-
PDF
- Split View
-
Views
-
Cite
Cite
David J. Kennaway, The role of circadian rhythmicity in reproduction, Human Reproduction Update, Volume 11, Issue 1, January/February 2005, Pages 91–101, https://doi.org/10.1093/humupd/dmh054
Close - Share Icon Share
Abstract
Circadian rhythmicity is evident in a wide range of physiological systems including the reproductive axis. The recent discoveries of rhythmic clock gene expression in peripheral tissues, including reproductive tissue, suggests that they may play an important role in optimizing fertility. The evidence for rhythmic control of reproduction from studies in laboratory animals is reviewed and where possible this includes evidence from human studies. Clock genes are highly conserved across species including humans and there is no reason to suggest that they are functionless in humans. The challenge issued here is for researchers to probe their function and the consequences of their disruption in both animal and human reproduction.
Introduction
In recent years our understanding of the origins, control and importance of biological rhythmicity has increased tremendously. In 1997, 1998 and 2002 for example the journal Science recognized advances in the field in its ‘Breakthrough of the Year’ lists. In 2003 the journal Nature published an Editorial entitled ‘Timing is everything’ (Anonymous, 2003), again highlighting the progress in our understanding of daily circadian rhythmicity and its role in normal physiological functions and in certain diseases. The aim of this review is to provide an update of our understanding of the relationship between circadian rhythms and reproductive processes.
Circadian rhythms
Before embarking on a review of rhythms and reproduction it is important to first establish some of the terminology and background in chronobiological research. A circadian rhythm is a biological rhythm that persists under constant environmental conditions (light, dark, temperature) with a period length of ∼24 h. Its phase can be reset by a brief interruption in the constant regimen. The persistence of rhythmicity in a constant environment such as continuous darkness implies that the rhythm is generated endogenously rather than in reaction to the external environment. The capacity for resetting is important in the context of seasonal adjustment to the duration of the daylength and becomes profoundly important for humans endeavouring to work and sleep outside normal working hours or travelling through multiple time zones.
The circadian timing system consists of an input pathway connecting the clock to the environment, the clock itself and output pathways. The site of the ‘master biological clock’ in mammals is the suprachiasmatic nucleus (SCN), which is a paired concentration of small neurons and glial cells in the anterior hypothalamus directly above the optic chiasm and divided by the third ventricle. The SCN cells express an endogenous precise rhythmicity of function that in most species is different from 24 h (as demonstrated by the periodicity of various systems in constant darkness (Middleton et al., 1996), following surgical isolation of the SCN in vivo (Inouye and Kawamura, 1979) and in SCN organ culture experiments in vitro (Green and Gillette, 1982) and so must be reset each day. Entrainment is achieved directly via neural input from the eyes via the optic tract (retino-hypothalamic tract), as well as indirectly via input from the intergeniculate leaflet, raphe nuclei and some other lesser important centres (Morin, 1994). The SCN cells contain a wide range of neurotransmitters, hormones and cytokines as well as receptors for an equally wide range of ligands (Table I). An obvious consequence of the localization of the neurotransmitter and hormone receptors in the SCN is that the biological clock and circadian rhythmicity can potentially be altered by drugs and endogenous hormones.
The output component of the circadian timing system translates internal time into physiological action. The SCN achieves this directly by secreted factor(s) and via multisynaptic neural pathways. The SCN has long been known to secrete material into the cerebrospinal fluid (CSF) that influences behaviour since circadian behavioural rhythms can be re-established in arrhythmic SCN-lesioned animals by transplantation of SCN tissue into the third ventricle (Silver et al., 1996). Secreted factor(s), not new neuronal connections, are responsible for the re-establishment of rhythmicity. Importantly not all rhythms were re-established and the rhythms were not entrained by light/dark. It is now known that vasopressin (AVP) (Schwartz and Reppert, 1985), transforming growth factor (TGFα) (Kramer et al., 2001) and prokineticin 2 (PK2) (Cheng et al., 2002) are secreted into the CSF. AVP is likely to be involved in the temperature rhythmicity; of the other two peptides, PK2 has the most potent effects on wheel-running behaviour and thus potentially on sleep/wakefulness (Cheng et al., 2002).
Neural pathways are the major means of controlling brain and peripheral tissue rhythmicity. The best known pathway controls the pineal gland production and secretion of melatonin. The SCN efferent neurons project to the paraventricular nuclei (PVN) which in turn send efferents to the intermediolateral column of the spinal cord and on to the superior cervical ganglia. Noradrenergic neurons of the nervi conari return to the brain and synapse on pinealocytes (Larsen et al., 1998; Teclemariam Mesbah et al., 1999). Contrary to early suggestions that SCN lesions lead to the abolition of production and rhythmicity of melatonin, it is now thought that SCN neural output tonically suppresses melatonin production during the day, since small carefully placed SCN lesions result in continuous arrhythmic melatonin production (Kalsbeek, Garidou et al., 2000a; Perreau-Lenz et al., 2003).
The timing of the melatonin rhythm and changes in amplitude of the rhythm are the result of SCN activity and as such secreted melatonin provides an additional link between the circadian timing system and peripheral organs and orchestrating seasonal changes in fertility (Lincoln et al., 2003b). Melatonin receptors are predominantly expressed in the SCN (Vanecek et al., 1987; Reppert et al., 1994) and the pars tuberalis (Williams and Morgan, 1988; Barrett et al., 1996). In the former case the hormone may provide a stabilizing feedback signal to the clock (Redman et al., 1983), whereas in the latter melatonin participates in the seasonal production and secretion of prolactin (Lincoln et al., 2003a).
The SCN projects to other parts of the brain involved in reproduction including the medial pre-optic area (MPOA) (Palm et al., 1999) and GnRH-positive cells (van der Beek et al., 1997b). Studies from the Buijs laboratory have suggested that SCN vasopressin release may play a role in GnRH secretion and consequently the timing and magnitude of the LH surge (Palm et al., 2001). Recent tract tracing studies have highlighted multisynaptic connections to the parasympathetic and sympathetic nervous system, resulting in SCN influences on adipocytes, heart, liver etc. (Kalsbeek, Fliers et al., 2000b; la Fleur et al., 2000; Buijs and Kalsbeek, 2001; Buijs et al., 2001; Scheer et al., 2001). It is reasonable to expect that the sympathetic innervation of the ovary and uterus may also be influenced by the SCN, although to our knowledge no such link has yet been investigated.
Molecular organization
As indicated previously, a feature of SCN rhythmicity is its endogenous nature with the near 24 h rhythmicity generated within cells of the nucleus. In the last few years the origin of this rhythmicity has been shown to be derived from gene transcription/translation feedback/feedforward loops (Reppert and Weaver, 2002). To date there are ≥10 genes that form the basis of cellular rhythmicity including per1, per2, per3, Clock, Bmal1, cry1, cry2, dec1, dec2 and Rev erb a (Table II).
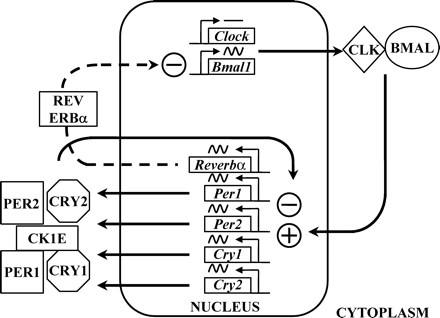
The key genes appear to be Bmal1 and Clock since their protein products form a heterodimer and bind to a specific gene sequence, CACGTG (called an enhancer or ‘E-box’) in the promoter region of per1, per2, cry1 and cry2 genes. There is some emerging evidence that aside from the E-Box itself, the context of the enhancer motif in the promoter may be important for circadian gene induction (Munoz et al., 2002). CLOCK/BMAL1 dimers drive the transcription of the period and cryptochrome genes resulting in a build up of the proteins in the cytoplasm. In the cytoplasm, phosphorylation by casein kinase 1E alters the stability of the period proteins and so limits the amount of PER protein available for dimerization with CRY and subsequent transport back into the nucleus. The entire PER1/PER2/CRY1/CRY2 complex is involved in displacing or inhibiting the transcription activity of the BMAL1/CLOCK dimer at the E-box and thus inhibiting the transcription of period and cryptochrome genes (Figure 1). This cycle takes ∼24 h to complete. The critical role of the casein kinase(s) in determining the period of the cycle was demonstrated in hamsters and humans through studies on naturally occurring mutations (Lowrey et al., 2000; Toh et al., 2001). In the tau mutant hamster the unusually short circadian period of these animals (22 h) was shown to be due to a mutation in casein kinase 1E which resulted in hypophosphorylation of PER proteins (Lowrey et al., 2000). With more protein available, the PER/CRY complex presumably was then able to suppress period and cryptochrome gene transcription earlier. In humans exhibiting Familial Advanced Sleep Phase Syndrome (ASPS), affected subjects were shown to have a mutation of per2 such that a phosphorylation cascade of the protein by casein kinase was inhibited (Toh et al., 2001). This again presumably resulted in a reduced rate of PER2 degradation and faster cycling of the feedback loop and an accompanying shorter circadian period and earlier onset/offset of physiological rhythms that included sleep and melatonin production.
The positive drive to the molecular rhythmicity in cells has been shown to involve PER2 induction of Bmal1 gene expression through an unknown site (Shearman et al., 2000a). To prevent this feed forward cycle from proceeding out of control, REV ERB alpha protein provides an inhibitory brake on Bmal1 transcription (Preitner et al., 2002). Further control over the cellular rhythmicity is afforded by another two genes, dec1 and dec2 (Honma et al., 2002; Kawamoto et al., 2004). Both dec1 and dec2 possess CACGTG E-boxes in their promoter regions and so are induced by CLOCK/BMAL1. As well as inhibiting their own transcription by interacting with CLOCK/BMAL1, DEC1 and DEC2 also repress per and cry transcription. Taken together these clock genes and their proteins provide an intricate molecular network that establishes the intracellular timing system.
The premier position of the SCN in the circadian timing system has been dogma until the era of clock genes commenced. In the first report of a mammalian per gene (Tei et al., 1997), it was clear that the gene was expressed widely (heart, brain, spleen, lung, liver, skeletal muscle, kidney and testis). Subsequently all of the other clock genes were shown to be expressed in a wide range of peripheral tissues, e.g. per2 (Sakamoto et al., 1999) and Bmal1 (Oishi et al., 1998). Indeed the expression of the clock genes is rhythmic and often of very high amplitude in peripheral tissues. Interestingly the phasing of the organ rhythms is often out of phase with the SCN rhythm (Yamazaki et al., 2000) although the phasing suggests that the rhythmicity is driven by the SCN. When peripheral tissues (liver, muscle and lung) were maintained in culture, luciferase transgene activity driven by a per1 promoter was rhythmic for only a few cycles before damping out, in contrast to the SCN in which rhythmic activity continued for >30 cycles. A recent study has, however, prompted a reassessment of the concept of a strict hierarchical control of rhythms by the SCN. Using a different per2:luciferase reporter, self-sustained circadian oscillations were maintained in vitro in liver, pituitary, kidney, lung and cornea for >20 cycles (Yoo et al., 2004). Lesion experiments confirmed that the circadian rhythms in the peripheral tissues did not depend on the SCN. The SCN maintains its ‘master’ role and plays a major role in the entrainment of the molecular timing of organ systems, but under certain circumstances, other influences such as stress (Balsalobre et al., 2000a) and food availability (Damiola et al., 2000) appear able to modify the timing/phasing of the tissue rhythmicity.
The demonstration of endogenous circadian rhythmicity in peripheral tissues is extremely important because several of the clock gene transcription factors form part of an output pathway. Microarray analysis of liver, heart, kidney, muscle as well as the SCN have shown that between 100 and 400 genes are rhythmically expressed (Akhtar et al., 2002; Panda et al., 2002; Storch et al., 2002; Oishi et al., 2003). Moreover, not all tissues express the same genes rhythmically although there is an overlap of a core set of ∼20–40 genes (Delaunay and Laudet, 2002). This astonishing situation stems from the fact that many transcription factors (e.g. dbp, usf2, myc, max, zfp36, sox3, tef and arnt2) and functional genes (e.g. vasopressin, pai-1) possess E-box motifs in their promoters that are activated by the cyclic appearance of the CLOCK/BMAL1 heterodimer complex (Akhtar et al., 2002; Panda et al., 2002; Storch et al., 2002; Oishi et al., 2003). DEC1 which has a role in rhythm generation is also known to be a potent repressor of genes (Grechez-Cassiau et al., 2004) as well as being itself induced by insulin (Yamada et al., 2003) and gonadotrophins (Yamada et al., 2004). The impact of these findings on the molecular biology of reproductive events is likely to be huge, but the field is only in its early stages. At the very least, it should no longer be considered acceptable to make or report measurements of gene expression in reproductive tissue without reporting the time of day the tissue was collected; preferably multiple time-points should be analysed to rule out the possibility of major circadian swings in activity and function.
As indicated previously, an important characteristic of circadian rhythmicity is the ability of rhythms to be entrained to the environment, but this aspect of molecular chronobiology is not completely understood. The most likely process is through induction of one or more of the core clock genes, e.g. the per or cry genes via a non-E-box site. A prime candidate for this re-setting is per1 since it appears to be easily induced by light in the SCN in vivo (Shigeyoshi et al., 1997) and by various inducers in cells maintained in vitro, including calcium and glucocorticoids (Balsalobre et al., 2000a,b), serum (Balsalobre et al., 1998), forskolin (Yagita and Okamura, 2000), 4β-phorbol-12-myristate-13-acetate (PMA) (Motzkus et al., 2000) and interleukin-6 (Motzkus et al., 2002).
Rhythms and reproduction
Circadian rhythmicity of physiological systems can have several purposes in reproduction. The most obvious is to provide an organism with the sense of time of day to ensure that physiological and behavioural events coincide. An example would be the coordination of ovulation, receptivity and wakefulness in the female with activity/wakefulness in the male. In many species, especially those with short life cycles or a duration of gestation and time to weaning of <1 year, circadian rhythms can provide a sense of time of year. Thus the changes in the time of the re-entraining stimulus of light may be associated with tightly controlled seasonal onset of puberty and adult infertility/fertility cycles.
Puberty
Since puberty reflects the maturation of all systems required for optimal reproductive performance it may be expected that circadian rhythmicity will have a role in puberty. In rodents, puberty onset is altered by daylength such that long durations of darkness inhibit sexual maturation (Reiter, 1980). The impact of short daylength is obvious in animals such as hamsters, which, outside the laboratory setting, live in higher latitudes and/or environments where food availability is highly seasonal. In longer-lived species such as sheep, the time of puberty of animals born late in the season may even be delayed until the following year (Foster et al., 1988). The advantages to a species of restricting onset of fertility and the resulting pregnancy and offspring care to favourable times of the year are obvious. In the case of laboratory rats and mice maintained for hundreds of generations in constant environmental conditions and selected for high fertility and fecundity, circadian or photoperiodic effects on puberty are far less common (Clark and Price, 1981; Lee and McClintock, 1986). Nevertheless some strains, e.g. Fisher 344 rats, do retain photo-responsiveness, although the basis for this is not known (Leadem, 1988). Some clues to the residual photosensitivity may lie in the well-known observation that some rat strains become responsive to short daylength when they are made anosmic (Blask and Nodelman, 1980) or fed a protein-restricted diet (Blask et al., 1980). It may be that these perturbations reinstate sensitivity to hormonal rhythms (e.g. melatonin) that have been selected against in animal breeding facilities. Note also that of the commonly used laboratory mouse strains, only CBA and C3H mice have functional AA-NAT and HIOMT enzymes that allow them to synthesize melatonin and so translate photoperiod information into a hormonal signal (Ebihara et al., 1986; Goto et al., 1989). As previously indicated there is new evidence that periodic food restriction in laboratory rodents can entrain circadian rhythms in gene expression (Damiola et al., 2000) and so a clue to photo-responsiveness may lie in future studies of clock gene expression in peripheral tissues of photo-responsive and non-responsive strains.
Human puberty
Studies on the seasonal occurrence of menarche in humans that are often reported (Bronson, 1995) are difficult to interpret in circadian or photoperiod terms since it is strongly influenced by size at birth (Adair, 2001) nutrition, climate, stress and living conditions (Wolanski et al., 1994; Gueresi, 1997). Nevertheless it has long been known that during puberty there is the appearance of remarkable rhythmicity in gonadotrophin secretion and steroid secretion in boys and girls (Boyar et al., 1972). Highest hormone levels are observed during the night, but this rhythmicity disappears in adult life. It is interesting, given the above discussion of nutritional sensitization to daylength in rodents, that hormonal rhythmicity often returns in women with anorexia nervosa. The return of menstrual function, however, does not show a simple relationship to weight, fatness, or maturity of LH pattern (Katz et al., 1978). It is worth noting that the early report of puberty in humans coinciding with a precipitous decrease in rhythmic melatonin production (Silman et al., 1979) has not been confirmed and in fact melatonin production is remarkably constant during puberty (Tetsuo et al., 1982).
Adult reproductive function
Males
There are strong seasonal rhythms in reproductive function in males of many species, best characterized in hamsters (Reiter, 1980) and sheep (Lincoln, 2003). Thus rams are able to decode the daily changes in light across the year and to translate these into hormonal signals that reduce and then reinitiate gonadal function and libido. During the sexual season, however, there is little evidence of circadian rhythmicity in hormone secretion, apart from prolactin and melatonin (Kennaway et al., 1981).
Several groups have investigated clock gene expression in the testes of laboratory animals. Remarkably the testis appears to be the only tissue studied to date that does not exhibit overt rhythmicity of clock gene expression. Morse et al. (2003) examined clock gene expression in the testes of mice on the premise that the developmental events occurring in spermatogonia, spermocytes and spermatids appear to be coordinated. They hypothesized that biological clocks might influence developmental decisions taken by each of the different cell types. They found that per1 was expressed in mouse tissue but there was no apparent change in expression across 24 h. Given the major role of Bmal1 in the control of rhythmic per1 expression, it was not surprising that Bmal1 expression was also non-rhythmic in the testis. PER1 protein was also constantly present across 24 h. Further analysis indicated that per1 expression was highest during stages VII–X of sperm development, suggesting that the expression may be developmentally regulated. Testis per1 expression was maintained in Clock mutant mice, suggesting that an alternate control pathway exists for testicular per1 expression compared to other peripheral tissues. These results were subsequently confirmed and per2, cry1 and npas2 added to the list of clock genes expressed in developing sperm and Leydig cells non-rhythmically (Alvarez et al., 2003; Bittman et al., 2003). Clock gene expression in Syrian hamster testis has also been investigated (Tong et al., 2004) and per1 transcription (in this case both a long and short transcript) peaked late in the subjective night. By contrast, rhythmicity of Bmal1 expression in long photoperiods was equivocal, while neither per2 and Clock expression showed significant variation with the time of day. The authors also showed in this photoperiodic species that long-term exposure to continuous darkness to produce gonadal regression altered the pattern of clock gene expression in the testis. Under these conditions, expression of the per1 transcripts became arrhythmic while Bmal1 expression was profoundly rhythmic. Thus although there is seasonality of male reproduction in many species, it would appear that if circadian rhythmicity at the gonadal level drives these changes it involves a different rhythm-generating mechanism.
There have not been any systematic studies of reproductive function in male mice with disrupted clock genes. All clock gene mutant and knockout mice are maintained as homozygous colonies and there have been no reports or comments implying impaired fertility in the males (Vitaterna et al., 1994; Thresher et al., 1998; van der Horst et al., 1999; Zheng et al., 1999, 2001; Bunger et al., 2000; Shearman et al., 2000b; Bae et al., 2001). One important knockout mouse, the Bmal1 null, deserves mention, however. In the paper describing the generation of this null mutant (Bunger et al., 2000), no mention was made of the fertility of the males or females; however, on establishing our own colony of Bmal1 knockout mice from the Bradfield laboratory we were advised that they must be maintained as heterozygotes due to impaired fertility. This has been confirmed in our colony and we have gone on to show that adult males have reduced seminal vesicle weight, normal testis weight and a very high incidence of abnormal sperm (M.J.Boden and D.J.Kennaway, unpublished data). Homozygous Bmal1 knockouts are rarely able (one out of 15 males in one study) to successfully fertilize normal females. The basis for this defect is not known, but is currently under investigation.
Females
Circadian rhythms are known to play a critical role in the estrous cycle of laboratory rodents. Rats and mice initiate their LH surge in the late afternoon of pro-estrus, with subsequent ovulation and mating occurring ∼6 h after darkness. There have been several excellent reviews on this topic (Turek et al., 1984; van der Beek, 1996; Barbacka-Surowiak et al., 2003), but a number of features need to be reiterated here. Lesions of the SCN block ovulation and abolish the LH surge in intact and ovariectomized steroid-treated animals. Administration of pentobarbital prior to pro-estrus acutely inhibits the LH surge and ovulation, but remarkably both events occur 24 h later at the expected time of day. The timing of the ovulatory process is clearly determined by the SCN.
The SCN is a target for gonadal steroids, is sexually dimorphic and the steroid milieu during development may influence SCN neurons, determining in part gender-specific rhythmicity (Swaab et al., 1995). An example of the effects of gender on rhythmicity is the change in wheel-running activity onset that occurs at estrus in laboratory rodents brought about by the elevated estrogen levels (Albers et al., 1981; Wollnik and Turek, 1988). Thus on the evening of pro-estrus the animals commence their circadian activity earlier than on other days of the estrous cycle. It is not known how the steroids affect SCN function, but one suggestion is that estradiol enhances the expression of immediate early gene and transcription factor responses to light in SCN cells (Abizaid et al., 2004) through estrogen receptor-positive neurons projecting to the SCN (de la Iglesia et al., 1999). Recent studies have confirmed the presence of estrogen receptors in the SCN (Shughrue et al., 1997; Su et al., 2001; Shima et al., 2003).
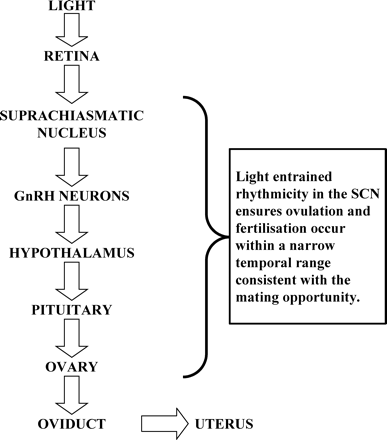
For the SCN to influence the LH surge and ovulation, it presumably affects hypothalamic production and/or secretion of GnRH. Connectivity with estrogen-sensitive medial pre-optic area neurons (Watson et al., 1995) and with GnRH-containing neurons has been described (van der Beek et al., 1993, 1997a; de la Iglesia et al., 1995). Previously in this review it was mentioned that clock gene rhythmicity occurred in cells and tissues not traditionally recognized as exhibiting circadian rhythmicity. Rhythmic per1 and per2 gene expression has been reported in an immortalized hypothalamic GnRH-producing cell line (GT1-7) following synchronization with serum (Gillespie et al., 2003) or forskolin (Chappell et al., 2003) as had been previously reported for fibroblast cells. When these cells were transfected with a mutated clock gene, ClockΔ19, the per1 rhythm was blunted (Chappell et al., 2003). Furthermore GnRH pulsatility was significantly reduced, but not absent, in the ClockD19-transfected cells. These results provide one of the first links between circadian clock gene expression and ultradian hormone pulses, although they do not provide clues to any of the underlying mechanisms. In summary, SCN rhythmicity ensures optimal temporal integration of behaviour, ovulation, fertilization and embryo development (Figure 2).
Clock gene disruption and female fertility
All known clock genes have been disrupted using conventional knockout techniques (Thresher et al., 1998; van der Horst et al., 1999; Zheng et al., 1999; Bunger et al., 2000; Shearman et al., 2000b; Bae et al., 2001; Zheng et al., 2001) except Clock and in most of these cases no protein is produced. In the case of Clock, a mutant has been produced through in vivoN-ethyl-N-nitrosourea (ENU) mutagenesis (Vitaterna et al., 1994). In the ClockΔ19 mutant mouse, an A→T transversion in the splice donor site downstream from exon 19 leads to the skipping of this exon in the Clock mRNA and elimination of 51 amino acids in the C-terminal glutamine-rich region of the CLOCK protein (King et al., 1997). The BMAL1/CLOCKD19 heterodimer lacks the ability to initiate transcription at an E-box (Gekakis et al., 1998). Even in those mutants that are arrhythmic in constant darkness such as cry1/cry2 double knockouts (van der Horst et al., 1999) there are apparently no major effects on fertility. By way of caution, however, it must be acknowledged that there have been no published systematic studies of fertility in these knockouts and so small changes and/or compensation may have been missed.
There have been some fertility concerns raised about the Clock mutant mouse. Mice with this mutation have been described as having altered fertility in that they could not be maintained through homozygous pairing due to ‘an as yet uncharacterized parturition defect’ (Low-Zeddies and Takahashi, 2001) although this was said to no longer be the case. There is also an anecdotal report of reduced fecundity and lack of response to ovulation induction in Clock mutant mice (Herzog et al., 2000). A ‘reduced breeding success rate’ in Clock mutant mice was reported (Chappell et al., 2003), but again there was insufficient experimental detail and very small numbers of pairings (n=4). These authors did, however, provide convincing data indicating that Clock mutant mice exhibit prolonged estrous cycles, spending a greater length of time in estrus than wild type or heterozygous mutant animals. The longer estrus cycle length apparently persisted in constant darkness.
The first extensive evaluation of reproduction in Clock mutant females (Miller et al., 2004) reported normal timing of puberty (vaginal opening) and confirmed the prolonged and irregular estrous cycles in these mutants. Despite apparently normal ovarian morphology and steroid levels on the afternoon of pro-estrus, Clock mutants apparently never exhibited an LH surge. The mutants had an increased rate of mid-gestation fetal resorption and pregnancy failure at full term, although this was previously reported to have disappeared from this colony (Low-Zeddies and Takahashi, 2001). One explanation given for the pregnancy loss was that lower estradiol levels observed in the mutants were responsible for the failure of Clock mutants to initiate labour. Interestingly estradiol administration failed to initiate an LH surge in ovariectomized Clock mutants despite a normal response to GnRH administration. In the former experiment, however, the steroid was administered and blood samples obtained at the same time of the day (at lights on) for both genotypes, which failed to take into account for the differences in residual rhythmicity reported in the two genotypes (Kennaway et al., 2003a). Despite these results, the reproductive phenotype of the Clock mutant is at best only minimally compromised. How the mice can ovulate in the apparent absence of an LH surge is not known and probably unprecedented.
In our laboratory we have monitored the reproductive performance of a Clock mutant colony derived from the original Vitaterna colony, as well as a colony of melatonin-proficient (Kennaway et al., 2003a) Clock mutants (Kennaway et al., 2004). We found that both colonies could be efficiently maintained through homozygous pairing. Upon acute introduction of males, both Clock mutant lines took 2–3 days longer to mate and deliver pups compared to their wild type control lines. Litter sizes were slightly but significantly reduced in Clock mutant mice (seven versus eight pups). While similar proportions of wild type and mutant mice failed to produce live births within 40 days of pairing, survival to weaning was poorer in both mutant lines (80–84%) compared to 94–96% survival in wild type lines. Our experience therefore indicates that the Clock mutation has significant, but subtle, effects on reproductive performance.
In view of the prominent role of circadian rhythmicity in estrous cycle function and CLOCK protein in the generation of rhythms, the subtle effects of the Clock mutation are difficult to explain. By introducing functional copies of the genes for the melatonin synthesizing enzymes AA-NAT and HIOMT [known to be mutated in all mice strains other than CBA and C3H (Goto et al., 1989)] into a Clock mutant background, we have shown that central, hypothalamic rhythmicity (i.e. the SCN signalling to the pineal gland to synthesise melatonin) is maintained in these mutants (Kennaway et al., 2003a). We argued that this may occur through the recruitment of NPAS2 to partner BMAL1 and initiate the clock gene rhythms at least centrally, although peripheral rhythmicity is lost (DJ Kereneway and A Voultsios, unpublished results).
The other key clock gene, Bmal1, has been targeted and a null mutant line produced (Bunger et al., 2000) and the evidence provided in that report suggested that it is a non-redundant partner of CLOCK despite the existence of a Bmal2 gene. Although it was not stated explicitly in the original description of the Bmal1 knockout line, it was stated subsequently that the homozygous null mutants are fertile (Cowden and Simon, 2002). Having established our own colony of Bmal1 null mutants from the original Bradfield laboratory colony, we have found this declaration of fertility to be incorrect (Boden and Kennaway, 2004). We have never observed live births from homozygous pairings of Bmal1 null mutants. Nevertheless the Bmal1 females ovulate (following irregular cycles), mate and fertilize ova following mating with wild type males, but they have either delayed embryo development or early embryo loss, such that no full term pregnancies have ever been achieved in our colony.
Circadian rhythms and early pregnancy
Following fertilization, the embryo spends up to 4–5 days traversing the oviduct prior to entering the uterus. There is considerable evidence that the environment within the oviduct plays a significant protective and nutritive role in embryo development (Leese et al., 2001). The importance of the environment during this period is evidenced by the enormous efforts to develop optimal supportive culture media for human in vitro embryo culture (Summers and Biggers, 2003). The role of circadian rhythmicity in early embryo development does not appear to have been widely studied. Delayed mating from 06:00 to 08:00 compared with 24:00 to 02:00 significantly decreased the percentage of morphologically normal embryos and increased the percentage of degenerated embryos (Sakai and Endo, 1988). While these deleterious effects may have been due to the delay in fertilization, they could also have been caused by the embryos reaching developmental milestones at inappropriate circadian times. Similarly it has been shown that the circadian period during early pregnancy influenced embryo development, since mice exposed to 22 or 26 h days had significantly higher percentages of dead or resorbed embryos 12 days post fertilization (Endo and Watanabe, 1989).
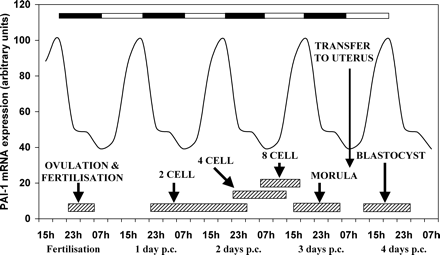
We have recently investigated rhythmicity in the rat oviduct using real-time RT–PCR analysis of clock genes and some selected functional genes (Kennaway et al., 2003b). The oviduct is like most other peripheral tissues and expresses clock genes rhythmically. We also observed that plasminogen activator inhibitor 1 (pai-1) gene expression is rhythmic. The promoter for the pai-1 gene is known to have the appropriate E-box for CLOCK/BMAL1 heterodimer binding (Maemura et al., 2000; Schoenhard et al., 2003) and so the rhythmicity is likely to be due to a direct effect of the clock gene rhythm in the oviduct cells. PAI-1 has been identified previously in the oviduct and is thought to play a role in protecting the embryo from protease damage during the traverse along the oviduct (Kouba et al., 2000). If the circadian change in pai-1 mRNA is reflected by changes in active protein, the embryo may experience periods of greater or lesser vulnerability to damage during development (Figure 3). Mis-matching of oviductal PAI-1 activity and stage of embryo development (for example, following delayed mating) may decrease viability. Other functional genes may be rhythmically expressed in the oviduct and influence embryo development.
Embryos express Bmal1, per1 and cry1 at all stages from oocyte to blastocyst (Johnson et al., 2002). Expression of Clock was apparent up to the 2-cell stage, then decreased below the assay sensitivity and reappeared later at the blastocyst stage. There have been no studies on the role/influence of rhythmicity on implantation events.
Humans
Despite the very strong evidence for a critical role of the circadian timing system in determining the time of ovulation in animal models, there have been few studies conducted in humans. The human SCN has, however, been shown to contain estrogen receptor α and β and progesterone receptors (Kruijver and Swaab, 2002). The possible role of the SCN in ovulation in humans could be inferred from studies on the timing of the LH surge. In such a study in women who were being monitored prior to natural cycle IVF with 4 hourly blood sampling from day 9, the surge occurred between 24:00 and 04:00 in 37% of the cycles and between 04:00 and 08:00 in 48% of the cycles (Cahill et al., 1998). In a subsequent report also using 4 hourly blood sampling in normal cycling women, 15 of 19 subjects ‘initiated’ their LH surge at 08:00 (Kerdelhue et al., 2002). These authors attempted to link the timing of the surge to the time of the SCN-mediated cortisol peak and found that four of four women with a cortisol peak at 04:00 initiated the LH surge at that time. Of the 15 women initiating the LH surge at 08:00, 12 had their cortisol peak at the same time while the remaining three had peak cortisol at 04:00. This latter study provides the strongest evidence, albeit indirect, that SCN may be involved in the timing of ovulation in humans. More rigorous studies are needed to definitively prove this link and to establish whether rhythm disruption affects the timing of the LH surge and ovulation. As for the actual time of day that the oocyte is released, to our knowledge this has never been reported, presumably because the procedures are so intrusive.
In humans we might expect that some clues to the role of daily rhythmicity on early pregnancy could come from studies of women working shifts and/or travelling regularly across time zones. Shiftwork disrupts sleep rhythmicity and even those shiftworkers who do manage to establish daytime sleep patterns, for many the melatonin rhythmicity remains nocturnal (Benhaberou et al., 1999). As a further example, a recent study reported an inverse relationship between the number of nights worked and melatonin levels (Schernhammer et al., 2004). Since melatonin is widely recognized as an accurate marker of SCN function, this study provides compelling evidence that the SCN of these shiftworkers was affected by their working conditions. Although no human studies have been conducted as yet, animal experiments indicate that timing of food presentation affects liver and other tissue rhythmicity without affecting central rhythm timing (Damiola et al., 2000). Thus shiftworkers who eat during the night when their SCN is maintaining normal melatonin secretion are likely to have conflicting central and peripheral cellular rhythmicity. The extent that the human reproductive axis is affected by shiftwork can really only be determined from epidemiological studies. Bisanti et al. (1996) attempted to analyse the effect of shiftwork on fecundity by recording the time of unprotected intercourse prior too pregnancy. They stated that despite some possible confounding factors, data from their study ‘are in favour of an association between shiftwork and prolonged waiting time to pregnancy’. A later, larger similar study, however, concluded that there ‘was no unequivocal evidence of causal association between shift work and subfecundity’ (Zhu et al., 2003). The small reduction they did observe, it was argued, may have been mediated by pregnancy planning bias or differences in opportunity for sexual contact. The evidence for a deleterious effect of shiftwork and time zone travel on pregnancy outcome is stronger (Cone et al., 1998; Aspholm et al., 1999; Knutsson, 2003) with an increased incidence of miscarriage, low birthweight and preterm birth. To what extent these adverse outcomes are the result of disrupted circadian timing rather than other lifestyle factors, including altered diet and stress, cannot be determined from these epidemiological studies.
Conclusion
The discovery of clock genes, the recognition of their widespread influence as transcription factors and their influence on a large number of functional genes should rekindle interest in the potential role of circadian rhythms in reproduction, including human reproduction. Clock genes are highly conserved from plants to Drosophila to rodents to humans. Given the role of rhythms in reproduction of other animals and the role of clock genes in the control of rhythmicity, it would seem unwise to ignore them in the quest for understanding and correcting fertility in humans. There is already emerging evidence that mutations in clock genes can alter human physiology and that polymorphisms can affect sleep behaviour. Contrary to previous beliefs, humans can respond to changes in photoperiod (Wehr et al., 1993) and so it is no longer valid to presume that circadian rhythms are of little importance in human physiology, especially reproduction. Humans are notorious for poor fertility as evidenced by the huge demand for assisted reproductive technologies and yet we still do not know when the LH surge commences with a precision of <12–24 h, and we have little information on the actual time of day that ovulation occurs. Furthermore, assisted reproductive technologies involving in vitro culturing or manipulation of embryos and subsequent transfer to the uterus tend to ignore the fact that the processes are occurring in a non-rhythmic environment. Similarly in the case of transfer, the embryo is returned potentially out of phase with the mother as a result of slower development in vitro. It is certainly time to consider seriously the temporal aspects of reproduction.
Schematic of the primary loops of clock gene transcription factor rhythms. Positive drive is afforded by the CLK/BMAL1 heterodimer complex which initiates transcription of per1, per2, cry1 and cry2. Proteins from these genes in complex with casein kinase 1E inhibit CLK/BMAL1 induction. Meanwhile, REV ERBα protein inhibits Bmal1 transcription. Clock expression is normally constitutive while Bmal1 is rhythmic and in antiphase to the period and cryptochrome gene expression. A secondary loop not shown here involves dec1 and dec2 (Honma and Honma, 2003).
Schematic of the link between the environment (light/dark) and the female reproductive tract. Rhythmicity at each level ensures optimal embryo development.
The relationship between the stages of mouse embryo development, the light dark cycle and oviduct expression of a putative embryo protective gene, pai-1. The light/dark cycle is illustrated by the closed and open bars at the top of the figure. The stages of embryo development are approximate boundaries of the various transitions. The graph shows the hypothetical pai-1 mRNA rhythm in the oviduct during early pregnancy, based on a study in rat oviduct (Kennaway et al., 2003b). PAI-1 is an example of many proteins expected to be rhythmically secreted into the oviduct that affect embryo development. It might be expected that perturbations in oviduct/uterus rhythmicity will alter embryo development. Similarly transfer of embryos to the oviduct/uterus at an inappropriate time of day for the stage of development may also compromise the embryo.
A short tabulation of some of the transmitters, receptors and hormones that have been identified in the suprachiasmatic nucleus
| Neurotransmitter . | Neurotransmitter receptors . | Hormone and cytokine receptors . |
|---|---|---|
| PACAP (pituitary adenylate cyclase- activating polypeptide.) | Serotonin (1A, 1B, 2C, 5, 7) | Melatonin |
| γ-Aminobutyric acid | N-Methyl-D-aspartate | Estrogen |
| Vasoactive intestinal peptide | Neurotensin | Insulin-like growth factor-I |
| Serotonin | NGF (Nerve growth factor) | Leptin |
| Glutamate | Vasopressin (V1A) | Interferon |
| Neuropeptide Y | Somatostatin | |
| Cholinergic (muscarinic and nicotinic VPAC2 (Vasoactive intestinal peptide receptor type 2) |
| Neurotransmitter . | Neurotransmitter receptors . | Hormone and cytokine receptors . |
|---|---|---|
| PACAP (pituitary adenylate cyclase- activating polypeptide.) | Serotonin (1A, 1B, 2C, 5, 7) | Melatonin |
| γ-Aminobutyric acid | N-Methyl-D-aspartate | Estrogen |
| Vasoactive intestinal peptide | Neurotensin | Insulin-like growth factor-I |
| Serotonin | NGF (Nerve growth factor) | Leptin |
| Glutamate | Vasopressin (V1A) | Interferon |
| Neuropeptide Y | Somatostatin | |
| Cholinergic (muscarinic and nicotinic VPAC2 (Vasoactive intestinal peptide receptor type 2) |
A short tabulation of some of the transmitters, receptors and hormones that have been identified in the suprachiasmatic nucleus
| Neurotransmitter . | Neurotransmitter receptors . | Hormone and cytokine receptors . |
|---|---|---|
| PACAP (pituitary adenylate cyclase- activating polypeptide.) | Serotonin (1A, 1B, 2C, 5, 7) | Melatonin |
| γ-Aminobutyric acid | N-Methyl-D-aspartate | Estrogen |
| Vasoactive intestinal peptide | Neurotensin | Insulin-like growth factor-I |
| Serotonin | NGF (Nerve growth factor) | Leptin |
| Glutamate | Vasopressin (V1A) | Interferon |
| Neuropeptide Y | Somatostatin | |
| Cholinergic (muscarinic and nicotinic VPAC2 (Vasoactive intestinal peptide receptor type 2) |
| Neurotransmitter . | Neurotransmitter receptors . | Hormone and cytokine receptors . |
|---|---|---|
| PACAP (pituitary adenylate cyclase- activating polypeptide.) | Serotonin (1A, 1B, 2C, 5, 7) | Melatonin |
| γ-Aminobutyric acid | N-Methyl-D-aspartate | Estrogen |
| Vasoactive intestinal peptide | Neurotensin | Insulin-like growth factor-I |
| Serotonin | NGF (Nerve growth factor) | Leptin |
| Glutamate | Vasopressin (V1A) | Interferon |
| Neuropeptide Y | Somatostatin | |
| Cholinergic (muscarinic and nicotinic VPAC2 (Vasoactive intestinal peptide receptor type 2) |
Genes shown to play a role in the generation of cellular rhythmicity in vertebrates
| Gene name . | Abbreviation . | Alternate name . | Homologue . |
|---|---|---|---|
| Period 1 | per1 | ||
| Period 2 | per2 | ||
| Period 3 | per3 | ||
| Circadian Locomotor Output Cycle Kaput | Clock | npas2 (MOP4) | |
| Brain Muscle ARNT-Like protein 1 | Bmal1 | MOP3 | Bmal2 |
| Cryptochrome 1 | cry1 | ||
| Cryptochrome 2 | cry2 | ||
| Differentiated Embryo Chondrocytes 1 | dec1 | Stra13, Sharp2, BHLHB2, Clast5 | |
| Differentiated Embryo Chondrocytes 2 | dec2 | Sharp1, BHLHB3 | |
| Rev Erb alpha | Rev erba |
| Gene name . | Abbreviation . | Alternate name . | Homologue . |
|---|---|---|---|
| Period 1 | per1 | ||
| Period 2 | per2 | ||
| Period 3 | per3 | ||
| Circadian Locomotor Output Cycle Kaput | Clock | npas2 (MOP4) | |
| Brain Muscle ARNT-Like protein 1 | Bmal1 | MOP3 | Bmal2 |
| Cryptochrome 1 | cry1 | ||
| Cryptochrome 2 | cry2 | ||
| Differentiated Embryo Chondrocytes 1 | dec1 | Stra13, Sharp2, BHLHB2, Clast5 | |
| Differentiated Embryo Chondrocytes 2 | dec2 | Sharp1, BHLHB3 | |
| Rev Erb alpha | Rev erba |
Genes shown to play a role in the generation of cellular rhythmicity in vertebrates
| Gene name . | Abbreviation . | Alternate name . | Homologue . |
|---|---|---|---|
| Period 1 | per1 | ||
| Period 2 | per2 | ||
| Period 3 | per3 | ||
| Circadian Locomotor Output Cycle Kaput | Clock | npas2 (MOP4) | |
| Brain Muscle ARNT-Like protein 1 | Bmal1 | MOP3 | Bmal2 |
| Cryptochrome 1 | cry1 | ||
| Cryptochrome 2 | cry2 | ||
| Differentiated Embryo Chondrocytes 1 | dec1 | Stra13, Sharp2, BHLHB2, Clast5 | |
| Differentiated Embryo Chondrocytes 2 | dec2 | Sharp1, BHLHB3 | |
| Rev Erb alpha | Rev erba |
| Gene name . | Abbreviation . | Alternate name . | Homologue . |
|---|---|---|---|
| Period 1 | per1 | ||
| Period 2 | per2 | ||
| Period 3 | per3 | ||
| Circadian Locomotor Output Cycle Kaput | Clock | npas2 (MOP4) | |
| Brain Muscle ARNT-Like protein 1 | Bmal1 | MOP3 | Bmal2 |
| Cryptochrome 1 | cry1 | ||
| Cryptochrome 2 | cry2 | ||
| Differentiated Embryo Chondrocytes 1 | dec1 | Stra13, Sharp2, BHLHB2, Clast5 | |
| Differentiated Embryo Chondrocytes 2 | dec2 | Sharp1, BHLHB3 | |
| Rev Erb alpha | Rev erba |
References
Abizaid A, Mezei G and Horvath TL (
Adair LS (
Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH and Kyriacou CP (
Albers HE, Gerall AA and Axelson JF (
Alvarez JD, Chen D, Storer E and Sehgal A (
Aspholm R, Lindbohm ML, Paakkulainen H, Taskinen H, Nurminen T and Tiitinen A (
Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM and Weaver DR (
Balsalobre A, Damiola F and Schibler U (
Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G and Schibler U (
Balsalobre A, Marcacci L and Schibler U (
Barbacka-Surowiak G, Surowiak J and Stoklosowa S (
Barrett P, MacLean A, Davidson G and Morgan PJ (
Benhaberou BD, Lambert C and Dumont M (
Bisanti L, Olsen J, Basso O, Thonneau P, Karmaus W, Juul S, Fletcher T, Bolumar F, Figatalamanca I, Pantelakis S, Spinelli A, Schaumburg I, Wulff M and Biczysko R (
Bittman EL, Doherty LS, Huang L and Paroskie A (
Blask DE and Nodelman JL (
Blask DE, Nodelman JL, Leadem CA and Richardson BA (
Boden MJ and Kennaway DJ (
Boyar R, Finkelstein J, Roffwarg H, Kapen S, Weitzman E and Hellman L (
Bronson FH (
Buijs RM, Chun SJ, Niijima A, Romijn HJ and Nagai K (
Buijs RM and Kalsbeek A (
Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS and Bradfield CA (
Cahill DJ, Wardle PG, Harlow CR and Hull MG (
Chappell PE, White RS and Mellon PL (
Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM and Zhou QY (
Clark BR and Price EO (
Cone JE, Vaughan LM, Huete A and Samuels SJ (
Cowden KD and Simon MC (
Damiola F, le Minh N, Preitner N, Kornmann B, Fleury-Olela F and Schibler U (
de la Iglesia HO, Blaustein JD and Bittman EL (
de la Iglesia HO, Blaustein JD and Bittman EL (
Delaunay F and Laudet V (
Ebihara S, Marks T, Hudson DJ and Menaker M (
Endo A and Watanabe T (
Foster DL, Ebling FJ and Claypool LE (
Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS and Weitz CJ (
Gillespie JM, Chan BP, Roy D, Cai F and Belsham DD (
Goto M, Oshima I, Tomita T and Ebihara S (
Grechez-Cassiau A, Panda S, Lacoche S, Teboul M, Azmi S, Laudet V, Hogenesch JB, Taneja R and Delaunay F (
Green DJ and Gillette R (
Gueresi P (
Herzog ED, Grace MS, Harrer C, Williamson J, Shinohara K and Block GD (
Honma S and Honma K (
Honma S, Kawamoto T, Takagi Y, Fujimoto K, Sato F, Noshiro M, Kato Y and Honma K (
Inouye ST and Kawamura H (
Johnson MH, Lim A, Fernando D and Day ML (
Kalsbeek A, Garidou ML, Palm IF, Van der Vliet J, Simonneaux V, Pevet P and Buijs RM (
Kalsbeek A, Fliers E, Franke AN, Wortel J and Buijs RM (
Katz JL, Boyar R, Roffwarg H, Hellman L and Weiner H (
Kawamoto T, Noshiro M, Sato F, Maemura K, Takeda N, Nagai R, Iwata T, Fujimoto K, Furukawa M, Miyazaki K, Honma S, Honma K and Kato Y (
Kennaway DJ, Obst JM, Dunstan EA and Friesen HG (
Kennaway DJ, Voultsios A, Varcoe TJ and Moyer RW (
Kennaway DJ, Varcoe TJ and Mau VJ (
Kennaway DJ, Voultsios A and Boden MJ (
Kerdelhue B, Brown S, Lenoir V, Queenan JTJ, Jones GS, Scholler R and Jones HWJ (
King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TD, Vitaterna MH, Kornhauser JM, Lowrey PL, Turek FW and Takahashi JS (
Knutsson A (
Kouba AJ, Burkhardt BR, Alvarez IM, Goodenow MM and Buhi WC (
Kramer A, Yang FC, Snodgrass P, Li XD, Scammell TE and Davis FC (
Kruijver FP and Swaab DF (
la Fleur SE, Kalsbeek A, Wortel J and Buijs RM (
Larsen PJ, Enquist LW and Card JP (
Leadem CA (
Lee TM and McClintock MK (
Leese HJ, Tay JI, Reischl J and Downing SJ (
Lincoln GA (
Lincoln GA, Andersson H and Hazlerigg D (
Lincoln GA, Andersson H and Loudon A (
Low-Zeddies SS and Takahashi JS (
Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M and Takahashi JS (
Maemura K, de la Monte SM, Chin MT, Layne MD, Hsieh CM, Yet SF, Perrella MA and Lee ME (
Middleton B, Arendt J and Stone BM (
Miller BH, Olson SL, Turek FW, Levine JE, Horton TH and Takahashi JS (
Morse D, Cermakian N, Brancorsini S, Parvinen M and Sassone-Corsi P (
Motzkus D, Albrecht U and Maronde E (
Motzkus D, Maronde E, Grunenberg U, Lee CC, Forssmann W and Albrecht U (
Munoz E, Brewer M and Baler R (
Oishi K, Sakamoto K, Okada T, Nagase T and Ishida N (
Oishi K, Miyazaki K, Kadota K, Kikuno R, Nagase T, Atsumi GI, Ohkura N, Azama T, Mesaki M, Yukimasa S et al. (
Palm IF, Van der Beek EM, Wiegant VM, Buijs RM and Kalsbeek A (
Palm IF, Van der Beek EM, Wiegant VM, Buijs RM and Kalsbeek A (
Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS and Hogenesch JB (
Perreau-Lenz S, Kalsbeek A, Garidou ML, Wortel J, Van der Vliet J, Van Heijningen C, Simonneaux V, Pevet P and Buijs RM (
Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U and Schibler U (
Redman J, Armstrong S and Ng KT (
Reiter RJ (
Reppert SM and Weaver DR (
Reppert SM, Weaver DR and Ebisawa T (
Sakai N and Endo A (
Sakamoto K, Nagase T, Fukui H, Horikawa K, Okada T, Tanaka H, Sato, Miyake Y, Ohara O, Kako K and Ishida N (
Scheer FA, ter Horst GJ and Buijs RM (
Schernhammer ES, Rosner B, Willett WC, Laden F, Colditz GA and Hankinson SE (
Schoenhard JA, Smith LH, Painter CA, Eren M, Johnson CH and Vaughan DE (
Schwartz WJ and Reppert SM (
Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der Horst GT, Hastings MH and Reppert SM (
Shearman LP, Jin X, Lee C, Reppert SM and Weaver DR (
Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, Moriya T, Shibata S, Loros JJ, Dunlap JC and Okamura H (
Shima N, Yamaguchi Y and Yuri K (
Shughrue PJ, Lane MV and Merchenthaler I (
Silman RE, Leone RM, Hooper RJ and Preece MA (
Silver R, LeSauter J, Tresco PA and Lehman MN (
Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH and Weitz CJ (
Su JD, Qiu J, Zhong YP and Chen YZ (
Summers MC and Biggers JD (
Swaab DF, Slob AK, Houtsmuller EJ, Brand T and Zhou JN (
Teclemariam Mesbah R, Ter Horst GJ, Postema F, Wortel J and Buijs RM (
Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M and Sakaki Y (
Tetsuo M, Poth M and Markey SP (
Thresher RJ, Vitaterna MH, Miyamoto Y, Kazantsev A, Hsu DS, Petit C, Selby CP, Dawut L, Smithies O, Takahashi JS and Sancar A (
Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, Ptacek LJ and Fu Y-H (
Tong Y, Guo H, Brewer JM, Lee H, Lehman MN and Bittman EL (
Turek FW, Swann J and Earnest DJ (
van der Beek EM (
van der Beek EM, Wiegant VM, van der Donk HA, van den Hurk R and Buijs RM (
van der Beek EM, Wiegant VM, van Oudheusden HJ, van der Donk HA, van den Hurk R and Buijs RM (
van der Beek EM, Horvath TL, Wiegant VM, van den Hurk R and Buijs RM (
van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker AP, van Leenen D, Buijs R, Bootsma D, Hoeijmakers JH and Yasui A (
Vanecek J, Pavlik A and Illnerova H (
Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW and Takahashi JS (
Watson RE, Langub MC, Engle MG and Maley BE (
Wehr TA, Moul DE, Barbato G, Giesen HA, Seidel JA, Barker C and Bender C (
Williams LM and Morgan PJ (
Wolanski N, Dickinson F and Siniarska A (
Wollnik F and Turek FW (
Yagita K and Okamura H (
Yamada K, Kawata H, Shou Z, Mizutani T, Noguchi T and Miyamoto K (
Yamada K, Kawata H, Mizutani T, Arima T, Yazawa T, Matsuura K, Shou Z, Sekiguch T, Yoshino M, Kajitani T and Miyamoto K (
Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M and Tei H (
Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M and Takahashi JS (
Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, Lee CC and Bradley A (
Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A and Lee CC (
Zhu JL, Hjollund NH, Boggild H and Olsen J (