-
PDF
- Split View
-
Views
-
Cite
Cite
R.G. Gosden, S.A. Treloar, N.G. Martin, L.F. Cherkas, T.D. Spector, M.J. Faddy, S.J. Silber, Prevalence of premature ovarian failure in monozygotic and dizygotic twins, Human Reproduction, Volume 22, Issue 2, 1 February 2007, Pages 610–615, https://doi.org/10.1093/humrep/del382
Close - Share Icon Share
Abstract
BACKGROUND: Premature ovarian failure (POF) before 40 years of age from natural causes affects ~1% of adult women, with minor variations between ethnic groups. A recent case of ovarian transplantation between young monozygotic (MZ) twins in which one had undergone unexplained POF at 14 years has prompted a study of the prevalence of POF. METHODS: Menopausal ages of 832 Australian and UK female twin-pairs were extracted from volunteer national twin registry databases containing medical, reproductive and lifestyle data surveyed by mail questionnaire. Surgical menopause was an exclusion criterion. RESULTS: The prevalence of POF in both MZ and dizygotic (DZ) twins was similar in both registries and 3- to 5-fold greater than the general population at age thresholds 40 and 45 years. No specific factors were found to account for the higher risk of early menopause. Some twins of both zygosities were highly discordant for menopausal age (≥10 years). Nevertheless, there was significant intra-twin dependence, especially for MZ twins, and the average age difference at last menses was greater in DZ twin-pairs. CONCLUSION: Both MZ and DZ twins are at higher risk of POF. Despite some striking differences within MZ twin-pairs, menopausal ages were more concordant than for DZ twin-pairs, confirming that the timing of menopause has a heritable component.
Introduction
Premature ovarian failure (POF) not only truncates the fertile lifespan but also has an impact on women’s long-term health and well-being, notably the risks of cardiovascular disease and osteoporosis (Goswami and Conway, 2005). POF is commonly, if arbitrarily, defined for clinical purposes as the spontaneous and irreversible cessation of menses before 40 years of age: it is a consequence of the failure of follicles to reach maturity, either because the store of primordial follicles is exhausted or because they are refractory to growth stimuli. Many factors are responsible for POF, including abnormal karyotype, translocations, point mutations, ovarian autoimmunity, pelvic infection, cytotoxic drugs and destructive ovarian surgery (Gosden and Faddy, 1998).
The numbers of follicles in the human ovary decline continuously throughout life; few remain at the time of the menopause and virtually none in post-menopausal ovaries (Richardson et al., 1987; Faddy et al., 1992). This transition normally occurs in mid-life and is conventionally defined retrospectively after 12 months of amenorrhoea. The age distribution of natural menopause has been described for many populations worldwide. Despite differences and limitations in survey designs (retrospective, cohort and prospective), results have been remarkably consistent, at least for well-nourished populations, indicating that the menopause is determined primarily by biological factors with minor modifications because of environmental or lifestyle factors (van Noord et al., 1997). The mean age of menopause is 50–52 years, and the median age is slightly higher because the distribution is negatively skewed. The prevalence of POF was correspondingly similar between surveys. Among mainly Caucasian women with an intact uterus and ovaries, the prevalence before 40 years of age was 0.9% in a survey from the Mayo Clinic (Coulam et al., 1986), 1.0% from the Study of Women Across the Nation (SWAN) (Luborsky et al., 2003) and 1.3% from the European Prospective Investigation into Cancer and Nutrition (EPIC) (Riboli et al., 2002). When the 40- to 44-year-old group was included, the prevalence of POF rose to 5–8%. The SWAN study reported that POF by the 40- and 45-year thresholds was more frequent in African and Hispanic Americans than Caucasians and less so in Japanese (Luborsky et al., 2003).
POF is extremely rare in healthy adolescents and young adults. Yet, we recently reported a case of an American woman aged 24 years who had undergone idiopathic POF 10 years earlier, but her monozygotic (MZ) twin sister remained fertile with a normal ovarian reserve (Silber et al., 2005). POF was reversed by sister → sister ovarian tissue transplantation, and a viable pregnancy was established after natural conception. Subsequently, several more MZ twin-pairs with POF have presented for investigation and prospective treatment, and we have received anecdotal information about other cases of ovarian discordancy. This expanding series prompted us to test the hypothesis that the prevalence of POF is higher in MZ twins than in the general population. We have now surveyed twin registries of two distinct, mainly Caucasian populations to establish the prevalence of POF and search for discordant cases among both MZ and dizygotic (DZ) twin-pairs.
Materials and methods
Study design
Data for a total of 428 and 404 same-sex, female twin-pairs who had reached menopause without intervention, surgical or otherwise, were extracted from data sets from the Australian (http://www.twins.org.au) and UK Twin Registries (http://www.twinsuk.ac.uk), respectively. Overall, the numbers of MZ twins (412) and DZ twins (420) were similar. These are voluntary registries representing 10–20% of the estimated numbers of mainly Caucasian twins in each national population. The data were collected independently and by postal questionnaire in several waves between 1980 and 1996 in the Australian sample to confirm whether amenorrhoea was temporary (even if >12 months) or permanent and to enable cross-checking between waves. Any inconsistencies were treated systematically according to Do et al. (1998). The databases included information on reproductive and health history, lifestyle factors, family structure and personality: details of the design of the questionnaires and sample characteristics have been published (Baker et al., 1996; Do et al., 1998; Treloar et al., 2000; www.twinsuk.ac.uk). The respondents were representative of their population of origin in respect of key social factors, including educational level and marital status (Baker et al., 1996; Andrew et al., 2001). The average age at menopause for both MZ and DZ twins was comparable with the general population (∼51 years), suggesting there had been no systematic bias when data were obtained by recalling dates (Do et al., 1998; Snieder et al., 1998). Menopausal age was conventionally defined as ≥12 months of amenorrhoea. The exclusion criteria were (i) absence of information about menopausal age in one or both twins, (ii) primary or secondary amenorrhoea and (iii) hysterectomy or ovarian surgery before natural menopause. The surveys did not include specific questions about medical or genetic factors that might be relevant to ovarian function and menopause, and birthweights were available in a minority of cases.
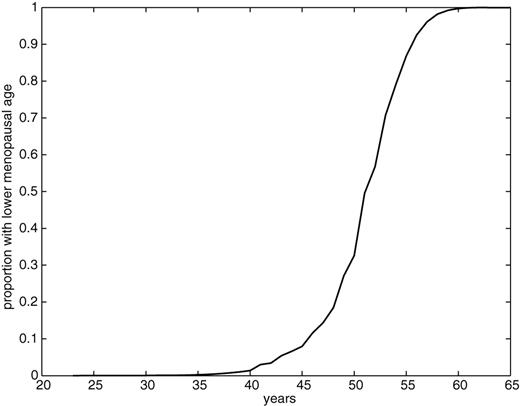
The data were compared with a large, contemporaneous survey of an ethnically comparable European population (EPIC) involving 3483 Dutch women recruited from a nationwide breast screening programme (Boker et al., 2001; Riboli et al., 2002). Virtually, all these subjects were post-menopausal, and the mean age of menopause was 50.3 years (Figure 1).
The empirical distribution of menopausal ages in the European Prospective Investigation into Cancer and Nutrition (EPIC) (adapted from Broekmans et al., 2004).
Statistics
The proportions of women who had reached the menopause at ages 40 and 45 years were calculated separately for zygosity and twin registry. Birthweights were compared using Student’s t-test for twin-pairs in which menopausal age was discordant by at least 10 years. Dependence between menopausal ages within twin-pairs was tested using a correlated binomial model based on the following rationale. If the probability of reaching menopause before a certain age is p, then the correlated binomial model has additional probabilities p0 and p1 with p = p0/(1 + p0 – p1), where p1 is the conditional probability of the second twin experiencing menopause before this age given that the first twin experienced this event and p0 is the conditional probability of the second twin experiencing this event given that the first twin did not experience it. The probability distribution of the number of these events then experienced by the twins is P (0 event) = [(1 – p0) (1 – p1)]/(1 + p0 – p1), P (1 event) = [2p0 (1 – p1)]/(1 + p0 – p1) and P (2 events) = p0p1/(1 + p0 – p1). If p0 = p1 = p then this reduces to the standard binomial distribution P (0 event) = (1 – p)2, P (1 event) = 2p (1 – p) and P (2 events) = p2. Based on menopausal age data, the probabilities p0 and p1 were estimated by maximum likelihood and the hypothesis p0 = p1, corresponding to no dependence between twin-pairs tested.
Results
Analysis of twin-pair data from both Australia and the UK confirmed the hypothesis that POF is more prevalent than in a general population. The proportion of menopausal women was 3‐fold higher at the 45-year threshold compared with 5 years younger (∼0.16 cf. ∼0.05), similar in the two registries and significantly higher than the general population (Tables I and II). Unexpectedly, the prevalence of POF for MZ twins was also higher than normal. Considering the survey data in turn, the proportion of Australian MZ twins who had reached menopause before 40 years was 0.046 (SE = 0.006), which is 3-fold higher and significantly different from 0.014 (0.002) for the same age threshold from the EPIC data set (P < 0.001) (Table I). There was a significant correlation for menopausal ages between MZ co-twins (P < 0.001). If one twin experienced menopause before 40 years then her sister was 6.9 times as likely to do so, but if she was menopausal at or after 40 years of age then her sibling was 1.4 times less likely to experience menopause before age 40. The proportion of Australian DZ twins who reached menopause before 40 years was 0.038 (0.011), significantly higher (P ≈ 0.027) than the proportion of women in the EPIC data set (0.014), but without a significant correlation between DZ twin-pairs (P > 0.5). The respective proportions of UK MZ and DZ twins reaching menopause by this age were also higher than expected (0.046 and 0.059) and similar to the Australian data (Table II).
Prevalence of POF before 40 and 45 years of age in an Australian database for MZ and DZ twins
| Zygosity . | twin-pairs (n) . | POF < 40 years [n (proportion)] . | Mean Δa and range (years) in co-twins discordant for POF < 40 years . | POF < 45 years [n (proportion)] . | Mean Δ and range (years) in co-twins discordant for POF < 45 years . |
|---|---|---|---|---|---|
| MZ | 270 | 25 (0.046) | 9.4 (0–25) | 86 (0.16) | 5.9 (0–25) |
| DZ | 158 | 12 (0.038) | 13.3 (10–19) | 52 (0.16) | 8.2 (0–19) |
| Zygosity . | twin-pairs (n) . | POF < 40 years [n (proportion)] . | Mean Δa and range (years) in co-twins discordant for POF < 40 years . | POF < 45 years [n (proportion)] . | Mean Δ and range (years) in co-twins discordant for POF < 45 years . |
|---|---|---|---|---|---|
| MZ | 270 | 25 (0.046) | 9.4 (0–25) | 86 (0.16) | 5.9 (0–25) |
| DZ | 158 | 12 (0.038) | 13.3 (10–19) | 52 (0.16) | 8.2 (0–19) |
DZ, dizygotic; MZ, monozygotic; POF, premature ovarian failure.
Δ is the mean difference in menopausal age between twin-pairs.
Prevalence of POF before 40 and 45 years of age in an Australian database for MZ and DZ twins
| Zygosity . | twin-pairs (n) . | POF < 40 years [n (proportion)] . | Mean Δa and range (years) in co-twins discordant for POF < 40 years . | POF < 45 years [n (proportion)] . | Mean Δ and range (years) in co-twins discordant for POF < 45 years . |
|---|---|---|---|---|---|
| MZ | 270 | 25 (0.046) | 9.4 (0–25) | 86 (0.16) | 5.9 (0–25) |
| DZ | 158 | 12 (0.038) | 13.3 (10–19) | 52 (0.16) | 8.2 (0–19) |
| Zygosity . | twin-pairs (n) . | POF < 40 years [n (proportion)] . | Mean Δa and range (years) in co-twins discordant for POF < 40 years . | POF < 45 years [n (proportion)] . | Mean Δ and range (years) in co-twins discordant for POF < 45 years . |
|---|---|---|---|---|---|
| MZ | 270 | 25 (0.046) | 9.4 (0–25) | 86 (0.16) | 5.9 (0–25) |
| DZ | 158 | 12 (0.038) | 13.3 (10–19) | 52 (0.16) | 8.2 (0–19) |
DZ, dizygotic; MZ, monozygotic; POF, premature ovarian failure.
Δ is the mean difference in menopausal age between twin-pairs.
Prevalence of POF before 40 and 45 years of age in a UK database for MZ and DZ twins
| Zygosity . | twin-pairs (n) . | POF < 40 years [n (proportion)] . | Δ and range (years) in co-twins discordant for POF < 40 years . | POF < 45 years [n (proportion)] . | Δ and range (years) in co-twins discordant for POF < 45 years . |
|---|---|---|---|---|---|
| MZ | 142 | 13 (0.046) | 4.3 (1–10) | 42 (0.15) | 3.1 (0–10) |
| DZ | 262 | 31 (0.059) | 7.9 (0–22) | 89 (0.17) | 6.3 (0–22) |
| Zygosity . | twin-pairs (n) . | POF < 40 years [n (proportion)] . | Δ and range (years) in co-twins discordant for POF < 40 years . | POF < 45 years [n (proportion)] . | Δ and range (years) in co-twins discordant for POF < 45 years . |
|---|---|---|---|---|---|
| MZ | 142 | 13 (0.046) | 4.3 (1–10) | 42 (0.15) | 3.1 (0–10) |
| DZ | 262 | 31 (0.059) | 7.9 (0–22) | 89 (0.17) | 6.3 (0–22) |
DZ, dizygotic; MZ, monozygotic; POF, premature ovarian failure.
Prevalence of POF before 40 and 45 years of age in a UK database for MZ and DZ twins
| Zygosity . | twin-pairs (n) . | POF < 40 years [n (proportion)] . | Δ and range (years) in co-twins discordant for POF < 40 years . | POF < 45 years [n (proportion)] . | Δ and range (years) in co-twins discordant for POF < 45 years . |
|---|---|---|---|---|---|
| MZ | 142 | 13 (0.046) | 4.3 (1–10) | 42 (0.15) | 3.1 (0–10) |
| DZ | 262 | 31 (0.059) | 7.9 (0–22) | 89 (0.17) | 6.3 (0–22) |
| Zygosity . | twin-pairs (n) . | POF < 40 years [n (proportion)] . | Δ and range (years) in co-twins discordant for POF < 40 years . | POF < 45 years [n (proportion)] . | Δ and range (years) in co-twins discordant for POF < 45 years . |
|---|---|---|---|---|---|
| MZ | 142 | 13 (0.046) | 4.3 (1–10) | 42 (0.15) | 3.1 (0–10) |
| DZ | 262 | 31 (0.059) | 7.9 (0–22) | 89 (0.17) | 6.3 (0–22) |
DZ, dizygotic; MZ, monozygotic; POF, premature ovarian failure.
The proportion of Australian MZ twins who had reached menopause before 45 years was 0.16 (0.02) and significantly >0.080 (0.005) in the EPIC data set (P < 0.001) (Table I). Again, there was a significant correlation between twin-pairs (P < 0.001). If one twin experienced menopause before 45 years then the other was 3.1 times as likely to do so, but if menopause was experienced by the twin at or after age 45 years, then her sibling was 1.6 times less likely to experience menopause before age of 45. The proportions of MZ and DZ twins experiencing menopause before age of 45 years were comparable and significantly higher than the proportion from the EPIC data set (0.080) (P < 0.001). The correlation between DZ twin-pairs was less striking, although significant (P ≈ 0.012). If one twin experienced menopause before age of 45 years then the other was 2.1 times as likely to do so, but if one twin experienced menopause at or after age of 45 years, then the other was 1.3 times less likely to experience menopause before age of 45. The respective proportions of UK twins reaching menopause by the age of 45 years were 0.15 (MZ) and 0.17 (DZ) (Table II).
Following these results, the menopausal ages from opposite sex pairs in the Australian registry were considered. From a total of 285 twin-pairs, 19 women (6.7%) had experienced menopause before age of 40, and there were 48 occurrences (16.8%) before 45 years. These proportions are also high compared with the EPIC data, like the same-sex twins.
Although definitions of ovarian discordancy are arbitrary, there were cases in which menopausal ages of some twin-pairs were manifestly different. The mean differences in menopausal age in years (Δ) were comparable in the two registries for MZ and DZ twin-pairs. Overall, Δ for DZ twins was about twice as high as in MZ twins, at both the 40- and 45-year thresholds (Tables I and II). The distributions were highly skewed because in a few cases the differences exceeded 20 years. To test whether prenatal growth is associated with an early menopause in the Australian data set, birth weights were compared between MZ and DZ twin-pairs discordant in menopausal age by at least 10 years (average 16 and 13 years, respectively). There was no significant difference for either zygosity (Table III, P = 0.07).
Relationship in the Australian data set between birthweight and zygosity in twins discordant in menopausal age by ≥10 years
| Zygosity . | n . | Twin 1 (earlier menopause) . | Twin 2 (later menopause) . | ||
|---|---|---|---|---|---|
| . | . | . | . | ||
| . | . | Mean menopausal age (years) . | Birthweight (g) Mean (95% CIa) . | Mean menopausal age (years) . | Birthweight (g) Mean (95% CI) . |
| MZ | 12 | 34 | 2840 | 50 | 2622 |
| (2214, 3465) | (1997, 3248) | ||||
| DZ | 10 | 38 | 2614 | 51 | 2331 |
| (2391, 2837) | (2108, 2554) | ||||
| Zygosity . | n . | Twin 1 (earlier menopause) . | Twin 2 (later menopause) . | ||
|---|---|---|---|---|---|
| . | . | . | . | ||
| . | . | Mean menopausal age (years) . | Birthweight (g) Mean (95% CIa) . | Mean menopausal age (years) . | Birthweight (g) Mean (95% CI) . |
| MZ | 12 | 34 | 2840 | 50 | 2622 |
| (2214, 3465) | (1997, 3248) | ||||
| DZ | 10 | 38 | 2614 | 51 | 2331 |
| (2391, 2837) | (2108, 2554) | ||||
DZ, dizygotic; MZ, monozygotic; POF, premature ovarian failure.
CI is the confidence interval for means.
Relationship in the Australian data set between birthweight and zygosity in twins discordant in menopausal age by ≥10 years
| Zygosity . | n . | Twin 1 (earlier menopause) . | Twin 2 (later menopause) . | ||
|---|---|---|---|---|---|
| . | . | . | . | ||
| . | . | Mean menopausal age (years) . | Birthweight (g) Mean (95% CIa) . | Mean menopausal age (years) . | Birthweight (g) Mean (95% CI) . |
| MZ | 12 | 34 | 2840 | 50 | 2622 |
| (2214, 3465) | (1997, 3248) | ||||
| DZ | 10 | 38 | 2614 | 51 | 2331 |
| (2391, 2837) | (2108, 2554) | ||||
| Zygosity . | n . | Twin 1 (earlier menopause) . | Twin 2 (later menopause) . | ||
|---|---|---|---|---|---|
| . | . | . | . | ||
| . | . | Mean menopausal age (years) . | Birthweight (g) Mean (95% CIa) . | Mean menopausal age (years) . | Birthweight (g) Mean (95% CI) . |
| MZ | 12 | 34 | 2840 | 50 | 2622 |
| (2214, 3465) | (1997, 3248) | ||||
| DZ | 10 | 38 | 2614 | 51 | 2331 |
| (2391, 2837) | (2108, 2554) | ||||
DZ, dizygotic; MZ, monozygotic; POF, premature ovarian failure.
CI is the confidence interval for means.
Discussion
This study validates the hypothesis that twins have a significantly higher prevalence of POF than women in the general population. The differences were large (3- to 5-fold), occurred at both the 40- and 45-year thresholds, and were similar in two independent national registries. Yet, despite these findings, POF is still uncommon, which may account for why the association was previously overlooked. The reference data to which the twin data were compared were obtained from a large survey of a European population, whose age distribution for menopausal age was comparable with other, predominantly Caucasian, surveys (Coulam et al., 1986; Luborsky et al., 2003). Although the higher incidence of POF in twins is a novel finding, it was hinted at in a previous study. Kaplan–Meier plots of menopausal age for the Australian registry did not reveal any significant difference, but the curves consistently stepped-down in twins ahead of singleton ‘controls’ at younger adult ages, indicating earlier menopause, and had mean menopausal ages of 50.3 and 50.7 years, respectively (Do et al., 1998).
The twin registries provided information about reproductive surgery, which was used to exclude cases of iatrogenic menopause. Other potentially relevant medical details that might account for cases of POF were unfortunately not obtainable in this survey. For example, treatment with alkylating agents and/or radiation for cancer can reduce or even totally ablate the follicle store in some patients. Full disclosure of this history was not available, although invasive cancer survivors (7 in 1000) would be a small proportion overall (National Cancer Institute, 2004). Ideally, potential cases should be screened for karyotype, fragile X premutations and skewed X-inactivation (Kline et al., 2006), though all these characteristics were normal in an independent series of seven MZ twin-pairs where one sister had idiopathic POF (Silber et al., 2005 and unpublished data). Smoking is another factor to be considered when comparing menopausal ages. But moderate-to-heavy smoking advances the menopause by only 2–3 years (Kinney et al., 2006), and this trend among Australian twins is similar to the population as a whole (Heath et al., 1999).
Although specific causes of POF cannot be determined for most cases in these registries, another surprising finding emerged, namely the equally high prevalence of POF in DZ as in MZ twins. Many years ago, there were already indications of earlier menopause in DZ twins, although there was no clear explanation (Wyshak, 1975). In theory, and for several reasons, an earlier menopause in DZ twins is unlikely to be because of accelerated follicle depletion. First, ovulating follicles are a minority of the cohort starting to grow at the beginning of the menstrual cycle (∼20) (Baird, 1983). Second, the total number of follicles potentially ovulating in a reproductive lifespan of 35 years (∼450) is a tiny fraction of the reserve of approximately a quarter of a million present at puberty (Faddy et al., 1992). The great majority at all ages undergo atresia (Faddy and Gosden, 1995) with twin ovulations probably occurring when serum FSH stimulation are sufficiently high to rescue a second follicle (Lambalk et al., 1998). Lastly, there is a complete lack of evidence that repeated ovarian superstimulation induces POF or, conversely, that amenorrhoea associated with grand multiparity and prolonged lactation have an ‘ovary-sparing’ effect. Rather, the explanation for the association of DZ twinning with POF may have a reverse causation, based on the widely held assumption that the peak incidence of twinning at 36 years of age is past the prime fecundity period and is related to the declining ovarian reserve (Bulmer, 1970) associated with the emergence of supernumerary follicles and slightly elevated serum FSH levels (Beemsterboer et al., 2006). The levels of inhibin A and B were found to be similar in a sample of mothers of DZ twins during monovular cycles and in controls, suggesting that any disturbances in negative feedback are at most subtle in the former group (Gilfillan et al., 2003). It may be posited that in women with a low ovarian reserve, the age distribution for twinning will be shifted to younger, peak child-bearing years, increasing the representation of twins among women prone to early menopause. Whether the magnitude of this effect explains the present observations is open to verification, but a different explanation must be sought for the more surprising excess of POF among MZ twins.
Studies of twins and mother–daughter pairs have shown a strong heritable component for menopause, although the genetic factors responsible are still largely unidentified (Snieder et al., 1998; de Bruin et al., 2001; van Asselt et al., 2004). Current data are consistent with this conclusion insofar that the intra-twin differences in menopausal age were greater in DZ twins. Moreover, there was strong evidence of dependency, especially between MZ twins (Do et al., 2000)—for example, when one had an early menopause her sibling was much more likely to have the same. In several diseases (e.g. familial breast cancer and Alzheimer’s disease), forms associated with mutations in single genes occur earlier in life than do sporadic cases. Therefore, we could expect that twins might be more concordant for POF than later menopause (Do et al., 2000).
There are limited data on age of menopause of first degree family members that tentatively indicate familial aggregation for MZ twin pedigrees (S. A. Treloar and N. G. Martin, unpublished observations). An increased twinning rate has been reported among women carrying premutations for fragile X (FRAXA) (Sherman, 2000), although one of our groups did not detect them by genotyping 21 mothers of twin-pairs where the mother reported natural menopause <40 years (Martin et al., 1997). Nor was there evidence of anything more than a possibly minor role of FRAXA in the inheritance of disposition to DZ twinning (Healey et al., 1997).
It is generally assumed that the ovaries of POF patients are depleted of follicles although, in the absence of endocrine, ultrasound or ovarian biopsy data, we cannot completely rule out the possibility of secondary ovarian failure from pathological factors or medication. In the seven aforementioned MZ cases, however, there was no doubt that the ovaries of the twin with POF were afollicular (including one case with ovarian dysgenesis), indicating that the cause was either germ cell hypoplasia/aplasia or perhaps accelerated follicular utilization because of mutations of a forkhead transcription factor (Castrillon et al., 2003). As the full quota of follicles for adult life is formed before birth (Eggan et al., 2006), it follows that menopausal age, and hence POF, may be determined prenatally as a function of their numbers (Gosden and Faddy, 1998). Thus, intrauterine growth restriction (IUGR) might be expected to lower peak numbers of primordial follicles and predispose women to early menopause (Cresswell et al., 1997). But this appealing hypothesis has not been borne out by quantitative studies of germ cells from fetuses with IUGR (de Bruin et al., 2001), nor in twin studies where lower birthweight was not associated with earlier menopause (Treloar et al., 2000). The present study was limited to a small number of cases above the IUGR weight range; nonetheless, heavier twins did not have significantly later menopause, but the evidence was rather to the contrary. Nor did menarcheal age predict menopausal age (apart from a suggestive trend at the extremes of the distribution) (Do et al., 1998), but this is not surprising as the timing of menarche is likely to be independent of follicle number above a threshold and is probably triggered by changes associated with body mass and composition.
Progress towards understanding the mechanism of POF in MZ twins is being hampered by the lack of suitable animal models. Yet, there are two hypotheses that might be open to experimental verification. First, discordant ovaries in MZ twins could develop sporadically from variations in the plane of splitting. Specifically, if early post-implantation embryos split unequally during the critical period when progenitor germ cells are forming in the epiblast (or extraembryonic ectoderm cells that provide morphogens for germ cell multiplication), a subnormal follicle store might be formed in one of the twins (Saitou et al., 2003). Second, stress from in utero competition between twins might encourage epigenetic abnormalities during a critical period of germ cell development when the epigenome is being reprogrammed (Young, 2001; Hajkova et al., 2002). The epigenome is more unstable than the genome, as indicated by loss of imprinting during assisted reproductive technologies (Thompson and Williams, 2005). The epigenome of MZ twins is relatively uniform at birth and diverges afterwards (Fraga et al., 2005); nonetheless, they can be discordant for Beckwith–Wiedemann syndrome, which is often associated with loss of imprinting of LIT1 (Weksberg et al., 2002), and there may be an epigenetic basis for psychotic illness in discordant twins (Petronis et al., 2003). High-throughput epigenomic strategies based on microarray platforms are now available for testing whether POF in MZ twins is because of variations in DNA methylation and/or chromatin modification (Callinan and Feinberg, 2006).
The great majority of twins of either zygosity can expect to reach menopause at a similar age to singletons, but for those prone to idiopathic POF there are clinical implications of having a discordant sibling. Women who have not yet attained their desired family size could have the possibility of sister → sister oocyte donation or ovarian tissue isografting. Although proportionately rare, the numbers of cases could be substantial in large countries. In the USA, for example, there are ∼150000 MZ female twin-pairs in the 20- to 40-year reproductive age bracket. About 6000 (4%) individuals will have POF before 40 years, and present data predict a substantial proportion have a sister with a larger ovarian reserve and prospectively later menopause. Besides the clinical implications of discordant POF in twins, research into this phenomenon may deepen understanding of largely unknown factors that determine the size of the follicle endowment and the timing of menopause.