-
PDF
- Split View
-
Views
-
Cite
Cite
Bruno Lunenfeld, Historical perspectives in gonadotrophin therapy, Human Reproduction Update, Volume 10, Issue 6, November/December 2004, Pages 453–467, https://doi.org/10.1093/humupd/dmh044
Close - Share Icon Share
Abstract
The 20th century witnessed the steady development of knowledge about the reproductive process in animals and humans. These advances led to the identification of higher centres governing the dynamics of ovarian function and to the discovery of gonadotrophic hormones. As the mechanisms of action of these hormones became increasingly understood, they began to be used in the management of infertility during the early 1930s. Hormone extracts were originally prepared from animal pituitaries and pregnant mare serum, as well as from human pituitaries, placenta and urine, with pregnancies reported following their use in the late 1930s. This review traces the constant quest to reduce risks and improve safety and efficacy of hormone preparations for patients. It describes the complex path and perils leading to the pure hormone preparations that are available today, concluding with an optimistic glimpse towards the future. Small molecules that are orally active and specific are currently being investigated, some with the capacity to bypass many parts of the receptor conformation. Here lies the immediate future of this field, utilizing low-cost, small, defined molecules to stimulate follicle growth, ovulation and corpus luteum formation. Perhaps one day the classical gonadotrophins will no longer be required in clinical treatment.
Introduction
Although gonadotrophin therapy is now taken for granted as an essential component in the routine management of infertility, a great deal of discovery and research was necessary in order to develop preparations that are safe and effective for clinical use. The history of this process originated with early attempts to extract and purify preparations from animals, human cadavers and human urine, eventually evolving to their production by recombinant DNA technology. Highly refined cell culture techniques are now used to prepare recombinant molecules derived from Chinese hamster ovary (CHO) cells. This process of evolution has been constantly driven by the need to make gonadotrophin products safe, pure, and effective not only in treatment but also in ease of use for the patient. Reliable batch-to-batch consistency is also needed in order to minimize the plethora of possible variables involved, and thus reduce variability in infertility treatment. An examination of the history in detail reveals that the road to efficient clinical use developed along a long and tedious pathway, which included many mistakes as well as important scientific encounters. This review will trace these events, from the past through to the present, and conclude with a glance towards the future (Table I).
Early understanding of the hypothalamic–pituitary–ovarian axis
The first experimental evidence suggesting that the pituitary has a role in regulating the gonads stems from the studies of Crowe et al. (1910). They showed that partial pituitary ablation resulted in atrophy of the genital organs in adult dogs, and a persistence of infantilism and sexual inadequacy in puppies. Thus, by 1910 these early studies launched the idea that the reproductive organs were governed by the pituitary gland.
Two years later, Aschner (1912) confirmed these findings and also postulated that pituitary function depends upon the function of higher centres in the brain. He observed that men and women with diseases, tumours or injuries of the hypophysis, pituitary stalk and centres including and above the medulla oblongata suffered hypopituitarism, and consequently, gonadal atrophy. He further demonstrated that sectioning of the pituitary stalk affected the genital organs, and therefore hypothesized that pituitary extracts may affect the gonads. He postulated that their use might have practical applications.
Another 15 years were to elapse before two different groups independently discovered the ‘gonadotrophic principle’. In 1926, Smith showed that daily implants of fresh anterior pituitary gland tissue from mice, rats, cats, rabbits and guinea-pigs into immature male and female mice and rats rapidly induced precocious sexual maturity, marked enlargement of the ovaries and superovulation (Smith, 1926; Smith and Engle, 1927). In the same year, Zondek (1926) implanted anterior pituitary glands from adult cows, bulls and humans into immature animals, and this evoked the rapid development of sexual puberty. These pioneering experiments revealed that ovarian function is regulated by the pituitary. Smith (1930) demonstrated that hypophysectomized immature male or female rats and mice failed to mature sexually, and that removal of the pituitary gland from adult animals without injury to the brain resulted in profound atrophy of genital organs, rapid regression of sexual characteristics and total loss of reproductive function in both sexes.
Only 3 years later, Zondek (1929) proposed the idea that the pituitary secretes two hormones that stimulate the gonads. He named these biological substances ‘Prolan A’ and ‘Prolan B’. The word ‘Prolan’ is probably derived from the Latin word ‘proles’, which means ‘descendant’. By introducing this name, Zondek undoubtedly wished to imply that these substances were the ‘spiritus movens’ of sexual function, the master hormones that control all the gonadal sex hormones, and are therefore responsible for maintaining the species.
Zondek (1930) then showed that the blood and urine of post-menopausal women contained gonadotrophins. He postulated that Prolan A stimulated follicular growth, that Prolan A together with Prolan B stimulated the secretion of ‘foliculin’, and that Prolan B induced ovulation, the formation of the corpus luteum and the secretion of lutein and foliculin. These two hormones induced the glandular transformation of the endometrium, with endometrial proliferation, and also caused changes in the vaginal epithelium. Zondek realized that the dynamics of Prolan A secretion by the anterior pituitary and the correct timing of Prolan B discharge are responsible for the rhythm of ovarian function: this in turn controlled the proliferation and function of the endometrium to create optimal conditions for nidation of the fertilized oocyte (Figure 1). If we merely change the names of Prolan A and B to FSH and LH, and the names of foliculin and lutein to estrogen and progesterone, we can see that by 1930 Zondek had described the pituitary–gonadal relationship as we know it today. This hypothesis was confirmed a year later with the extraction of two different hormones from the pituitary, one of which acted as a follicle-stimulating factor and one as a luteinizing factor (Fevold et al., 1931).
The discovery of hCG
Ascheim and Zondek (1927) demonstrated that the blood and urine of pregnant women contained a gonad-stimulating substance: injecting this substance subcutaneously into intact immature female mice produced follicular maturation, luteinization and haemorrhage into the ovarian stroma—this became known as the Ascheim Zondek pregnancy test. Ascheim and Zondek believed that this gonadotrophic substance was produced by the anterior pituitary. Subsequent work by Seegar-Jones et al. (1943) showed that this gonadotrophin was produced in vitro in placental tissue culture, proving conclusively that the placenta, not the pituitary, was responsible for the elaboration of the hormone. This gonad-stimulating property was exhibited by the chorionic villi, and was especially marked in the cytotrophoblastic Langhans cells.
Marius Tausk's book on the history of Organon (Tausk, 1978) describes the gonadotrophic hormone hCG (extracted from human placenta) as being very similar to the pituitary hormone ‘Prolan B’ (= LH). Organon launched this extract on the market in 1931, under the name ‘Pregnon’. However, because of similarity to another trademark, the name was later changed to ‘Pregnyl’. Pregnyl was released in 1932, when regulatory authorities for the evaluation and approval of medicines did not yet exist, and has survived until the present day. According to Tausk this preparation was used for the ‘stimulation of the ovaries’ at first, and the initial hCG products were calibrated in animal units. A rat unit was defined as the amount that produced vaginal opening together with estrus when injected into female immature rats. The International standard for hCG was established in 1939, under the auspices of the League of Nations. The International Unit (IU) was defined as the activity contained in 0.1 mg of the standard preparation. Purified urinary preparations of hCG became available in 1940 (Gurin et al., 1940). Urine obtained during the first half of pregnancy is chilled, filtered and acidified to pH 3.5 with glacial acetic acid. The clear filtrate is percolated through a column containing permutit, and adsorption is complete when 10 litres of urine per hour are passed through a 4 inch diameter column containing 2 kg of permutit. The active principle is eluted from the column with an alcoholic solution of ammonium acetate, and the hormone is precipitated from the eluate by increasing the concentration of alcohol. The potency of these preparations ranged from 6000 to 8500 IU/mg (Katzman et al., 1943). Clinical studies with hCG began as early as 1930 (Hamblen, 1933,1935) and were summarized by the same author 15 years later (Hamblen et al., 1945): women who were scheduled for non-gynaecological abdominal surgery were injected with hCG, and the ovaries were inspected during the operation. When hCG was administered in the follicular phase of the cycle, their ovaries showed no evidence of follicle stimulation, ovulation or corpus luteum formation, i.e. in the absence of FSH, no visual effect of hCG could be seen. Hamblen and Ross (1937) confirmed these results.
The introduction of hog and sheep gonadotrophins for clinical use
These early discoveries revealing the physiological action of gonadotrophins in the normal ovarian cycle tempted many scientists to seek gonadotrophic extracts with sufficient purity to allow their use in the treatment of infertile patients suffering from gonadotrophin insufficiency. In 1930, gonadotrophins extracted from swine pituitaries were produced by IG Farbenindustrie A-G, Leverkusen, Germany, and used clinically to treat patients.
Several years later a hog preparation became available from The Armour Laboratories, and GD Searle then produced a commercially available sheep pituitary FSH preparation (Gonadophysin; Maddock et al., 1956). Two animal pituitary gonadotrophin extracts are quoted in the 1959 French drug index (Vidal, 1959): Gonadohormone (Laboratoires Byla) and Hormone Gonadotrope Hypophysaire Choay (Laboratoires Choay). Both were reimbursed by the French social security system. Maddock et al. (1956) described the increase in urinary estrogen excretion during FSH administration. Enlarged cystic ovaries measuring 7–10 cm in diameter were observed in some of the patients, and clear cysts as large as 2 cm in diameter were seen in other patients; microscopically these were lined with granulosa cells undergoing early lutein changes (Maddock et al., 1956). Netter (1959) also described a spectacular increase in urinary estrogen following short treatments (1–3 ampoules of 10 mouse units on alternate days for up to 9 days) with a commercially available animal pituitary gonadotrophic extract (Gonadohormone; Laboratoires Byla). Gonadotrophin extracts from animal pituitaries continued to be used in Europe and the USA until the early 1960's; their use began to decline after the discovery of a new phenomenon, the ‘anti-hormones’.
Two important monographs were published independently in 1942: ‘Antigonadotrophic substances’ (Ostergaard, 1942) and ‘The antigonadotrophic factor with consideration of the anti-hormone problem’ (Zondek and Sulman, 1942). Both claimed that gonadotrophins from animal origin produced ‘anti-hormones’, which decreased ovarian responsiveness in humans. To quote Zondek and Sulman: ‘It was noted in 1930, during chronic treatment with gonadotrophic hormone, that the effector organ, i.e. the ovary, maintains its response only in a limited period of time, at the end of which the response becomes increasingly weaker and finally disappears’. They further stated in the book: ‘Chronic treatment of animals with gonadotrophic hormones evokes in them the formation of a new blood substance, called an anti-hormone. This is capable of inactivating gonadotrophin hormone both in vivo and in vitro’. Thus, more than two decades before the nature of immunological phenomena was fully recognized, the authors had actually described the formation of antibodies to animal gonadotrophins in women. Maddock (1956) confirmed this previous work and described the detection of ‘Antihormones’ between the 44th and 76th days following prolonged treatment with animal pituitary FSH preparations. The ‘Antihormones’ prevented the action of each of the gonadotrophins against which they were tested (hog FSH, human pituitary gonadotrophin, hCG), and remained at detectable levels for 2–3 months after stopping gonadotrophin administration. A new treatment course provoked a prompt and striking increase in antihormone titres. Following an editorial by Wilkins (1953) describing ‘the need for an inhibitor of gonadotrophins’ for certain gynaecological diseases, Maddock (1956) concluded that ‘the induction of antihormone formation by the administration of animal pituitary FSH may have therapeutic applications in cases in which it is desirable to inhibit pituitary gonadotrophins’.
Pregnant mare serum gonadotrophin (PMSG)
PMSG is secreted by structures known as endometrial cups in pregnant mares, and was first described by Cole and Hart (1930). Endometrial cups, circular structures on the surface of the endometrium around the point of attachment of the fetus, secrete a highly viscous gel. PMSG first appears in this gel between days 37 and 42 of pregnancy, and reaches its highest concentration between days 50 and 70. It virtually disappears after the 4th month of gestation. The hormone produced in the endometrial cups is also present in the blood, and PMSG was prepared by collecting blood from pregnant mares around the 65th day of pregnancy. This procedure involved fractional precipitation with acetone or alcohol, removal of impurities by proper adjustment of acidity (pH 7.0–7.5) and final precipitation of the hormone fraction in the presence of increased concentrations of acetone or alcohol. The hormone fractions were obtained as dry white, water-soluble and remarkably stable powders, and furnished a satisfactory basis for preparing sterile solutions that could be used for laboratory and clinical studies (Cartland and Nelson, 1937). This material stimulated follicular growth, ovulation and corpus luteum formation in the ovary, and estrus changes in the uterus and vagina. Cartland and Nelson defined their rat unit as the total dose of PMSG which will produce a 5-fold increase in ovarian weight.
In 1938 an international standard for PMSG was established. One International Unit was defined as 0.25 mg of the standard preparation (Burn, 1950). Soon afterwards commercial preparations of PMSG appeared on the market. Schering Corporation in the USA marketed Anteron, and Organon and Roussell in Europe marketed ‘Gestyl’ and ‘Gonadotrophine Serique’. Both preparations were reimbursed by ‘Social Security’ in France. Clinical trials in women demonstrated an ovarian response to these gonadotrophins (Fevold et al., 1931; Fevold, 1937; Hamblen, 1940), but attempts to induce ovulation produced inconsistent results. In 1939, Hamblen showed that ‘cyclic administration of PMSG during the follicular phase of the cycle, in amounts judged to be adequate, failed to result in progestational bleeding or progestational changes in the endometrium (as judged by endometrial biopsy studies) or in pregnancy’ (Hamblen, 1935, Hamblen, 1939).
The two-step protocol
The concept of the ‘two-step protocol’ was introduced in 1941: ovarian stimulation using gonadotrophins (PMSG, or hog or sheep pituitary gonadotrophins) to stimulate follicular growth and development, followed by the induction of ovulation using hCG. Mazer and Ravetz (1941) used ‘Synapoidin’, a mixture of a human pregnancy urine extract and an extract of animal anterior pituitary lobe tissue (commercially available from Parke, Davis & Co.). The authors state: ‘The combination of chorionic gonadotrophin and the anterior pituitary extract evoked one or more menstrual flows in 19 of 23 severely amenorrhhoeic women, some of whom had not menstruated for years’. The authors also claimed that ‘the degree of the ovarian response seems to depend upon the receptivity of the ovaries, the total dosage and the duration of treatment. On the other hand, the ovaries of three regularly menstruating young women were over-stimulated to a pathological degree. Each of the ovaries was 8–10 cm in diameter and studded with numerous haemorrhagic follicles which ruptured and bled at the slightest touch’. Thus, as early as 1941, the hyperstimulation syndrome was described.
Hamblen et al. (1945) defined the ‘ideal treatment’: ‘To permit effective therapy of hypo-functioning ovaries, a gonadotrophin should evoke, in sequence, follicle stimulation, ovulation and corpus luteum development, and these phenomena should be in physiologic order compatible with fertility and conception.’ They demonstrated that administration of PMSG during the follicular phase, followed by the application of hCG 12–18 days later, resulted in secretory endometrium and pregnancies following correctly planned coitus.
Although the antibody formation provoked by pregnant mare serum gonadotrophin caused biological neutralization of the injected material, it did not cause anaphylactic shock or severe allergic reactions. PMSG and other gonadotrophic preparations from animal pituitary sources were therefore used for many years. Despite the fact that during the 1940s gonadotrophins of animal origin (formerly marketed as Synapoidin Steri-Vial in the USA) were shown to produce allergic reactions, approval of the US Food and Drug Administration for Synapoidin Steri-Vial was not withdrawn until July 6, 1972 (see the Federal Register of July 6, 1972: 37 FR 13284). As late as 1962, Folistiman (VEB Arneimittelwerk Dresden), a highly purified, standardized FSH preparation from pig pituitaries, was introduced on the East German market. These preparations were used in the hope that perhaps ovulation and consequent pregnancy could be evoked within the first few months of treatment, before the immune response and its consequences had fully developed. A number of pregnancies were indeed reported (Vesell, 1938; Rydberg and Madsen, 1949; Rydberg and Ostergaard, 1939; Daume, 1970; Groot-Wassink and Blawert, 1973). Daume compared the results of ovulation induction by sheep gonadotrophin extract with those obtained using urinary hMG. The pregnancy rate per treatment cycle was 11.5 and 12.7% for the animal and human preparations respectively. Groot-Wassink and Blawert (1973) compared the results (pregnancy rate) of an animal and a human FSH preparation, and found that gonadotrophins derived from animal sources gave significantly better results. They explained this difference on the basis that poor results obtained with preparations derived from human sources were due to an excess of LH: the FSH:LH ratio was 11:1 in the human preparation, and 70:1 in the animal preparation. Groot-Wassink and Blawert (1973) were the first to claim that excessive LH could have adverse effects on reproductive performance.
PMSG eventually had to be withdrawn from the market because of the potential dangers as a consequence of provoking antibody formation. However, animal gonadotrophins under the trade name Folistiman (VEB Arzneimittelwerk, Dresden) were still available in some East European countries until 1998. Recognizing the fact that animal gonadotrophins might produce antibodies in humans which could neutralize not only the preparation applied, but also the endogenous gonadotrophins, scientific and technological efforts were focused on extracting and purifying gonadotrophins from human sources. During the summer of 1953, Dr Rudi Borth and myself, with the help of Prof. H. de Watteville invited a number of scientists to Geneva in order to exchange information and to coordinate research on gonadotrophins. Egon Diczfalusy, Jim Brown, John Loraine and others were amongst those invited. The ‘G Club’ was founded during this meeting, and the basic and clinical goals of gonadotrophic research defined. These included development of specific assay procedures, as well as bioassay standards and purification methods required to obtain gonadotrophic preparations suitable for therapeutic purposes.
Human pituitary gonadotrophins (hPG)
In 1958 Carl Gemzell extracted gonadotrophins from human pituitary glands. The lyophilized glands were extracted with calcium oxide solution, and gonadotrophins precipitated with ammonium sulphate, dissolved in water, dialysed and lyophilized. The clinical results achieved with the use of this preparation were published in 1958 (Gemzell et al., 1958). Buxton and Hermann (1961) tested a pituitary FSH preparation prepared by Merck & Co. from lyophilized pituitaries; gonadotrophins were extracted with 40% ethanol and precipitated by increasing the alcohol concentration. Bettendorf (1963) demonstrated that ovarian stimulation is possible in hypophysectomized individuals. Between 1958 and 1988, hPG preparations were successfully used for ovulation induction in the treatment of ovulation disturbances in several centres throughout the world. However, it soon became clear that the reservoir of human pituitaries was too limited to cover the constantly growing demand for gonadotrophin preparations. Moreover, more than 20 years after its commercial introduction, hPG made the headlines when cases of iatrogenic Creutzfeld–Jakob disease (CJD) were discovered and linked to the use of hPG or human pituitary growth hormone—cases of CJD were identified in Australia, in France, and in the UK (Cochius et al., 1990; Dumble and Klein, 1992).
It is interesting to note that none of these cases arose from the use of products registered by pharmaceutical companies—they were traced to the use of products produced by government agencies: the Pituitary Agency in Australia, the Pituitary Agency in the UK, and France-Hypophyse. hPG was subsequently withdrawn from the market, bringing to an end another era in the history of gonadotrophin use.
Human menopausal gonadotrophins
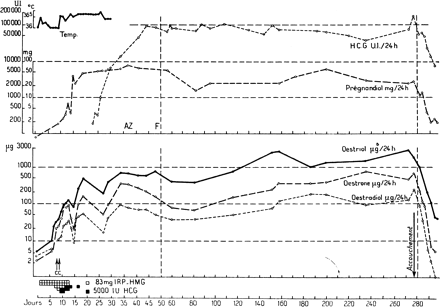
Human menopausal gonadotrophins were purified and isolated from crude extracts of large urine pools, and a number of extraction and concentration procedures were proposed. The method of Bradbury et al. (1949), modified by Albert (1955), used kaolin for adsorption and acetone for precipitation of proteins (gonadotrophins), and this method was accepted by many laboratories and used successfully for many years, with minor variations (Figure 2). After numerous significant alterations, this procedure was subsequently used to produce considerable amounts of hMG for clinical use. Production of hMG is a relatively simple procedure (Donini and Montezemolo, 1949; Donini et al., 1964; Lunenfeld and Donini, 1966). Menopausal urine is mixed with activated kaolin and shaken, the suspension is left to settle at room temperature and then centrifuged. The supernatant is discarded, and the kaolin cake is eluted twice with 1 mol/l NH4. The eluate containing adsorbed urinary proteins, including gonadotrophins, is washed thoroughly, acidified to pH 5.5 and the proteins are then precipitated with acetone. The precipitate, after washing with acetone, ethanol and ether, is subjected to treatment with diethyl-aminoethyl cellulose and passed through a permutit chromatography column. The pH must be accurately controlled during all production stages to ensure a good yield of gonadotrophins: pH must be ∼4.0 at the adsorption phase and between 11.0 and 11.5 during the elution phase. Final stages of production include washing, filtering, adjustment of the FSH and LH contents and lyophilization. The first hMG preparation for clinical use ‘Pergonal 25 Serono’ was registered in Italy on May 22, 1950. The definition of 1 Unit was based upon the capacity of the product to induce estrus in 28 day old pre-pubertal female rats. In 1953, hMG was successfully used for ovarian stimulation of hypophysectomized rats (Borth et al., 1954). The second meeting of the G club took place in Birmingham during 1955, and a large batch of of a menopausal urine kaolin extract (hMG 20) donated by Dr J.Dekansky from Organon Newhouse in Scotland was designated as a laboratory standard. Borth et al. (1957a,b) described the transition from animal units to the expression of results in relation to reference preparations. The Unit was initially defined in relation to the reference preparations hMG 20, hMG 20a and hMG 24 (Albert et al., 1958; Benz et al., 1959). By 1959 most of the reference preparation hMG 24 had been used, and Dr Dekansky could not make available the amount originally agreed upon. Dr Donini from Serono declared his willingness to donate 50 g of the preparation ‘Pergonal 23’, and this material became the ‘International Reference Preparation’ (IRP-hMG) for the quantification of hMG (Lunenfeld, 1961). Clinical trials using hMG preparations were then initiated. The purity of the available preparations was only 5%, containing both FSH and LH. However, in the absence of an alternative, they were accepted both by the regulatory agencies and the scientific community. In 1960 the use of this preparation in humans led to the expected and desirable changes in endometrium, vaginal epithelium and steroid excretion (Lunenfeld et al., 1960). In 1961 we noted that amenorrhoeic hypogonadotrophic women needed 6.8–13.6 mg of Pergonal, depending on individual sensitivity (120–240 mg equivalent of the international reference preparation) in order to stimulate their ovaries to produce estrogens (Lunenfeld et al., 1961). Thereafter we reported the first successful induction of ovulation followed by pregnancies in hypogonadotrophic anovulatory women using a sequential step-up/step-down regime. The starting dose in these women was 240 mg IRP-hMG daily, increased to 360 mg and then to 480 mg IRP-hMG, and then gradually reduced to 360 mg and finally to 240 mg IRP-hMG daily. Ovulation was induced by administration of 10 000 IU of hCG followed by 10 000 and 5000 IU of hCG on consecutive days (Figure 3). This hCG dose was sufficient to maintain corpus luteum function until endogenous hCG appeared ∼13 days later (Lunenfeld et al., 1962a,b; Lunenfeld, 1963). Our results were confirmed by Palmer and Dorangeon (1962) in France, and Rosenberg et al. (1962) in the USA. In 1964 the WHO Expert Committee of biological Standardization defined the international unit (IU) for FSH and the IU for ICSH Interstitial cell stimulating hormone (LH) as the respective activities contained in 0.2295 mg of the IRP hMG.
A retrospective conversion of IRP-hMG to IU of FSH and LH showed that the doses we had used were between 55 and 110 IU/daily injection. Following numerous reports on the successful induction of ovulation, Pergonal 75 was registered in Israel in 1963 and in Italy in 1965. One ampoule of this hMG preparation contained ∼75 IU of FSH and 75 IU of LH as measured by standard bioassays.
In 1972 the World Health Organization convened a scientific group meeting in Geneva, which I had the privilege to chair. During this meeting, guidelines for the diagnosis and management of infertile couples were developed (World Health Organization, 1973). The effective daily dose for hypogonadotrophic patients was reported to be in the range of 150–225 IU, and for anovulatory normogonadotrophic patients 75–150 IU. It was also noted that the FSH:LH ratio varies in different hMG and hPG preparations, but the available evidence indicated that preparations with ratios of 0.1–10 would be acceptable therapeutic agents provided that a sufficient total dose of FSH is administered to the patient.
Two years later, in 1975 the WHO Expert Committee of Biological Standardization met under my chairmanship (TRT 565) and noted that ‘since preparations of hMG are administered to man in many countries, it is desirable to have an international standard for the control of potency of such preparations’. The Committee defined the International Unit for human urinary FSH for bioassay as the activity contained in 0.11388 mg and the International Unit for human urinary LH (ICSH) for bioassay as the activity contained in 0.13369 mg of the international standard. The committee also stressed that the new standard, future standards and preparations calibrated against it should have their separate activities [FSH and LH (ICSH)] individually assessed.
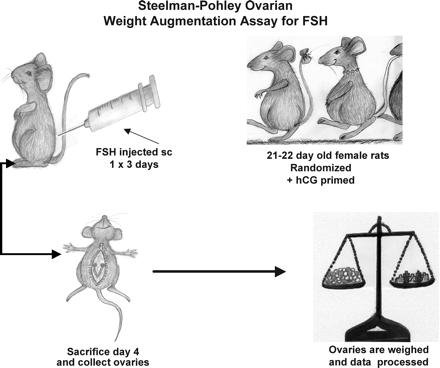
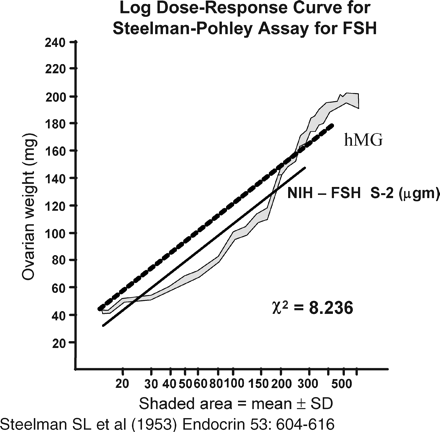
The Steelman and Pohley (1953) assay for FSH estimation became the gold standard. In this assay, a group of immature rats is injected subcutaneously with hCG, and then injected with the international standard of FSH once daily for 3 days. A second group of animals is injected with the same amount of hCG, and then with the preparation to be tested once daily for 3 days. Autopsy is performed 72 h after the first injection, at which time the ovaries are dissected and weighed (Figure 4). The FSH content of the preparation is calculated from the curve obtained with the standard (Figure 5). When preparations with varying FSH:LH ratios were tested by this method, the LH content did not interfere (Lunenfeld, 1967). However, although the assay method is specific, its precision depends on the number of animals used and is relatively low even with large numbers. A source of homogeneous FSH that could be standardized with respect to mass and bioactivity (Keene et al., 1989) would bring a significant advantage.
The search for pure preparations
In the early 1970s, clinicians began to voice the opinion that different patient groups and individuals may need different treatment regimes, with variations in protocols and in dosages of FSH and LH. Such individually adjusted treatment regimes would require therapeutic gonadotrophin preparations that contained pure, or almost pure, FSH and LH. Attempts to separate FSH from LH in gonadotrophin extracts were pursued via a multitude of modifications in various methods. Proteins containing FSH and LH extracted from pituitaries or from urine were subjected to digestion with trypsin (Jutisz, 1965), pancreatic enzymes (Segaloff and Steelman, 1959) or urea (Ellis, 1961). Butt et al. (1964) supplemented the digestion process with removal of inert proteins by starch gel electrophoresis. Other authors proposed purifying FSH from the LH contamination by zone electrophoresis or by chromatography on diethylamino ethyl cellulose (DEAE-C) (Porath, 1964; Reichert and Parlow, 1964). Gemzell's group used chromatography and Sephadex gel filtration (Roos and Gemzell, 1964). Donini et al. (1966) combined electrophoresis and chromatography with lyophilization of the efluent and its subsequent filtration on a Sephadex column. This method yielded a gonadotrophin preparation containing 384.4 IU of FSH activity and 5.6 IU of LH activity per mg of protein. However, none of the above efforts brought a real solution, being either too cumbersome, too complicated and expensive, or not sufficiently accurate and efficient. New developments in immunological techniques (Lunenfeld et al., 1961a,b) opened a virtually limitless horizon for measuring and producing several hormones, including purified FSH. These new procedures were devised through the synergy of four different aspects: advances in knowledge of theoretical immunology; technical developments in macromolecular chemistry; progress in nuclear physics which enabled in vitro iodination using radioactive isotopes (125I and 131I) and availability of highly purified and potent hormone preparations. Specific hyperimmune anti-hCG serum preparation had already been attempted in the 1930s (Bachman, 1935; Twombly, 1936). However, the gonadotrophin preparations available at that time were crude, and contained large amounts of non-specified proteins, each of which served as a separate antigen. This resulted in the formation of a ‘mixed’ antiserum, which was neither sufficiently specific to accurately recognize the hCG antigen, nor sensitive enough to allow detection of small amounts of this hormone. This method was refined in the 1960s (Eshkol and Lunenfeld, 1967) by using an immuno-column with polyclonal anti-LH antibodies. A urinary hMG preparation containing both FSH and LH was filtered through the column; all of the proteins contained in hMG, including FSH, pass through the column, and LH is retained in the column, bound by the specific antibodies. The eluted FSH is then purified and lyophilized to a biologically pure FSH preparation, with only minimal LH activity (Lunenfeld and Eshkol, 1970). The final product (Metrodin) contained 150 IU of FSH and 1 IU of LH per milligram of protein.
Preparation of purified gonadotrophins, coupled with the availability of macromolecules permitting the development of a whole array of efficient immunoabsorbents, enabled the pharmaceutical industry to introduce purified FSH preparations almost free from LH contamination (Donini et al., 1966).
Highly purified urinary FSH
Further technological advances made it possible to replace polyvalent antibodies with highly specific monoclonal antibodies. The production of purified urinary FSH was essentially a ‘passive’ process, in which LH was separated from bulk material and FSH, together with some other urinary proteins, were collected and lyophilized for use. ‘Third generation gonadotrophin’, i.e. highly purified urinary FSH (FSH-HP), is produced by a more direct process. The affinity column uses highly specific monoclonal antibodies to selectively bind the FSH molecules in the hMG bulk material. The unbound urinary protein and LH pass through the column and are removed, leaving pure FSH retained by the column. This is then extracted as a highly purified product, devoid of both LH and contaminating urinary proteins. As a result of the improved processing, this FSH preparation (Metrodin HP) contains <0.1 IU of LH activity and <5% of unidentified urinary proteins. The specific activity of the FSH is increased from ∼100–150 IU/mg of protein in purified urinary FSH preparations (Metrodin) to ∼9000 IU/mg protein in the highly purified product (‘Metrodin HP’). The purity is also increased from 1–2% to 95%. This enhanced purity means that the total amount of injected protein is very small, making the highly purified urinary FSH preparation suitable for subcutaneous administration. Not only is batch-to-batch variability virtually eliminated, but the product now lends itself to detailed analysis by physico-chemical methods in addition to the classical in vivo bioassay. The technical developments that led to the production of highly purified FSH, together with a deeper understanding of the pharmacodynamics and pharmacokinetics of these preparations, have all made it possible to redesign ovulation-inducing protocols (for example, low dose regimens, low dose increments, subcutaneous injection route). New protocols that use pure hormone preparations with complete batch-to-batch consistency offer the potential of improved efficiency by facilitating a more effective treatment plan that can be adjusted to a predictable response. Although we cannot control ‘intra-patient variability’, prospective management after assessing response to an initial treatment cycle can be more reliably planned, without the concern of an inconsistent response due to batch-to-batch variations in the drug preparations. This allows the design of ‘tailor made’ protocols for individual patients, so that the number of developing follicles can be better controlled, reducing the risk of multiple pregnancies in ovulation induction cycles, and of hyperstimulation in both ovulation induction and assisted reproductive treatment cycles.
The use of highly purified FSH preparations indicated that the role of estrogen as a marker of follicular development required re-evaluation. Animal experiments (Eshkol and Lunenfeld, 1967) and studies in a patient with 17α-hydroxylase deficiency (Rabinovici, 1989) demonstrated that follicular growth and development can take place despite extremely low levels of estrogens, and that quantitative estrogen levels do not necessarily represent an accurate assessment of follicular growth and development. When pure FSH is administered, the effect on follicular estrogen production will depend on the presence and the amount of LH produced by the patient. Since ultrasound examination can be used to assess ovarian follicular growth (a measure of FSH activity) and uterine endometrial thickness (a marker of estrogenic stimulation), ultrasound assessment of the ovaries and uterus will suffice to monitor the effects of FSH administration. A single estrogen determination prior to the planned ovulation induction can be used to predict hyperstimulation, and to decide on further management (withholding hCG, etc.).
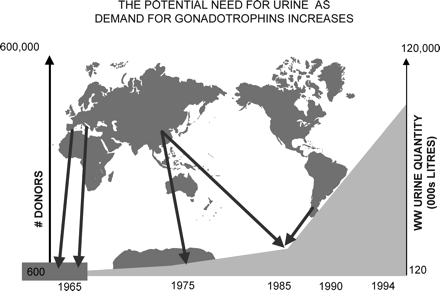
In the past, human pituitaries and menopausal urine were the sole source for production of human-derived gonadotrophin preparations (hPG); hPG preparations were abandoned when cases of iatrogenic Creutzfeld–Jakob disease (CJD) were recognized, and until recently, menopausal urine represented the only primary source. It then became evident that there are serious shortcomings in the use of menopausal urine as a source. When the urinary extraction process was started, there were four urine collecting centres: one in The Netherlands, one in Spain, one in Israel and one in Italy. Altogether, 600 women participated in these collection centres, and each single woman was well known by the collectors. If any of these women fell ill or was treated with drugs such as antibiotics, their urine samples were rejected. Over a period of 1 year, these groups of women produced 120 000 litres of urine, an amount absolutely sufficient for treating hypopituitary–hypogonadotrophic amenorrhoeic women (WHO I) worldwide at that time. At the beginning of this millenium, 120 000 000 litres of urine were necessary to satisfy the worldwide need—an increase of 100-fold, which required 600 000 donors (Figure 6). These donors were recruited from countries in Europe, Korea, China, India and South America. Since this process was no longer based upon individual collections, an increasing number of safety measures had to be included.
The shortcomings of the urine extraction process can be summarized:In addition to these concerns, a protease-resistant form of prion protein has been found in the urine of scrapie-infected hamsters, bovine spongiform encephalopathy (BSE)-infected cattle, and humans suffering from CJD (Shaked, 2001; Gabizon, 2003). The prion protein was also identified in 29 human urine samples from 38 individuals with probable prion disease, but not in 20 patients diagnosed with Alzheimer's disease (Furukawa et al., 2003). Experimental studies show that high levels of infectivity can be found in the brain and spleen of animals that do not develop clinically apparent disease during a normal lifespan, and it now seems that subclinical forms of prion disease exist (Hill and Collinge, 2003). Such asymptomatic prion ‘carrier states’, as well as preclinical/clinical prion disease, may be relevant when analysing potential risks associated with biological products. If asymptomatic carriers of vCJD prion infection exist in the human population, they represent a potential risk to others via iatrogenic routes of prion transmission, although they might not develop clinical prion disease themselves.
Lack of regulatory control.
Impossible to trace donor source.
Quality cannot be checked during transportation.
Urine sources cannot be validated.
Decontamination may denature proteins.
Cross-contamination cannot be avoided.
Poor quality control.
Limited source.
Concerns about potential disease transmission led a number of countries to apply the ‘precautionary principle’: in 1996 the Australian Drug Evaluation Committee published its resolution on replacement of urinary with recombinant gonadotrophins in view of their higher standard of purity and safety. In the same year, France introduced a class warning regarding viral safety risk on all urinary gonadotrophin leaflets. In 2003, The UK Medicines Control Agency withdrew highly purified FSH (Metrodin HP) from the UK market as a precaution against the theoretical risk of vCJD transmission ((SCRIP, 2003). The Swissmedic letter (2003) stated: ‘Urine from countries, which belong to a GBR-class with a higher risk or in which no secured knowledge concerning status and monitoring system of transmissible spongiform encephalopathy (TSE), such as China and Korea, should, as a precaution to improve safety, no longer be used. In addition, it has to be taken into account that, for certain preparations, recombinant products are now available. For those reasons, Swissmedic considers preventive measures to be reasonable and necessary.’
The future of infertility therapy clearly relies on the capacity to produce pharmaceutical grade gonadotrophins in sufficient quantities to meet the ever-increasing worldwide demand (Figure 7) and to reduce the risk of biological contamination, small as it may be. The detailed information now available regarding the physiological processes involved in the synthesis of gonadotrophins by pituitary cells, along with the development of recombinant DNA technology, now carries the potential to produce pharmacologically active FSH preparations in unlimited quantities.
Recombinant gonadotrophins
The general principle behind obtaining recombinant human gonadotrophins relies first of all upon identification and separation of the appropriate protein molecules. The amino acid sequences of the FSH α- and β-subunits were described by Rathnam and Saxena (1975) and Saxena and Rathnam (1976) respectively, and the gene encoding the FSH molecule was subsequently cloned, Howles, (1996). Under appropriate conditions, this gene is incorporated into the nuclear DNA of a host cell via a plasmid vector, using spliced DNA strings containing the FSH gene and segments of bacterial DNA. This molecular biology ‘trick’ allowed the FSH gene to be expressed in mouse fibroblasts. However, the task of producing recombinant gonadotrophin molecules proved to be more difficult. Early technology focused on producing biological molecules in bacterial cells (usually Escherichia coli). Bacteria efficiently produce non-glycosylated peptides such as insulin, and yeast has been cloned for the production of certain vaccines. However, the structural complexity of human gonadotrophins such as FSH, and the need for post-translational modification of the molecule by protein folding and glycosylation, made functional protein production impossible in prokaryotes. The sites and extent of glycosylation determine tertiary structure, degradation time, the regions of the molecule exposed to target cell receptors, and exposure of the molecule to mechanisms that regulate metabolism in vivo.
Recombinant glycosylated peptides may be synthesized by certain mammalian cell lines, and this led to the investigation of mammalian cell culture systems that were capable of producing functional molecules (Chappell, 1992). The genes coding for the human FSH α-subunit and β-subunit were inserted into cloning vectors (plasmids) to enable efficient transfer into recipient cells. These vectors also contained promoters that could direct transcription of foreign genes in recipient cells. CHO cells were selected as recipient cells, since they are easily transfected with foreign DNA, and are capable of synthesizing glycoproteins. Furthermore, they can be grown in cell cultures on a large scale.
The world's first recombinant human FSH (rhFSH; follitropin alfa) preparation for clinical use was produced by Serono laboratories in 1988, and was licensed for marketing in the European Union as Gonal-F® in 1995. A similar rhFSH (follitropin beta, Puregon®) product was licensed by Organon laboratories in 1996. In the manufacture of Gonal F, Serono used two separate vectors to construct an FSH-producing cell line, one vector for each sububnit (Howles, 1996). Puregon was manufactured by NV Organon, using a single vector containing the coding sequences of both subunit genes (Olijve, 1996). Following transfection, a genetically stable tranformed cell line producing biologically active FSH was isolated. The CHO line used for the production of Puregon had 150–450 gene copies present.
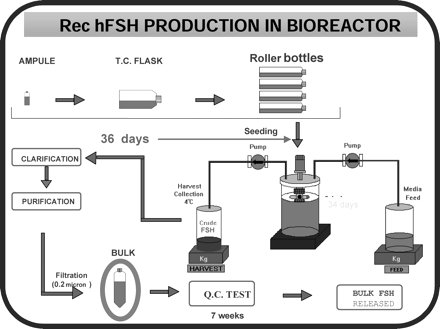
For the purpose of bioproduction, stable cell lines were selected that expressed FSH dimer in relatively abundant amounts. A master cell bank (MCB) was established, which contains identical cell preparations of the clone that was selected on the basis of high FSH productivity. The resulting recombinant FSH was more homogenous than the most highly purified pituitary FSH preparations, providing a basis for clinical use. Specific cell clones have now been selected for large scale production of recombinant FSH, LH and hCG. The resultant preparations have high purity and high biological potency (FSH >10 000, LH 9000 and hCG 20 000 IU/mg protein respectively). These cell preparations are stored in individual vials and cryopreserved until needed. A working cell bank is established by growing cells from a single vial of the MCB, and aliquots of this culture are then cryopreserved in vials. Cells from one or more vials are cultured for each production cycle. Figure 8 summarizes the steps involved in bulk production of rhFSH. The FSH-producing CHO cells are grown on microcarriers in a bioreactor, perfused with growth promoting medium for a period of up to 3 months. The cell culture supernatant medium is collected from the bioreactor for isolation of recombinant FSH.
The downstream purification process differs for the two comercially available recombinant FSH preparations. The Puregon process uses a series of anion and cation exchange chromatographic steps, hydrophobic chromatography and size exclusion chromatography. The Gonal F process uses a similar series of five chromatographic steps (Figure 7), and also includes an immunoaffinity step with a specific monoclonal antibody. Each purification step is rigorously controlled in order to ensure batch to batch consistency of the purified product.
Clinical use of recombinant gonadotrophins
During the early 1990s, several IVF programmes reported pregnancies following the use of recombinant FSH to induce ovulation for IVF (Devroey et al., 1992; Germond et al., 1992; Out et al., 1995). The recombinant preparations were shown to be at least as efficient as the urinary preparations in the management of assisted reproduction cycles (Daya et al., 2001).
Agrawal et al. (1997) reported the first birth following stimulation of follicular growth in a hypopituitary–hypogonadotrophic woman (WHO Group I) with recombinant FSH and recombinant LH; recombinant hCG was used to induce ovulation. Donderwinkel et al. (2002) reported a pregnancy following ovulation induction with rFSH in a patient with polycystic ovaries.
Recent advances in the manufacturing process for the rFSH (follitropin alfa) result in high batch-to-batch consistency in both isoform profile and glycan species distribution. The most significant advantage of this preparation over urinary-derived FSH is that it permits FSH to be quantified reliably by protein content (mass in μg) rather than by biological activity; this indication of purity offers optimal risk reduction as well as superior quality assurance and batch-to-batch consistency. The coefficient of variation for an in vivo bioassay is typically ±20%, compared with 2% for physico-chemical analytical techniques such as size-exclusion high-performance liquid chromatography (SE-HPLC; Driebergen et al., 2002). As a result, Serono International now quantify their rFSH (Gonal-F®) protein by SE-HPLC, a precise and robust assay that results in a significant improvement in batch-to-batch consistency over batches quantified by the Steelman–Pohley bioassay (Hugues et al., 2003).
Future developments
Protein engineering offers an approach for expanding the range of recombinant gonadotrophins available for infertile women. Using today's medications, daily injections of FSH are necessary for effective ovarian follicle stimulation; for the future, new FSH molecules may be developed that are engineered to possess an extended half-life and duration of therapeutic action. Such molecules will enable the physician to provide single injections to drive follicle growth for up to a week, in a controlled and predictable fashion.
Recombinant DNA technology permits the design of potent therapeutically active gonadotrophin agonists and antagonists by altering key proteins and carbohydrate regions in the α- and β-subunits of FSH and LH (Boime et al., 1990). FSH has a relatively short half-life, and hCG has a relatively long half-life. The long half-life of hCG is in part due to the presence of four serine O-linked oligosaccharides attached to an extended hydrophilic carboxyterminus. Site-directed mutagenesis and gene transfer techniques made it possible to fuse the carboxyterminal extension of hCGβ (CTP) to the 3′ end of the FSH coding sequence. The recombinant FSH-CTP fusion protein retained the same biological activity as native FSH in vivo, but had a prolonged circulating half-life. This gives the molecule an in vivo potency significantly higher than native FSH, making it an obvious candidate as a long-acting FSH agonist (Fares et al., 1992). The results achieved with this long-acting FSH molecule in an international, multicentre trial were published recently (Bouloux et al., 2001). No severe drug-related adverse events were observed when the molecule was administered to 13 hypogonadotrophic males, although symptoms of mild local reaction occurred in 39% of the subjects. Antibodies to either component of the FSH molecule were not observed, and the elimination half-life was reported to be 94.7±26.2 h, which is two to three times longer than that of natural FSH (Bouloux et al., 2001). A study in healthy female volunteers showed that a single dose of rFSH-CTP induced multiple follicular growth accompanied by a dose-dependent rise in serum inhibin-B (Duijkers et al., 2002). The first live birth after ovarian stimulation using a chimeric long-acting human rFSH agonist (rFSH-CTP) was reported by Beckers et al. (2003). Ovarian stimulation was initiated on day 3 of a spontaneous menstrual cycle with 180 μg rFSH-CTP. This was followed by 150 IU rFSH, concomitant with a GnRH antagonist for another 2 days. hCG 10 000 IU was administered on cycle day 12, and 12 oocytes were subsequently retrieved. Ten oocytes were fertilized, and, following the transfer of two embryos, a healthy child was spontaneously delivered at term.
A recombinant human FSH analogue with an extended half-life has been identified at the Serono Reproductive Biology Institute (SRBI). Powerful bioinformatics computer modelling approaches were applied to the three-dimensional structure of FSH in order to predict how new molecular structures might ‘resist’ the physiological mechanisms that inactivate and clear it from the body. This protein, termed GM-1, displays an extended half-life: when delivered to rats via subcutaneous injection, the circulating levels of the molecule 2 days later remain ∼6-fold higher than those seen after identical administration of rFSH (Gonal-F®).
Serono International S.A. is also developing a novel, long-acting formulation of recombinant FSH. The new formulation is based on Alkermes' ProLease injectable sustained-release drug delivery technology, which involves the encapsulation of a drug into small polymeric microspheres. These degrade slowly and release the encapsulated drug at a controlled rate following subcutaneous or intramuscular injection. The new formulation is designed to offer patients the alternative of a single injection rather than multiple daily injections. Data on this formulation are yet to be published.
It may be of historical interest to note that in 1966 our group (Insler et al., 1966) attempted to prolong the effect of hMG by using a synthetic plasma expander as a vehicle. The activity of hMG dissolved in saline and injected into mice increased up to 24 h followed by a significant decrease. The activity of hMG in Dextran increased up to 24 h, levelled off between 24 and 48 h and decreased significantly 72 h after the injection.
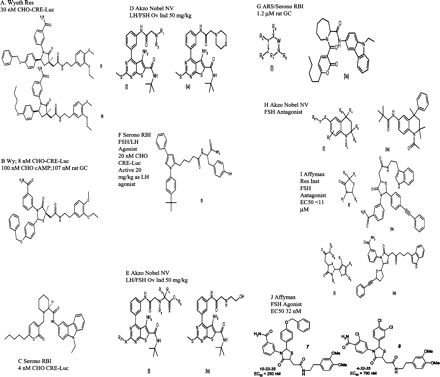
For companies committed to developing a wider range of innovative medicines for infertility, a generation of orally bioavailable gonadotrophin mimetics has been the ‘holy grail’ of drug development research for several years. As knowledge about the activating sites of gonadotrophins and GnRH analogues has increased, it has become possible to create small, non-peptide molecules that induce signal transduction without binding to the extracellular domains of membrane proteins. Such molecules will ultimately be converted into highly potent orally active therapeutic preparations, and will either replace the dimeric glycoprotein hormones or act as antagonists. One classical approach is high-throughput screening of large chemical libraries to find small molecule (<500 Da) agonists of human FSH or LH receptors. Indeed, work on the human LH receptor illustrates that this approach has met with some success: a pyrazolyl tyrosine amide molecule has been shown to activate LH receptors expressed either heterologously in CHO cells or naturally on the surface of testicular Leydig cells. Organon recently reported the first human exposure study using a low molecular weight LH agonist (Org 43553) in female volunteers of reproductive age. In this study, oral administration of Org 43553 up to single doses of 2700 mg was well tolerated and safe. The mean peak concentrations of Org 43553 were at 0.5–1 h and the mean elimination half-life (t½) varied between 30 and 47 h. Based on follicle rupture observed by ultrasound and rises of serum progesterone (>15 nmol/l), treatment with a single oral dose of Org 43553 resulted most frequently in ovulation in the 300 mg and 900 mg dose groups (Mannaerts, 2004). Figure 9 illustrates the range of non-peptide molecules currently under investigation that are gonadotrophin-mimetic and orally active.
It is therefore feasible that the future of ovarian stimulation will be a simple treatment schedule composed of different tablets, taken at certain time-points during the ovarian stimulation cycle.
Conclusion
As summarized in Figure 10, major advances in technology have brought the field of gonadotrophin therapy a very long way since the era of animal-, human pituitary-, and urinary-derived hormones. It seems clear that new pharmaceutical products will offer the safest option for ovarian stimulation. Pure FSH preparations with ∼13000 IU FSH/mg protein are now accessible, offering significant risk reduction for patients as well as the assurance of superior quality control over the final product. We must therefore ask ourselves if it is medically prudent and ethically admissible to use preparations derived from urine of untraceable donor source, contaminated with extraneous proteins.
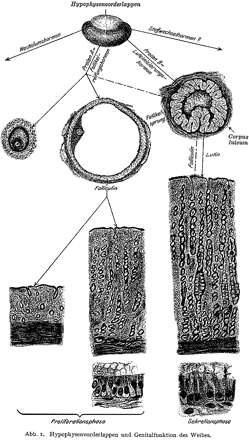
Zondek's illustration of the relationship between the hypothalamus, pituitary, ovaries, and endometrium (Zondek, 1930). Reprinted by permission from Zondek (1930), Ueber die Hormones des Hypophysenvorderlappens. Klin Wochenschrift 9,245–248, Copyright Springer Verlag.
Extraction of gonadotrophins from menopausal urine. (A) Collection of pooled urine. (B) Menopausal urine filtered through a kaolin cake.
The chart illustrating the first successful induction of ovulation followed by pregnancies in hypogonadotrophic anovulatory women, using a sequential step-up/step-down regime. The starting dose of 240 mg (equivalent to 55 IU) IRP-hMG daily was increased to 360 mg, and then to 480 mg (=110 IU) IRP-hMG, gradually reduced to 360 and finally to 240 mg IRP-hMG daily. Ovulation was induced by administration of 10 000 IU of hCG followed by 10 000 and 5000 IU of hCG on consecutive days.
Diagrammatic illustration of the Steelman–Pohley FSH bioassay. A group of immature rats is injected subcutaneously with hCG, and then injected with the international standard of FSH once daily for 3 days; a second group of animals is injected with the same amount of hCG, and then with the preparation to be tested once daily for 3 days. Autopsy is performed 72 h after the first injection, at which time the ovaries are dissected and weighed.
Standard curve used to calculate FSH content (μg) in samples tested with the Steelman–Pohley bioassay. NIH=National Institutes of Health Standard.
The potential requirement for urine as demand for gonadotrophins increased between 1965 and 1995. In the 1960s, 600–1000 donors were sufficient to supply the urine necessary for the production of hMG. The development of new clinical indications and the expansion of infertility treatment on a worldwide basis resulted in an exponential increase in demand, and the number of donors required exceeded 100 000 by the early 1990s. These were recruited from Europe, Korea, China, India and South America; donor sources and urine collection could no longer be traced, controlled or regulated, leading to major concerns about safety.
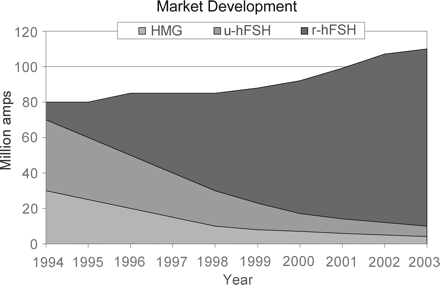
The change in worldwide demand for gonadotrophins. Approximately 2–3 litres of urine are required to prepare one vial of hMG, and one vial of purified FSH requires 6–9 litres. As recombinant gonadotrophins became available, the number of donors required began to decrease, and by 2003, ∼40 × 106 donors were needed to produce 20 × 106 ampoules of urinary gonadotrophins. The benefits offered by recombinant technology, and the application of precautionary principles, are reflected in the increased use of rhFSH from 1994 onwards.
Preparation of recombinant human FSH. An aliquot from the selected clone of Chinese hamster ovary cells is first grown in T-flasks, then subcultured into roller bottles and allowed to expand for up to 36 days. The cells are then mixed with a suspension of microcarrier beads and transferred to a bioreactor vessel with continuous culture media infusion for an average duration of 34 days. The harvested ‘crude FSH’ is stored at 4°C until purification. The final product is released after extensive quality control testing over a period of 7 weeks.
Molecular structure of a number of gonadotrophin-mimetic and orally active non-peptide molecules that are currently under investigation.
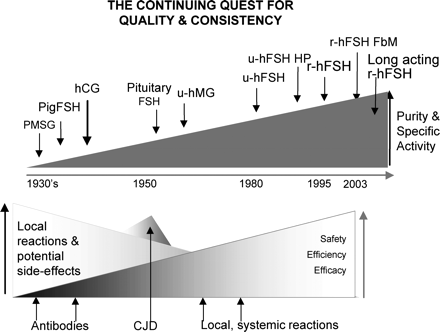
The continuing quest for quality and consistency. Between 1930 and 1972, gonadotrophins from animal pituitaries and pregnant mare serum were used; due to allergic reactions and lack of efficacy, they were abandoned. Between 1958 and 1988, gonadotrophins from the pituitaries of human cadavers were used; they were also abandoned, due to the threat of Creutzfeld–Jacob disease. In the early 1960s, the purity of hMG was 5%, the remaining 95% consisted of significant amounts of known and unknown contaminating proteins. By the mid-1960s, improved purification processes were implemented that led to uFSH containing less LH, but contaminated with 95% of unwanted proteins. Improved purification processes still only achieved a purity of 40–90%. Between 1996 and 2003, precautionary principles were applied in a number of countries leading them to restrict or abandon the use of urinary gonadotrophins. FbM=Filled by Mass.
Milestones in gonadotrophin therapy, indicating the first pregnancies reported after the use of increasingly refined products
| First pregnancy achieved with: . | Author(s) . |
|---|---|
| ‘Antophysin’ | Vesell (1938) |
| Pregnant mare serum gonadotrophin/hCG | Hamblen et al. (1945) |
| hMG | Lunenfeld et al. (1962) |
| Human pituitary gonadotrophin (hPG) | Gemzell (1962) |
| hPG in a hypophysectomized patient | Bettendorf (1963) |
| Recombinant hFSH in IVF (rhFSH, follitropin alfa) | Germond et al. (1992) |
| Recombinant hFSH (follitropin beta) in IVF | Devroey et al. (1992) |
| Recombinant FSH in a PCO patient | Donderwinkel et al. (2002) |
| Recombinant FSH-CTP | Beckers et al. (2003) |
| First pregnancy achieved with: . | Author(s) . |
|---|---|
| ‘Antophysin’ | Vesell (1938) |
| Pregnant mare serum gonadotrophin/hCG | Hamblen et al. (1945) |
| hMG | Lunenfeld et al. (1962) |
| Human pituitary gonadotrophin (hPG) | Gemzell (1962) |
| hPG in a hypophysectomized patient | Bettendorf (1963) |
| Recombinant hFSH in IVF (rhFSH, follitropin alfa) | Germond et al. (1992) |
| Recombinant hFSH (follitropin beta) in IVF | Devroey et al. (1992) |
| Recombinant FSH in a PCO patient | Donderwinkel et al. (2002) |
| Recombinant FSH-CTP | Beckers et al. (2003) |
PCO = polycystic ovaries; CTP = c-terminal peptide.
Milestones in gonadotrophin therapy, indicating the first pregnancies reported after the use of increasingly refined products
| First pregnancy achieved with: . | Author(s) . |
|---|---|
| ‘Antophysin’ | Vesell (1938) |
| Pregnant mare serum gonadotrophin/hCG | Hamblen et al. (1945) |
| hMG | Lunenfeld et al. (1962) |
| Human pituitary gonadotrophin (hPG) | Gemzell (1962) |
| hPG in a hypophysectomized patient | Bettendorf (1963) |
| Recombinant hFSH in IVF (rhFSH, follitropin alfa) | Germond et al. (1992) |
| Recombinant hFSH (follitropin beta) in IVF | Devroey et al. (1992) |
| Recombinant FSH in a PCO patient | Donderwinkel et al. (2002) |
| Recombinant FSH-CTP | Beckers et al. (2003) |
| First pregnancy achieved with: . | Author(s) . |
|---|---|
| ‘Antophysin’ | Vesell (1938) |
| Pregnant mare serum gonadotrophin/hCG | Hamblen et al. (1945) |
| hMG | Lunenfeld et al. (1962) |
| Human pituitary gonadotrophin (hPG) | Gemzell (1962) |
| hPG in a hypophysectomized patient | Bettendorf (1963) |
| Recombinant hFSH in IVF (rhFSH, follitropin alfa) | Germond et al. (1992) |
| Recombinant hFSH (follitropin beta) in IVF | Devroey et al. (1992) |
| Recombinant FSH in a PCO patient | Donderwinkel et al. (2002) |
| Recombinant FSH-CTP | Beckers et al. (2003) |
PCO = polycystic ovaries; CTP = c-terminal peptide.
I would like to express my gratitude to B. Fauser for reviewing and commenting on the first draft of this review, and to many of my colleagues for their comments and criticism. Special thanks to William Mac Donald and his colleagues for helping to obtain old and important documents, and to Kay Elder for her precious expert help in editing this review.
References
Agrawal R, West C, Conway GS, Page ML and Jacobs HS (
Albert A (
Albert A, Borth R, Diczfalusy E, Loraine JA, Lunenfeld B, Mcarthur JW and Rosemberg E (
Ascheim S and Zondek B (
Aschner B (
Bachman C (
Beckers NG, Macklon NS, Devroey P, Platteau P, Boerrigter PJ, Fauser BC (
Benz F, Borth R, Brown PS, Crooke AC, Dekanski JB, Diczfalusy E, Loraine A, Lunenfeld B, Schuler W (
Bettendorf G (
Boime I, Keene J, Galway AB and Fares FM (
Borth R, Lunenfeld B and de Watteville H (
Borth R, Diczfalusy E and Heinrichs HD (
Borth R, Lunenfeld B and et Watteville de H (
Bouloux PM, Handelsman DJ, Jockenhovel F et al. (
Bradbury JT, Brown ES and Brown WE (
Burn JH, Finney DJ and Goodwin LG (
Butt WR, Cunningham FJ and Hartree A (
Buxton CL and Hermann W (
Cartland GF and Nelson JW (
Chappel S, Kelton C, Nugent N, et al. (
Cochius JI, Mack K and Burns RJ (
Cole HH and Hart GH (
Crowe SJ, Cushing H and Homans J (
Daume E (
Daya S, Ledger W, Auray JP, Duru G, Silverberg K, Wikland M, Bouzayen R, Howles CM and Beresniak A (
Devroey P, Van Steirteghem A, Mannaerts B and Coelingh Bennink H (
Donderwinkel PFJ, Schoot DC, Coelingh I, Bennink HJT and Fauser CJM (
Donini P and Montezemolo R (
Donini P, Puzzuoli D and Montezemolo R (
Donini P, Puzzuoli D, D'Alessio I, Lunenfeld B, Eshkol A and Parlow AF (
Driebergen R, Basset R, Baer G et al. (
Duijkers IJ, Klipping C, Boerrigter PJ et al. (
Dumble LD and Klein RD (
Ellis S (
Eshkol A and Lunenfeld B (
Fares FA, Suganuma N, Mishimori K, LaPolt P, Hsue AJ and Boime W (
Fauser BC (
Fevold HL (
Fevold SL, Hisaw FL and Leonard SL (
Furukawa H, Shirabe S and Niwa M (
Gabizon R (
Gemzell CA (
Gemzell CA, Diczfalusy E and Tillinger G (
Germond M, Dessole S, Senn A et al. (
Groot-Wassink K and Blawert H (
Gurin S, Bachman G and Wilson DW (
Hamblen EC (
Hamblen EC (
Hamblen EC (
Hamblen EC (
Hamblen EC and Ross RA (
Hamblen EC, Davis CD and Durham NC (
Hill AF and Collinge J (
Howles CM (
Hugues J-N, Barlow DH, Rosenwaks Z et al. (
Insler V, Rikover M, Lunenfeld B and Toledo R (
Katzman PA, Godfried M, Cain CK and Doisy EA (
Keene JL, Matzuk MM, Otani T, Fauser BCJM, Galway AB, Hsueh AJW and Boime I (
Lunenfeld B (
Lunenfeld B (
Lunenfeld B (
Lunenfeld B and Donini P (
Lunenfeld B and Eshkol A (
Lunenfeld B, Menzi A and Volet B (
Lunenfeld B, Rabau E, Rumney G and Winkelsberg G (
Lunenfeld B, Givol D and Sela M (
Lunenfeld B, Sulimovici S and Rabau E (
Lunenfeld B, Sulimovici S, Rabau E and Eshkol A (
Maddock WO, Leach RB, Tokuyama I, Paulsen A and Roy WR (
Mannaerts (2004) Personal communication.
Mazer C and Ravetz E (
Netter A (
Olijve W, de Boer W, Mulders JW, van Wezenbeek PM (
Out HJ, Mannaerts BMJL, Driessen SGA, Coelingh Bennink H. (
Palmer R and Dorangeon P (
Porath J (
Rabinovici J, Blankstein J, Goldman B, Rudak E, Dor Y, Pariente C, Geier A, Lunenfeld B and Mashiach S (
Rathnam P and Saxena B (
Reichert LE and Parlow AF (
Roos P and Genzell CA (
Rosenberg E, Coleman J, Damani M and Garcia CR (
Rydberg E and Madsen V (
Rydberg E and Ostergaard E (
Saxena BB and Rathnam P (
Seegar-Jones GE, Gey GO and Ghisletta M (
Segaloff A and Steelman SL (
SCRIP (2003) Metrodin HP withdrawn in the UK. Scrip World Pharmaceutical News (a PJB publication.) Vol 2824,18.
Shaked GM, Shaked Y, Kariv-Inbal Z, Halimi M, Avraham I and Gabizon R (
Smith PE (
Smith PE (
Smith PE (
Smith PE and Engle ET (
Steelman SL and Pohley FM (
Swissmedic letter (2003) TSE risk of medicines manufactured from human urine, foreseen measures to ensure medical safety, December 15, 2003.
Tausk M (
Twombly GH (
Vesell M (
Vidal L (
World Health Organization (
Wilkins L (
Zondek B (
Zondek B (
Zondek B and Sulman F (
- pregnancy
- chorionic gonadotropin
- gonadotropins
- hormones
- corpus luteum of ovary
- hair follicle
- infertility
- menotropins
- molecular conformation
- ovarian follicle
- ovulation
- reproductive physiological process
- safety
- urinary tract
- pharmacokinetics
- pituitary gland
- placenta
- urine
- bypass
- ovarian function
- molecule