-
PDF
- Split View
-
Views
-
Cite
Cite
A.S. Lopes, S.E. Madsen, N.B. Ramsing, P. Løvendahl, T. Greve, H. Callesen, Investigation of respiration of individual bovine embryos produced in vivo and in vitro and correlation with viability following transfer, Human Reproduction, Volume 22, Issue 2, 1 February 2007, Pages 558–566, https://doi.org/10.1093/humrep/del404
Close - Share Icon Share
Abstract
BACKGROUND: Quantification of oxygen consumption by individual preimplantation embryos has the potential to improve embryo selection. This study investigated whether respiration rates of individual embryos are useful indicators of embryo viability. The effect of the Nanorespirometer on embryo viability was also evaluated. METHODS: The respiration rates of individual day 7 bovine in vivo- (n = 44) and in vitro-produced (n = 156) embryos were measured using the Nanorespirometer. In vivo-produced embryos were individually transferred to recipients. RESULTS: The respiration rates of in vivo-produced embryos increased with increasing morphological quality and stage of development (P < 0.05). Pregnancy rates on days 35 and 60 were 65 and 60%, respectively. The mean respiration rate did not differ significantly between embryos producing and not producing a pregnancy, but the transfer of embryos with respiration rates <0.78 nl/h, between 0.78 and 1.10 nl/h, and >1.10 nl/h resulted in 48, 100 and 25% pregnancy rate, respectively. The mean respiration rate of in vitro-produced embryos was higher than that of in vivo-produced embryos because of differences in the morphological quality and stage of development. CONCLUSION: The Nanorespirometer does not adversely influence embryo viability, but the sample size was too small to confirm the significance of the correlation observed between respiration rates and viability.
Introduction
The ability to accurately and rapidly assess the quality and viability of individual preimplantation embryos has been considered highly important for many years. In animal embryo transfer units, the selection of those embryos that are most likely to implant successfully will obviously assist in reducing time and financial losses associated with keeping recipients for long periods. Furthermore, the commercial value of the selected embryos would be increased (Donnay, 2002). In human medicine, an increased chance of pregnancy by transfer of high-quality embryos would improve the success rates of IVF treatments. High success rates will likely cause a reduction in the number of transferred embryos by treatment cycle, thereby reducing the risk of multiple births, which are often associated with obstetric and neonatal complications (Neuber et al., 2003; Bergh, 2005).
Morphological evaluation using a standard stereomicroscope is still the most widely used technique for rapid routine assessment of embryo quality and viability before transfer (Boiso et al., 2002; Merton, 2002). Although a direct relationship between morphological quality [using the International Embryo Transfer Society (IETS) evaluation system] and embryo viability measured in terms of pregnancy rates has been demonstrated previously (Hasler, 1998), the subjectivity of such evaluation is widely recognized (Farin et al., 1995; Overström, 1996; Farin et al., 1999). A combination of several quality parameters will most likely result in improved embryo selection criteria and in a better distinction between viable and less- or non-viable embryos.
Oxygen consumption is a potential quality parameter in itself, as it provides a valuable indication of the overall metabolic activity of a single embryo (Leese, 2003). Consequently, the quantification of oxygen consumption by single embryos combined with a morphological assessment is likely to improve the selection of embryos by facilitating the evaluation of their developmental competence. In fact, correlation between oxygen consumption and morphological quality has previously been demonstrated for in vitro-produced embryos (Shiku et al., 2001; Lopes et al., 2005a) but not for in vivo-produced bovine embryos (Overström, 1992).
Previous studies reported important differences at the morphological, ultrastructural, physiological, transcriptional and metabolic levels between bovine embryos produced in vivo and those produced in vitro (Holm et al., 1998; Khurana and Niemann, 2000; Bertolini et al., 2002; Lazzari et al., 2002; Lonergan et al., 2003). Morphological differences between these two types of embryos are often attributed to the way embryo age is determined. In vivo recoveries are performed 7 days after estrus (day 0) and thus ∼6 days after fertilization, whereas the age of in vitro-produced embryos is determined from the time of IVF (day 0). Therefore, in vitro-produced embryos are at least 1 day older than the corresponding in vivo embryos (Hasler et al., 1995). This difference is reflected in the stage of development of the respective embryos, where early blastocyst and expanded blastocyst are the most common stages for day 7 in vivo- and in vitro-produced embryos, respectively (Merton, 2002). Moreover, the distinct culture conditions in which both embryo types develop and the presence of certain media components also influence the observed differences (Yoshioka et al., 1997, 2000; Peippo et al., 2001; Holm et al., 2002).
Previous studies have provided evidence that embryonic respiration rates are directly correlated with viability following embryo transfer. Overström et al. (1989, 1992) measured the oxygen consumption of single mouse and bovine in vivo-produced blastocysts before transfer, and the highest survival rates were observed in the group with the highest respiration rates. Trimarchi et al. (2000) reported the birth of Aguti (B6C6F1) mouse pups, resulting from the transfer of day 4 in vitro-cultured blastocysts previously measured with the self-referencing microelectrode technique (Smith, 1995; Land et al., 1999). Nevertheless, all the previous studies comprised a pooling of embryos before transfer which makes it impossible to identify which embryo (and corresponding respiration rate) produces the observed pregnancy, making the interpretation of these results difficult.
We have earlier described and validated the nanorespirometer system (Nanorespirometer), which is a non-invasive and highly sensitive technology developed for the measurement of individual embryo respiration rates (Lopes et al., 2005a). However, that study did not assess embryo viability directly, as embryo transfer to recipients following the measurements was not performed. Nor was the effect of the measurement procedure itself on the subsequent viability of the embryos properly evaluated.
In the present study, we determined the respiration rates of day 7 in vivo-produced bovine embryos under the conditions normally used for embryo transfer. We have investigated whether embryos with higher respiration rates yield higher pregnancy rates and consequently whether the measurement of respiration rate will be a useful indicator of subsequent embryo viability. We have further evaluated the relationship between respiration rates and morphological quality, the stage of development and the diameter of day 7 embryos produced in vivo. Moreover, we have demonstrated that the oxygen measurements performed with the Nanorespirometer did not influence the subsequent viability of the embryo. Finally, we compared the respiration rates of day 7 bovine in vivo- and in vitro-produced embryos. Preliminary results of this work have been presented earlier (Lopes et al., 2005b).
Materials and methods
Day 7 in vivo-produced embryos
In vivo embryo production
A total of 11 Danish Holstein–Friesian heifers (∼14–16 months) and cows (∼30 months) from a single herd (milk production of 12 500 kg per cow) were monitored daily for detection of estrous signs (estrus = day 0) and subsequently superovulated between days 9 and 13 of the estrous cycle. Superovulation was induced with a total of 760 IU of FSH + 760 IU of LH (Pluset®, Laboratorios Calier S.A., Barcelona, Spain), administered i.m. in decreasing doses over 4 days at 12 h intervals. Estrus was induced by a single i.m. injection of 3 ml (75 µg/ml) d-cloprostenol (Genestran Vet, Scanvet Animal Health A/S, Fredensborg, Denmark), a prostaglandin analogue, administered in the morning of the fourth day of superovulation. Estrus was detected ∼48 h later (estrus was monitored twice daily), and artificial insemination (AI) was carried out 12 h after the beginning of standing estrus with frozen–thawed semen from Holstein–Friesian bulls of proven fertility (10 × 106 sperm cells/straw). AI was normally performed once in heifers but twice in cows, 12 h apart and with semen from the same bull.
On day 7 of the estrous cycle, bovine in vivo-produced embryos (average of 4.55 embryos/donor; n = 50) were non-surgically recovered by flushing the uterine horns of the donors with phosphate-buffered saline (PBS; Danish Veterinary Laboratory, Frederiksberg, Denmark) supplemented with 10% cattle serum (CS; Danish Veterinary Laboratory) and maintained at room temperature as previously described (Greve, 1981). The recovered embryos were washed in PBS and classified according to the stage of development (compact morula or blastocyst) and morphological quality (quality I, II, III or IV) by an experienced practitioner using a stereomicroscope with a ×50 magnification and based on the definitions specified in the IETS Manual (Wright J, 1998). Only transferable in vivo embryos, i.e. embryos likely to produce a pregnancy (quality I, II and III; n = 47), were used in this study, and thus, quality IV embryos (dead, degenerating or 1-cell embryos; n = 3) were excluded.
Subsequently, embryos in PBS plus 10% CS were individually loaded into 0.25-ml sterilized straws. The straws containing the embryos were placed in a portable incubator (37.5°C) and transported to the laboratory, 1 h away from the embryo transfer facilities.
Digital records and diameter assessment
Upon arrival to the laboratory, the in vivo-produced embryos were unloaded from the straws into a 4-well dish, and their outer diameter was measured under a stereomicroscope (SMZ800; Nikon, Tokyo, Japan) using an ocular micrometer eyepiece. Digital images of each embryo were acquired using a digital still camera (GC-X3E; JVL, Yokohama, Japan) mounted on an inverted optical microscope (TDM, Nikon), with a thermal control microscope stage (CO 102; Linkam Scientific Instruments Ltd, Tadworth, Surrey, UK).
Measurement of oxygen consumption
The Nanorespirometer (Unisense A/S, Aarhus, Denmark) used to measure the oxygen consumption of single embryos has previously been described (Lopes et al., 2005a). Briefly, the rosette with the embryos was submerged into a beaker (80 ml) containing the culture medium, which was composed of synthetic oviduct fluid (SOF) medium including amino acids, citrate and inositol (SOFaaci; Holm et al. 1999) supplemented with antibiotics (gentamycin sulphate, 50 µg/ml) and 5% CS. The medium was maintained in a semi-closed system at 38.5°C and under a constant flow of humidified 5% CO2 in 19.5% O2. Two rounds of measurements were performed to determine the oxygen consumption of each embryo twice. Two empty glass capillaries served as reference measurement without respiratory activity (= control). Due to technical problems, accurate respiration rates were only determined in 44 of the 47 embryos. Respiration rates, determined twice with a 43 ± 6 min interval between measurements, were similar, as the regression line between the first and second determination was close to unity [y2 = 0.93 (±0.06) y1 + 0.09 (±0.05); R2 = 0.89, P < 0.001; Proc Reg, SAS]. Furthermore, the background noise of the nanorespirometer system was negligible (P > 0.05, two-tailed Student’s t-test), with the empty control capillaries showing an apparent oxygen consumption of 0.007 ± 0.005 nl/h (n = 18), which was not significantly different from zero (P > 0.1). These results confirm the accuracy and consistency of the measurements performed by the Nanorespirometer, and hence, the background noise on each day of measurement was not subtracted from the calculated embryonic respiration rates.
Embryo processing after the measurements
After the measurements, the embryos were individually transferred to single wells of a 4-well dish (Nunc, Roskilde, Denmark) containing SOFaaci culture medium supplemented with antibiotics and 5% CS. After this, embryos were moved to a 4-well dish containing PBS plus 10% CS and individually loaded into 0.25-ml straws, which were transported to the embryo transfer facilities in a portable incubator maintained at 37.5°C.
Non-surgical embryo transfer
Forty-three Danish Holstein–Friesian heifers (∼14–18 months) were used as recipients. Recipients were pre-selected based on normal estrous activities and synchronized by i.m. injection of 2 ml (75 µg/ml) d-cloprostenol ∼12 h before the corresponding injection in the donor animals. Only recipients with detected signs of estrus and with a palpable corpus luteum at the time of embryo transfer were used.
Single fresh day 7 bovine embryos produced in vivo (n = 43) were non-surgically transferred to randomly assigned synchronized recipient heifers on day 7 (±12 h) after estrus. Pregnancy was diagnosed by palpation per rectum on day 35 and confirmed by ultrasonography on day 60 after estrus. The annual pregnancy rate observed in the herd where this study was carried out is ∼65%, following the transfer of an average of 600 fresh embryos/year (Madsen SE, personal communication).
The present study was considered to be part of the routine work performed in relation to embryo transfer in practice. Embryos were flushed in the morning, loaded into straws, transported to and from the laboratory and transferred back to recipients ∼8 h later. As oxygen measurements were non-invasive and expected not to interfere with the subsequent viability of the embryos, permission from the Danish experimental committee was not required for this study.
Day 7 in vitro-produced embryos
In vitro embryo production
The method used for in vitro production has been reported elsewhere (Holm et al., 1999). In brief, bovine immature cumulus–oocyte complexes were aspirated from slaughterhouse-derived ovaries, selected and matured for 24 h in 4-well dishes. Each well contained 400 µl of bicarbonate-buffered TCM-199 medium (Gibco BRL, Paisley, UK) supplemented with 15% CS, 10 IU/ml of equine CG and 5 IU/ml of HCG (Suigonan Vet; Intervet Scandinavia, Skovlunde, Denmark). The oocytes were matured under mineral oil at 38.5°C in 5% CO2 in humidified air. Fertilization was performed in modified Tyrode’s medium (Parrish et al., 1986) using frozen–thawed, Percoll-selected sperm, which was pre-tested for in vitro production and different from the semen used for AI of the superovulated donors. After 22 h, cumulus cells were removed by vortexing, and presumptive zygotes were transferred to 400 µl of culture medium and incubated at 38.5°C in 5% CO2, 5% O2, 90% N2 atmosphere with 100% humidity. The embryos were produced in vitro over 18 independent replicates by using ∼50 oocytes/replicate. Mean cleavage and blastocyst rates were 78.4 and 41.5%, respectively.
In vitro embryo selection, evaluation and oxygen measurements
A total of 156 in vitro-produced embryos were measured during 18 experimental days. On each of the 18 experimental days, 5–10 embryos at the blastocyst stage (day 7 of culture) were randomly selected to represent all morphological qualities and individually transferred to one well of a 4-well dish containing culture medium.
Embryos were classified according to the stage of development: blastocysts (n = 17), expanded blastocysts (n = 123; 27 early expanded, 68 expanded or 28 late expanded), collapsed blastocysts (n = 10) or hatching blastocysts (n = 6). Classification into four morphological qualities (quality I, II, III or IV), digital records, diameter assessment as well as oxygen measurements were performed as previously described for in vivo-produced embryos.
Statistical analysis
Analysis of in vivo-produced embryos
Pregnancy status on day 35 was analysed using a linear mixed model (Mixed Procedure, SAS). The models included the stage of embryonic development and morphological quality as fixed effects and donor as random effect. The random effect of donor was tested by a chi-square analysis, before being introduced into the random part of the model.
Respiration rates were tested for the distributional properties, and deviation from normal distribution was not significant (Univariate Procedure, SAS). Respiration rates of day 7 embryos were analysed using a linear mixed model (Mixed Procedure, SAS), including the stage of embryonic development, diameter and morphological quality as fixed effects and donor as random effect. Least squares means (LSMs) for treatment factors were produced by this model.
The effect of embryonic respiration rate on pregnancy was tested using a linear mixed model (Mixed Procedure, SAS) and included pregnancy status on day 35 or 60 as fixed effect and donor as random effect. Using an extended model, we subsequently introduced the factors morphological quality, stage of embryonic development and embryo diameter as fixed effects. Mean embryo respiration rate for cows subsequently diagnosed pregnant or non-pregnant were analysed by the two-tailed Student’s t-test.
The embryos were divided into two and three even-sized categories according to respiration rates, and differences in rates according to the subsequent pregnancy status were estimated by chi-square analysis (Freq Procedure, SAS). Embryos were subsequently divided into three categories where the threshold values (0.78 and 1.10 nl/h) were defined based on the pregnancy status on day 35. The degree of significance of these three categories was also tested using the chi-square analysis (Freq Procedure, SAS).
Analysis of in vivo- and in vitro-produced embryos (combined)
Respiration rates of in vivo- and in vitro-produced embryos were tested jointly for the distributional properties (Univariate Procedure, SAS). Data were subsequently transformed using a square-root transformation, as this was effective in stabilizing variance and obtaining approximate normal distribution of data and residuals.
Following transformation, the respiration rates of in vivo- and in vitro-produced embryos were evaluated by a linear mixed model (Mixed Procedure, SAS), including the following fixed factors: embryo morphological quality, the stage of development, type (in vivo versus in vitro) and all interactions between the type, morphological quality and stage of development. Effects regarded as non-significant were subsequently removed from the analysis. The significance of the results was assessed on the transformed data, but final results (LSM ± SEM) were generated by back transforming (√x) to the original scale.
All statistical procedures were performed using the computational software of SAS (SAS Institute, 1999). The level of significance was P < 0.05, unless otherwise indicated.
Results
Respiration rates of in vivo-produced embryos
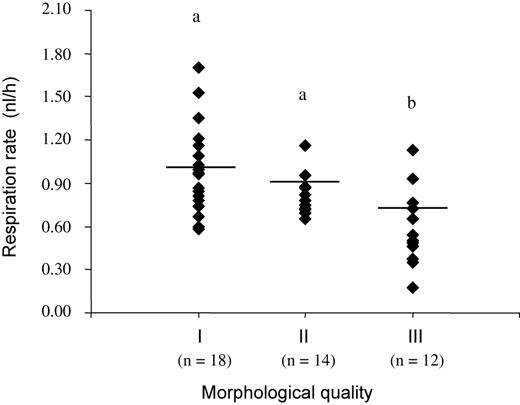
The overall mean respiration rate of day 7 in vivo-produced embryos was 0.82 ± 0.05 nl/h (n = 44). The mean respiration rate decreased with decreasing morphological quality, being 1.01 ± 0.06a (n = 18), 0.89 ± 0.07a (n = 14) and 0.73 ± 0.08b nl/h (n = 12) for quality I, II and III embryos, respectively (a,b: P < 0.05; values with different superscripts differ significantly; Figure 1). Blastocysts had a 35% higher oxygen consumption than compact morulae (P < 0.01). On the contrary, the respiration rates of in vivo-produced embryos were not related to embryo diameter.
Respiration rates (nl/h) of day 7 in vivo-produced embryos according to the morphological quality (a,b: P < 0.05; n = 44). The horizontal bars in the figure represent the mean respiration rates for each morphological quality.
Embryo viability following transfer of in vivo-produced embryos
Pregnancy rates following the transfer of pre-measured in vivo-produced embryos were 65% (28/43) and 60% (26/43), on days 35 and 60 post-estrus, respectively. This is in accordance with the rates routinely observed following embryo transfer in the herd where this study was carried out. Of the blastocysts transferred, 50% (3/6) produced a pregnancy on days 35 and 60 as opposed to 68% (25/37) and 62% (23/37) pregnancy rates observed on days 35 and 60 for the compact morula stage, respectively. After the transfer of quality I, II and III embryos, the associated pregnancy rates on day 35 were 75, 57 and 62%, respectively. Neither the morphological quality nor the stage of embryonic development influenced pregnancy rates on days 35 and 60. A total of 26 normal, viable calves were born following the transfers.
The mean respiration rate for embryos producing a pregnancy was slightly higher but not significantly different from the mean respiration rate of embryos that did not produce a pregnancy (0.79 ± 0.04 versus 0.73 ± 0.07 nl/h; P > 0.05). Neither was the variance of the measured respiration rates significantly different in embryos producing a pregnancy versus those not producing a pregnancy on day 35. Pregnancy on days 35 and 60 was not influenced by respiration rates of day 7 in vivo-produced embryos (P < 0.05). When embryos were divided into two even-sized groups according to their respiration rates, the probability of producing a pregnancy was not significantly different between embryos with high and low respiration rates (P = 0.33; Table I). A similar result was found for three even-sized groups.
Pregnancy status according to respiratory category (high versus low) of bovine in vivo-produced embryos
| Respiratory category . | Pregnant . | Non-pregnant . |
|---|---|---|
| High (>0.75 nl/h) | 70% (n = 14) | 30% (n = 6) |
| Low (<0.75 nl/h) | 45% (n = 11) | 55% (n = 9) |
| Respiratory category . | Pregnant . | Non-pregnant . |
|---|---|---|
| High (>0.75 nl/h) | 70% (n = 14) | 30% (n = 6) |
| Low (<0.75 nl/h) | 45% (n = 11) | 55% (n = 9) |
Threshold limit of 0.75 nl/h was chosen to generate two equal-sized groups.
Pregnancy status according to respiratory category (high versus low) of bovine in vivo-produced embryos
| Respiratory category . | Pregnant . | Non-pregnant . |
|---|---|---|
| High (>0.75 nl/h) | 70% (n = 14) | 30% (n = 6) |
| Low (<0.75 nl/h) | 45% (n = 11) | 55% (n = 9) |
| Respiratory category . | Pregnant . | Non-pregnant . |
|---|---|---|
| High (>0.75 nl/h) | 70% (n = 14) | 30% (n = 6) |
| Low (<0.75 nl/h) | 45% (n = 11) | 55% (n = 9) |
Threshold limit of 0.75 nl/h was chosen to generate two equal-sized groups.
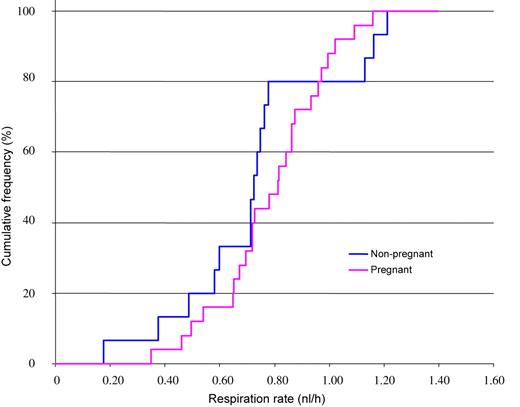
The cumulative frequency curves for respiration rates of embryos associated with pregnant and non-pregnant animals are shown in Figure 2. The pink curve for respiration rate of embryos associated with pregnancy closely followed the sigmoid pattern expected for a normal distribution. However, the blue curve—showing respiration rates for embryos that did not induce pregnancy in their recipient—was different. Embryos subsequently associated with non-pregnant animals seemed to show both very low and very high respiration rates. However, all of the 13 embryos with a respiration rate between 0.78 and 1.10 nl/h resulted in a pregnancy on day 35. The blue curve in Figure 2 thus contains a conspicuous vertical line segment from 0.78 to 1.10 nl/h. This is in agreement with the hypothesis that a normal range of respiration rates exists and that embryos with respiration rates below or above this range have a reduced probability of generating a pregnancy. Thus, three biologically meaningful categories are likely to exist, and the selection of boundaries for the three categories is therefore reasonable. We defined a low, a medium and a high respiratory category, which included embryos with respiration rates <0.78 nl/h, between 0.78 and 1.10 nl/h and >1.10 nl/h, respectively. The pregnancy status of the recipients following transfer of embryos of distinct respiratory categories is summarized in Table II, and no statistically significant differences among the three categories were detected.
Cumulative frequency (%) curves of the respiration rates (nl/h) of day 7 in vivo-produced embryos retrospectively categorized by the pregnancy status of the recipient. The pink and blue curves represent pregnant (n = 28) and non-pregnant animals (n = 15), respectively. The curves indicate the percentage of the given pool of embryos that had a respiration rate less than or equal to a given value (e.g. from the blue curve, it can be observed that 80% of the embryos that did not produce a pregnancy had a respiration rate <0.8 nl/h).
Pregnancy status according to respiratory category (high versus medium versus low) of bovine in vivo-produced embryos
| Respiratory category . | Pregnant . | Non-pregnant . |
|---|---|---|
| High (>1.10 nl/h) | 25% (n = 1) | 75% (n = 3) |
| Medium (0.78–1.10 nl/h) | 100% (n = 13) | 0% (n = 0) |
| Low (<0.78 nl/h) | 48% (n = 11) | 52% (n = 12) |
| Respiratory category . | Pregnant . | Non-pregnant . |
|---|---|---|
| High (>1.10 nl/h) | 25% (n = 1) | 75% (n = 3) |
| Medium (0.78–1.10 nl/h) | 100% (n = 13) | 0% (n = 0) |
| Low (<0.78 nl/h) | 48% (n = 11) | 52% (n = 12) |
Threshold limits (0.78 and 1.1 nl/h) were chosen to reflect the apparent pattern shown in the cumulative frequency curves from Figure 2.
Pregnancy status according to respiratory category (high versus medium versus low) of bovine in vivo-produced embryos
| Respiratory category . | Pregnant . | Non-pregnant . |
|---|---|---|
| High (>1.10 nl/h) | 25% (n = 1) | 75% (n = 3) |
| Medium (0.78–1.10 nl/h) | 100% (n = 13) | 0% (n = 0) |
| Low (<0.78 nl/h) | 48% (n = 11) | 52% (n = 12) |
| Respiratory category . | Pregnant . | Non-pregnant . |
|---|---|---|
| High (>1.10 nl/h) | 25% (n = 1) | 75% (n = 3) |
| Medium (0.78–1.10 nl/h) | 100% (n = 13) | 0% (n = 0) |
| Low (<0.78 nl/h) | 48% (n = 11) | 52% (n = 12) |
Threshold limits (0.78 and 1.1 nl/h) were chosen to reflect the apparent pattern shown in the cumulative frequency curves from Figure 2.
Comparison of respiration rates of in vivo- and in vitro-produced embryos
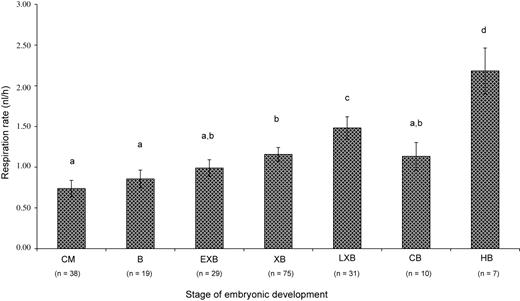
The overall mean respiration rate of day 7 in vitro-produced embryos was 1.35 ± 0.06 nl/h (n = 156), which was considerably higher than the 0.82 ± 0.05 nl/h (n = 44) observed for day 7 in vivo-produced embryos. After square-root transformation, the corresponding back-transformed values were 1.26 ± 0.05 and 0.79 ± 0.05 nl/h, respectively. Regardless of the apparent difference in mean respiration rates between in vivo- and in vitro-produced embryos, embryo type was found not to influence embryonic oxygen consumption. Instead, the stage of embryonic development and morphological quality within type significantly affected respiration rates, with embryos of better morphological quality and at more advanced stages of development showing higher respiration rates (Table III; Figure 3).
Respiration rates (nl/h; mean ± SEM, back-transformed from square root) of in vivo- and in vitro-produced embryos according to the morphological quality
| Morphological quality . | Embryo type . | |||
|---|---|---|---|---|
| . | . | |||
| . | In vivo . | In vitro . | ||
| . | . | . | ||
| . | n . | Respiration rate . | n . | Respiration rate . |
| I | 18 | 1.26 ± 0.14a,x | 38 | 1.70 ± 0.11a,y |
| II | 14 | 1.24 ± 0.20a,x | 52 | 1.27 ± 0.08b,x |
| III | 12 | 0.96 ± 0.19b*,x | 47 | 1.15 ± 0.08b,x |
| IV | – | – | 19 | 0.79 ± 0.11c |
| Morphological quality . | Embryo type . | |||
|---|---|---|---|---|
| . | . | |||
| . | In vivo . | In vitro . | ||
| . | . | . | ||
| . | n . | Respiration rate . | n . | Respiration rate . |
| I | 18 | 1.26 ± 0.14a,x | 38 | 1.70 ± 0.11a,y |
| II | 14 | 1.24 ± 0.20a,x | 52 | 1.27 ± 0.08b,x |
| III | 12 | 0.96 ± 0.19b*,x | 47 | 1.15 ± 0.08b,x |
| IV | – | – | 19 | 0.79 ± 0.11c |
a,b,c,x,y: P < 0.05, b*: P = 0.07; values with different superscripts within the same column (a, b, b*, c) and within the same row (x, y) vary significantly.
Respiration rates (nl/h; mean ± SEM, back-transformed from square root) of in vivo- and in vitro-produced embryos according to the morphological quality
| Morphological quality . | Embryo type . | |||
|---|---|---|---|---|
| . | . | |||
| . | In vivo . | In vitro . | ||
| . | . | . | ||
| . | n . | Respiration rate . | n . | Respiration rate . |
| I | 18 | 1.26 ± 0.14a,x | 38 | 1.70 ± 0.11a,y |
| II | 14 | 1.24 ± 0.20a,x | 52 | 1.27 ± 0.08b,x |
| III | 12 | 0.96 ± 0.19b*,x | 47 | 1.15 ± 0.08b,x |
| IV | – | – | 19 | 0.79 ± 0.11c |
| Morphological quality . | Embryo type . | |||
|---|---|---|---|---|
| . | . | |||
| . | In vivo . | In vitro . | ||
| . | . | . | ||
| . | n . | Respiration rate . | n . | Respiration rate . |
| I | 18 | 1.26 ± 0.14a,x | 38 | 1.70 ± 0.11a,y |
| II | 14 | 1.24 ± 0.20a,x | 52 | 1.27 ± 0.08b,x |
| III | 12 | 0.96 ± 0.19b*,x | 47 | 1.15 ± 0.08b,x |
| IV | – | – | 19 | 0.79 ± 0.11c |
a,b,c,x,y: P < 0.05, b*: P = 0.07; values with different superscripts within the same column (a, b, b*, c) and within the same row (x, y) vary significantly.
Respiration rates (mean ± SEM; nl/h) of individual embryos according to the stage of embryonic development (in vivo and in vitro embryos analysed together; back-transformed data from square-root transformation; CM, compact morula; B, blastocyst; EXB, early expanded blastocyst; XB, expanded blastocyst; LXB, late expanded blastocyst; CB, collapsed blastocyst; HB, hatching blastocyst; a,b,c,d: P < 0.05, values with different superscripts vary significantly).
Discussion
For the first time, the respiration rates of individual day 7 in vivo-produced embryos were accurately measured with the Nanorespirometer and the measured embryos subsequently individually transferred fresh to synchronized recipients. Hence, this study allowed us to compare the respiration rate and viability of a single embryo following transfer.
The Nanorespirometer was accurate and consistent in measuring individual embryonic respiration rates, as demonstrated by the high correlation between the first and the second measurement of the same embryo as well as by the negligible background noise of the empty control capillaries (which is likely attributed to the extremely small oxygen consumption by the electrochemical microsensor during the measurements). Furthermore, this technology did not interfere with embryo viability, as pregnancy rates resulting from the transfer of embryos after measurements of respiration rate were in agreement with those published by others working with similar systems (Hasler, 1998; Farin et al., 1999) and with the results routinely achieved following embryo transfer in the herd where this study was carried out. In fact, the pregnancy rates obtained in this study following measurements of embryo respiration rate were slightly higher than the rates normally observed for each morphological quality. These normally range between 65 and 70%, 50–70% and 30–50% following the transfer of quality I, II and III embryos, respectively (Madsen SE, personal communication).
The respiration rates obtained in this study for in vivo-produced embryos are considerably different from those documented by Thompson et al. (1995) and Overström (1996), as they were 0.7 ± 0.1 and 1.47 ± 0.44 nl/h, respectively. The observed difference might be attributed to differences in methodology, techniques and approaches, and hence, direct comparisons may not be possible. The respiration rates of in vivo-produced embryos decreased with decreasing morphological quality, indicating that morphological differences are at least partly reflected at the level of oxygen consumption. Nevertheless, the respiration rates of quality I and II embryos were not significantly different. A similar result was found by Overström (1992), who did not observe any differences in oxygen consumption among embryos of different morphological qualities. The significant but limited correlation between embryo morphological quality and respiration rate observed in this study and in a previous study using in vitro-produced embryos (Lopes et al., 2005a) suggests that combining measurements of oxygen consumption with assessment of morphological quality might improve embryo selection.
As opposed to our previous observations for in vitro-produced embryos (Lopes et al., 2005a), we could not detect a significant correlation between embryo diameter and respiration rate for in vivo-produced embryos. Correlation between embryo diameter and oxygen consumption has been reported earlier (Shiku et al., 2001). The lack of correlation between respiration and diameter observed in this study could be related to the fact that in vivo-produced embryos are a more homogeneous population with a more even size distribution as compared with in vitro-produced embryos (Holm et al., 1998). Another explanation could be that very few in vivo-produced embryos were at the blastocyst stage (n = 9), where the diameter and associated respiration rate increase considerably.
The higher respiration rates observed for blastocysts as compared with morulae have previously been described (Thompson et al., 1996; Harvey et al., 2002, 2004; Abe et al., 2005) and seem to be associated with a higher energy demand for protein synthesis and activity of the plasma membrane Na+-K+-dependent ATPase during blastocoel formation. However, this association can also reflect the difference in cell number between these two stages of embryonic development, as the number of cells of an embryo appears to be correlated with oxygen consumption (Donnay, 2002).
No significant correlation between individual respiration rates and subsequent viability of the embryos was demonstrated in our study, where embryo viability was assessed by evaluating the pregnancy status of the recipients receiving the pre-measured embryos. No single threshold value beyond which more pregnancies could be observed was established. However, such a simple interpretation is in most cases inadequate for crucial physiological parameters like respiration. For such key parameters, we frequently observe an optimal range, and adverse conditions as well as a poor prognosis are likely to occur when the observed parameter is below or above this range.
The cumulative frequency distribution of the pregnancies according to respiration rate in Figure 2 (and notably the observation that three out of four embryos with the highest respiration were associated with non-pregnancy) led us to speculate that both very high and very low levels of embryonic respiration rates could be associated with non-pregnancy, whereas the best chance for pregnancy would be found among embryos with middle values of respiration. This interpretation of the data is reflected in the two chosen threshold limits that form the basis for Table II. In this case, very low respiration rates would correlate with adverse alterations in oxidative phosphorylation and in other oxygen-related processes, and consequently with reduced embryo viability and increased mortality. Moreover, a very high respiration rate could represent a manifestation of metabolic stress, which is associated with reduced embryo viability. The fact that two of the three embryos that did not produce a pregnancy in the high respiratory category (Table II) were at the blastocyst stage suggests that the lower pregnancy rates obtained in this group could also be associated with the reduced capacity of fast-cleaving embryos to produce a pregnancy, as previously reported (Ziebe et al., 1997). Nevertheless, none of the previous hypotheses could be statistically verified with the limited number of embryos investigated in this study. Previous attempts to correlate respiration rate and embryo viability suggested that higher survival rates of mouse and bovine embryos were achieved with the highest respiration rates (Overström et al., 1989, 1992). Yet, in these studies, the survival rates were evaluated after the transfer of groups of, and not individual, embryos.
Various factors can account for the observed difference in respiration rates even for in vivo-produced embryos that subsequently produced a pregnancy. Among these factors is the precise time of fertilization, which cannot be accurately determined, particularly when cows were used as donors where insemination was performed twice. Indeed, embryos were collected either at the compact morula or at the blastocyst stage, and 60% of the donors yielding compact morulae with respiration rates <0.78 nl/h and resulting in pregnancy were cows re-inseminated 12 h after the first insemination. The possibility of these embryos being only produced after the second insemination, thus being substantially younger, could partly explain the successful implantation of embryos with low respiration rates. Although most of the embryos were collected at the compact morula stage, the degree of compaction possibly differed, and this could also partly explain the surprisingly low respiration rates of some of the embryos that were subsequently associated with a pregnancy.
The recipients are obviously a potential confounding factor, when the evaluation of embryo viability is based solely on the subsequent pregnancy status. Pregnancy rates are highly influenced by the quality of the recipients. In fact, the ability of the recipients to produce and maintain a pregnancy following embryo transfer has been regarded as more important than the morphological quality of the embryos transferred (McMillan, 1998).
Moreover, as oxygen consumption correlates with the number of cells of an embryo (Kaidi et al., 2001) and the cell number does not apparently reflect the viability of an embryo after transfer (Donnay, 2002), it is likely that some bias has been introduced. The limited number of embryos transferred in this study has likewise limited the statistical significance of the observations, and therefore, further investigations involving a higher number of individual transfers are required to clarify the nature of the relationship between embryonic respiration and viability.
Overall, the respiration rates of in vivo-produced embryos were not significantly lower than those of day 7 in vitro-produced embryos. The apparent difference in mean respiration rates between in vivo- and in vitro-produced embryos was mostly explained by the different ratio of embryos of each morphological quality within embryo type. Differences between these two embryo types were only observed for quality I embryos and are likely associated with differences in the stage of development. These results are somewhat surprising, as in vivo-produced embryos have previously been described as being different from their in vitro counterparts also at the metabolic level (Partridge and Leese, 1996; Khurana and Niemann, 2000). Furthermore, it could be expected that the culture conditions and media components such as glucose, amino acids, pyruvate and serum, which are known to affect embryo metabolism (Eckert et al., 1998; Lane and Gardner, 2000; Donnay, 2002; Abe et al., 2005), would have had quantifiable effects on the resulting embryos, which would have been detected during the respiration measurements.
Consistent with previous observations for in vivo-produced embryos, when both embryo types were jointly analysed, a strong correlation between respiration rate and the stage of embryonic development could be established. Such correlation has previously been described (Thompson et al., 1996; Donnay and Leese, 1999; Abe et al., 2005). Moreover, in line with previously reported data (Thompson et al., 1995; Sturmey and Leese, 2003), reduced levels of embryo respiration were observed after maximum expansion, i.e. at the collapsing stage before hatching. The reduced respiration rate of the collapsed embryos has earlier been explained by the reduced activity of Na+-K+-dependent ATPase after complete formation of the blastocoel. One could also speculate that embryos enter a stage of ‘temporary quietness’ before the strong metabolic outburst occurs, at the time of hatching.
The presented experiment allows us to conclude that measuring the respiration rates of individual bovine embryos using the Nanorespirometer did not influence their subsequent viability, as embryos developed into viable fetuses at rates considered normal under farm conditions and all calves born were normal and viable. As no statistically validated correlation between respiration rate and pregnancy status could be demonstrated, it is possible that the use of this equipment will not help in identifying the most viable in vivo-produced embryos just by measuring the respiration rates, both because in vivo-produced embryos are known to be a more homogeneous population and because confounding factors such as the quality of the recipients seem to play a major role in the pregnancy outcome. On the contrary, it is possible that the use of this technology can bring new possibilities and prove beneficial in improving selection of in vitro-produced embryos, especially in the human field where the selection of proper recipients is obviously not an issue.
Conflict of interest
Niels Ramsing holds shares of Unisense FertiliTech A/S.
Acknowledgements
The authors thank Anette Pedersen, Klaus Villemoes, Lars Hauer Larsen, Lars Borregard Pedersen and Ruth Kristensen for their technical assistance. Dr P. Horn is appreciated for critically reviewing the manuscript. Novo Nordisk Foundation financially supported the acquisition of the nanorespirometer technology and the Danish Directorate for Food, Fisheries and Agri Business (TFH03-8) the embryo work. Ana Sofia Lopes is supported by a grant from the Foundation for Science and Technology (FCT), Ministry of Science and Technology, Portugal (SFRH/BD/8486/2002).