0; range 0–8) in the SET group and 0.9 (SD 1.8; range 0–8) in the DET group. Unfortunately, the frozen–thawed embryo transfers did not contribute to the overall live birth rate in our study. Two Finnish studies reported an increase of the pregnancy rate due to cryopreserved embryos in patients treated with elective SET of 18% (Vilska et al., 1999) to 26% (Tiitinen et al., 2001). Martikainen and colleagues observed an increase in live birth rate from 30% to 39% after frozen–thawed embryo transfers in their SET group (Martikainen et al., 2001). It seems worthwhile to put effort in improving cryopreservation programmes, which will reduce the number of second cycles with fresh SET and as a consequence result into a considerable costs reduction.
If SET is to be implemented, more patient-friendly IVF strategies might be considered. This will result into a lower number of redundant embryos, but more importantly, also in reduction of the risk of ovarian hyperstimulation syndrome. Minimal ovarian stimulation regimens or even natural cycle IVF can be applied as a low-risk, low-cost and patient-friendly procedure. Pelinck and colleagues reported an ongoing pregnancy rate of 7.2% in a systematic literature review of 1800 natural IVF cycles (Pelinck et al., 2002). Lukassen and colleagues showed a live birth rate of 10.3% per cycle in 29 natural ICSI cycles in patients <37 years of age (Lukassen et al., 2003). On the other hand, we have to consider that there will be no, or a restricted, selection of embryos available for transfer, which will result in a decrease of the cryoaugmentation effect. A cost-effectiveness study is yet to be carried out.
The psychological and social impact of multiple births on the host family must also not be underestimated. Mothers of IVF twins experience more parenting stress as compared with mothers of IVF singletons and naturally conceived singletons (Pinborg et al., 2003; Glazebrook et al., 2004). Parents of multiples are more likely to get divorced and mothers of twins suffer more often from fatigue and depression (Hay et al., 1990; Thorpe et al., 1991). In our study, many couples refused to participate, because they desired a twin over a singleton gestation. Ryan and colleagues recently showed in a prospective analysis that 20% of women with infertility desired multiples, which was associated with nulliparity and lower family income (Ryan et al., 2004). It is questionable whether the patients in our study were aware of the practical, financial and emotional impact that the birth of twins can have on their lives. On the other hand, we did not study whether there was more emotional stress for the couples in cases where more than one cycle was needed to become pregnant of a singleton or to undergo several IVF cycles to have two singletons instead of one twin. Moreover, insurance policies may play an important role in the acceptance of SET. At the time this study was performed the Dutch government reimbursed a maximum of three IVF cycles; nowadays, the patient has to pay for the first out of three IVF cycles. As a consequence, probably more patients will insist on DET, which will cost the government relatively much more money.
We are aware of the rather small sample size of our study. Retrospectively, to show a significant difference of 10% between the live birth rate of SET (26%) and DET (36%), 90 patients per group would have been needed (α error 0.10, β error 0.20). Therefore, it is necessary to keep monitoring the success rate thoroughly in the future after implementing SET in daily practice and through well designed randomized control trials and possible meta-analysis to get more precise estimates of the success rate.
In conclusion, two cycles with SET are equally effective as one cycle with DET, and the medical costs per live birth up to 6 weeks after delivery are about the same. This favours the SET strategy because of the dramatic difference in multiple pregnancy rates. When the lifetime costs for severe handicaps due to multiple pregnancies are included, almost €7000 per live birth could be saved after implementing SET in this patient group. Therefore, the DET strategy should be abandoned completely in this high-risk group, in favour of the strategy with two cycles of SET. This will save the government not only a large amount of money, but most of all, the patients and their offspring will be saved from a large range of medical and socio-emotional problems.
Baseline characteristics of the study subjects and the non-participants of the first cycle
| Variable . | SET (n=54) . | DET (n=53) . | Non-participants (n=217) . | |||
|---|---|---|---|---|---|---|
| Age (years) [mean (SD; range)] | 30.2 (3.2; 20–34) | 31.2 (2.9; 25–34) | 30.6 (3.0; 20–34) | |||
| Duration of infertility (months) [mean (SD; range)] | 37 (17; 6–99) | 42 (23; 7–123) | 41.2 (23.3; 5.7–139.5) | |||
| Primary infertility [n (%)] | 40 (74) | 38 (72) | 162 (72) | |||
| Basal FSH level (IU/l) | 6.6 (1.5) | 6.6 (1.8) | NA | |||
| Etiology of infertility [n (%)] | ||||||
| Male factor | 36 (67) | 26 (49) | 140 (64) | |||
| Tubal | 5 (9) | 9 (17) | 24 (11) | |||
| Unexplained | 5 (9) | 14 (27) | 32 (15) | |||
| Other female | 8 (15) | 4 (8) | 21 (10) | |||
| ICSI [n (%)] | 28 (52) | 21 (40) | 117 (54) | |||
| Oocytes [mean (SD; range)] | 13.4 (6.1; 3–29) | 12.6 (6.4; 3–26) | 11.8 (5.7; 2–43) | |||
| Embryos [mean (SD; range)] | 8.0 (4.1; 2–18) | 7.8 (4.1; 2–18) | 7.0 (4.0; 2–24) | |||
| ≥1 good embryo [n (%)] | 40 (74) | 35 (66) | 127 (59) | |||
| Cryopreserved [mean (SD; range)] | 1.8 (2.0; 0–8) | 0.9 (1.8; 0–8) | ||||
| Variable . | SET (n=54) . | DET (n=53) . | Non-participants (n=217) . | |||
|---|---|---|---|---|---|---|
| Age (years) [mean (SD; range)] | 30.2 (3.2; 20–34) | 31.2 (2.9; 25–34) | 30.6 (3.0; 20–34) | |||
| Duration of infertility (months) [mean (SD; range)] | 37 (17; 6–99) | 42 (23; 7–123) | 41.2 (23.3; 5.7–139.5) | |||
| Primary infertility [n (%)] | 40 (74) | 38 (72) | 162 (72) | |||
| Basal FSH level (IU/l) | 6.6 (1.5) | 6.6 (1.8) | NA | |||
| Etiology of infertility [n (%)] | ||||||
| Male factor | 36 (67) | 26 (49) | 140 (64) | |||
| Tubal | 5 (9) | 9 (17) | 24 (11) | |||
| Unexplained | 5 (9) | 14 (27) | 32 (15) | |||
| Other female | 8 (15) | 4 (8) | 21 (10) | |||
| ICSI [n (%)] | 28 (52) | 21 (40) | 117 (54) | |||
| Oocytes [mean (SD; range)] | 13.4 (6.1; 3–29) | 12.6 (6.4; 3–26) | 11.8 (5.7; 2–43) | |||
| Embryos [mean (SD; range)] | 8.0 (4.1; 2–18) | 7.8 (4.1; 2–18) | 7.0 (4.0; 2–24) | |||
| ≥1 good embryo [n (%)] | 40 (74) | 35 (66) | 127 (59) | |||
| Cryopreserved [mean (SD; range)] | 1.8 (2.0; 0–8) | 0.9 (1.8; 0–8) | ||||
NA=not available.
Baseline characteristics of the study subjects and the non-participants of the first cycle
| Variable . | SET (n=54) . | DET (n=53) . | Non-participants (n=217) . | |||
|---|---|---|---|---|---|---|
| Age (years) [mean (SD; range)] | 30.2 (3.2; 20–34) | 31.2 (2.9; 25–34) | 30.6 (3.0; 20–34) | |||
| Duration of infertility (months) [mean (SD; range)] | 37 (17; 6–99) | 42 (23; 7–123) | 41.2 (23.3; 5.7–139.5) | |||
| Primary infertility [n (%)] | 40 (74) | 38 (72) | 162 (72) | |||
| Basal FSH level (IU/l) | 6.6 (1.5) | 6.6 (1.8) | NA | |||
| Etiology of infertility [n (%)] | ||||||
| Male factor | 36 (67) | 26 (49) | 140 (64) | |||
| Tubal | 5 (9) | 9 (17) | 24 (11) | |||
| Unexplained | 5 (9) | 14 (27) | 32 (15) | |||
| Other female | 8 (15) | 4 (8) | 21 (10) | |||
| ICSI [n (%)] | 28 (52) | 21 (40) | 117 (54) | |||
| Oocytes [mean (SD; range)] | 13.4 (6.1; 3–29) | 12.6 (6.4; 3–26) | 11.8 (5.7; 2–43) | |||
| Embryos [mean (SD; range)] | 8.0 (4.1; 2–18) | 7.8 (4.1; 2–18) | 7.0 (4.0; 2–24) | |||
| ≥1 good embryo [n (%)] | 40 (74) | 35 (66) | 127 (59) | |||
| Cryopreserved [mean (SD; range)] | 1.8 (2.0; 0–8) | 0.9 (1.8; 0–8) | ||||
| Variable . | SET (n=54) . | DET (n=53) . | Non-participants (n=217) . | |||
|---|---|---|---|---|---|---|
| Age (years) [mean (SD; range)] | 30.2 (3.2; 20–34) | 31.2 (2.9; 25–34) | 30.6 (3.0; 20–34) | |||
| Duration of infertility (months) [mean (SD; range)] | 37 (17; 6–99) | 42 (23; 7–123) | 41.2 (23.3; 5.7–139.5) | |||
| Primary infertility [n (%)] | 40 (74) | 38 (72) | 162 (72) | |||
| Basal FSH level (IU/l) | 6.6 (1.5) | 6.6 (1.8) | NA | |||
| Etiology of infertility [n (%)] | ||||||
| Male factor | 36 (67) | 26 (49) | 140 (64) | |||
| Tubal | 5 (9) | 9 (17) | 24 (11) | |||
| Unexplained | 5 (9) | 14 (27) | 32 (15) | |||
| Other female | 8 (15) | 4 (8) | 21 (10) | |||
| ICSI [n (%)] | 28 (52) | 21 (40) | 117 (54) | |||
| Oocytes [mean (SD; range)] | 13.4 (6.1; 3–29) | 12.6 (6.4; 3–26) | 11.8 (5.7; 2–43) | |||
| Embryos [mean (SD; range)] | 8.0 (4.1; 2–18) | 7.8 (4.1; 2–18) | 7.0 (4.0; 2–24) | |||
| ≥1 good embryo [n (%)] | 40 (74) | 35 (66) | 127 (59) | |||
| Cryopreserved [mean (SD; range)] | 1.8 (2.0; 0–8) | 0.9 (1.8; 0–8) | ||||
NA=not available.
The cumulative outcome of fresh embryo transfers
| Variable . | SET (n=54) . | . | . | DET (n=53) . | P . | ||
|---|---|---|---|---|---|---|---|
| . | 1st cycle . | 2nd cycle . | Cumulative . | . | . | ||
| No. of subjects | 54 | 40 | 54 | 53 | NS | ||
| No. of transfers | 54 | 35a | 89 | 53 | NS | ||
| Clinical pregnancy [n (%)] | 20 (37) | 10 (25) | 30 (56) | 25 (47) | NS | ||
| Miscarriage [n (%)] | 6 (11) | 2 (5) | 8 (15) | 5 (9) | NS | ||
| Ectopic pregnancy [n (%)] | 0 | 0 | 0 | 1 (2) | NS | ||
| Live birth [n (%)] | 14 (26) | 8 (20) | 22 (41) | 19 (36) | NS | ||
| Singleton [n (%) of live births] | 14 (100) | 8 (100) | 22 (100) | 12 (63) | NS | ||
| Twin [n (%) of live births] | 0 | 0 | 0 | 7b (37) | 0.002 | ||
| Perinatal death (n) | 0 | 0 | 0 | 1c,d | NS | ||
| Preterm birth < 37 weeks [n (%)] | 2 (14) | 0 | 2e (10) | 5d (20) | NS | ||
| Low birthweight infants (<2500 g) [n (%)] | 1 (7) | 0 | 1e (5) | 10d (40) | 0.002 | ||
| Variable . | SET (n=54) . | . | . | DET (n=53) . | P . | ||
|---|---|---|---|---|---|---|---|
| . | 1st cycle . | 2nd cycle . | Cumulative . | . | . | ||
| No. of subjects | 54 | 40 | 54 | 53 | NS | ||
| No. of transfers | 54 | 35a | 89 | 53 | NS | ||
| Clinical pregnancy [n (%)] | 20 (37) | 10 (25) | 30 (56) | 25 (47) | NS | ||
| Miscarriage [n (%)] | 6 (11) | 2 (5) | 8 (15) | 5 (9) | NS | ||
| Ectopic pregnancy [n (%)] | 0 | 0 | 0 | 1 (2) | NS | ||
| Live birth [n (%)] | 14 (26) | 8 (20) | 22 (41) | 19 (36) | NS | ||
| Singleton [n (%) of live births] | 14 (100) | 8 (100) | 22 (100) | 12 (63) | NS | ||
| Twin [n (%) of live births] | 0 | 0 | 0 | 7b (37) | 0.002 | ||
| Perinatal death (n) | 0 | 0 | 0 | 1c,d | NS | ||
| Preterm birth < 37 weeks [n (%)] | 2 (14) | 0 | 2e (10) | 5d (20) | NS | ||
| Low birthweight infants (<2500 g) [n (%)] | 1 (7) | 0 | 1e (5) | 10d (40) | 0.002 | ||
Data given are mean (%).
In five patients no embryo transfer took place: one patient got divorced, one became pregnant spontaneously, one experienced total fertilization failure; two still have to undergo the second cycle.
One dizygotic triplet.
Prenatal death: onefetus from a twin pregnancy died in utero at 36 weeks amenorrhoe.
Number of live born children in DET group=26.
Number of live born children in SET group=22.
NS=not significant.
The cumulative outcome of fresh embryo transfers
| Variable . | SET (n=54) . | . | . | DET (n=53) . | P . | ||
|---|---|---|---|---|---|---|---|
| . | 1st cycle . | 2nd cycle . | Cumulative . | . | . | ||
| No. of subjects | 54 | 40 | 54 | 53 | NS | ||
| No. of transfers | 54 | 35a | 89 | 53 | NS | ||
| Clinical pregnancy [n (%)] | 20 (37) | 10 (25) | 30 (56) | 25 (47) | NS | ||
| Miscarriage [n (%)] | 6 (11) | 2 (5) | 8 (15) | 5 (9) | NS | ||
| Ectopic pregnancy [n (%)] | 0 | 0 | 0 | 1 (2) | NS | ||
| Live birth [n (%)] | 14 (26) | 8 (20) | 22 (41) | 19 (36) | NS | ||
| Singleton [n (%) of live births] | 14 (100) | 8 (100) | 22 (100) | 12 (63) | NS | ||
| Twin [n (%) of live births] | 0 | 0 | 0 | 7b (37) | 0.002 | ||
| Perinatal death (n) | 0 | 0 | 0 | 1c,d | NS | ||
| Preterm birth < 37 weeks [n (%)] | 2 (14) | 0 | 2e (10) | 5d (20) | NS | ||
| Low birthweight infants (<2500 g) [n (%)] | 1 (7) | 0 | 1e (5) | 10d (40) | 0.002 | ||
| Variable . | SET (n=54) . | . | . | DET (n=53) . | P . | ||
|---|---|---|---|---|---|---|---|
| . | 1st cycle . | 2nd cycle . | Cumulative . | . | . | ||
| No. of subjects | 54 | 40 | 54 | 53 | NS | ||
| No. of transfers | 54 | 35a | 89 | 53 | NS | ||
| Clinical pregnancy [n (%)] | 20 (37) | 10 (25) | 30 (56) | 25 (47) | NS | ||
| Miscarriage [n (%)] | 6 (11) | 2 (5) | 8 (15) | 5 (9) | NS | ||
| Ectopic pregnancy [n (%)] | 0 | 0 | 0 | 1 (2) | NS | ||
| Live birth [n (%)] | 14 (26) | 8 (20) | 22 (41) | 19 (36) | NS | ||
| Singleton [n (%) of live births] | 14 (100) | 8 (100) | 22 (100) | 12 (63) | NS | ||
| Twin [n (%) of live births] | 0 | 0 | 0 | 7b (37) | 0.002 | ||
| Perinatal death (n) | 0 | 0 | 0 | 1c,d | NS | ||
| Preterm birth < 37 weeks [n (%)] | 2 (14) | 0 | 2e (10) | 5d (20) | NS | ||
| Low birthweight infants (<2500 g) [n (%)] | 1 (7) | 0 | 1e (5) | 10d (40) | 0.002 | ||
Data given are mean (%).
In five patients no embryo transfer took place: one patient got divorced, one became pregnant spontaneously, one experienced total fertilization failure; two still have to undergo the second cycle.
One dizygotic triplet.
Prenatal death: onefetus from a twin pregnancy died in utero at 36 weeks amenorrhoe.
Number of live born children in DET group=26.
Number of live born children in SET group=22.
NS=not significant.
References
Callahan TL, Hall JE, Ettner SL, Christiansen CL, Greene MF and Crowley WF Jr (
Coetsier T, Devroey P, Dhont M, Edwards RG, Evers H, Hagglund L, Handyside A, Gerris J, Koudstaal J, Vilska S et al. (
De Sutter P, Gerris J and Dhont M (
Gardner CK and Schoolcraft WB (
Gardner DK, Surrey E, Minjarez D, Leitz A, Stevens J and Schoolcraft WB (
Gerris J, De Neubourg D, Mangelschots K, Van Royen E, Van de Meerssche M and Valkenburg M (
Gerris J, De Neubourg D, Mangelschots K, Van Royen E, Vercruyssen M, Barudy-Vasquez J, Valkenburg M and Ryckaert G (
Gerris J, De Sutter P, De Neubourg D, Van Royen E, Vander Elst J, Mangelschots K, Vercruyssen M, Kok P, Elseviers M, Annemans L et al. (
Glazebrook C, Sheard C, Cox S, Oates M and Ndukwe G (
Hay DA, Gleeson C, Davies C, Lorden B, Mitchell D and Paton L (
Lukassen HGM, Kremer JAM, Lindeman EJM, Braat DDM and Wetzels AMM (
Lukassen HGM, Schönbeck Y, Adang E, Braat DDM, Zielhuis G and Kremer JAM (
Luke B and Keith LG (
Martikainen H, Tiitinen A, Tomas C, Tapanainen J, Orava M, Tuomivaara L, Vilska S, Hyden-Granskog C and Hovatta O (
Martin JA, Hamilton BE, Ventura SJ, Menacker F, Park MM and Sutton PD (
Pelinck MJ, Hoek A, Simons AHM and Heineman MJ (
Pinborg A, Loft A, Schmidt L and Andersen AN (
Quinn P, Kerin JF and Warnes GM (
Ryan GL, Zhang SH, Dokras A, Syrop CH and Van Voorhis BJ (
Scher AI, Petterson B, Blair E, Ellenberg JH, Grether JK, Haan E, Reddihough DS, Yeargin-Allsopp M and Nelson KB (
Sebire NJ, Jolly M, Harris J, Nicolaides KH and Regan L (
Senat MV, Ancel PY, Bouvier-Colle MH and Breart G (
Steer CV, Mills CL, Tan SL, Campbell S and Edwards RG (
Strandell A, Bergh C and Lundin K (
Testart J, Lassalle B, Belaischallart J, Hazout A, Forman R, Rainhorn JD and Frydman R (
Thorpe K, Golding J, Macgillivray I and Greenwood R (
Thurin A, Hausken J, Hillensjö T, Jablonowska B and Pinborg A (
Tiitinen A, Halttunen M, Harkki P, Vuoristo P and Hyden-Granskog C (
Van Steirteghem A, Tournaye H, Van der Elst J, Verheyen G, Liebaers I and Devroey P (
Vilska S, Tiitinen A, Hyden Granskog C and Hovatta O (
Author notes
1Department of Obstetrics and Gynecology, University Medical Centre Nijmegen, Geert Grooteplein 10, 6525 GA, Nijmegen, Departments of 2Epidemiology and Biostatistics, and 3Medical Technology Assessment, University Medical Centre Nijmegen, Nijmegen and 4Department of Obstetrics and Gynecology, Gelderse Vallei Ziekenhuis, Willy Brandtlaan 10, 6716 RP, Ede, The Netherlands