-
PDF
- Split View
-
Views
-
Cite
Cite
Eric A Engels, Elizabeth Clark, Louis M Aledort, James J Goedert, Denise Whitby, Kaposi’s sarcoma-associated herpesvirus infection in elderly Jews and non-Jews from New York City, International Journal of Epidemiology, Volume 31, Issue 5, October 2002, Pages 946–950, https://doi.org/10.1093/ije/31.5.946
Close - Share Icon Share
Abstract
Background Worldwide, Kaposi’s sarcoma (KS) occurs in immunocompetent elderly adults, especially men. Elderly Jews have relatively high KS risk, but it is unclear whether this indicates heightened prevalence of KS-associated herpesvirus (KSHV), the KS agent. We studied Jewish and non-Jewish patients at a New York City geriatrics clinic.
Methods We measured plasma antibodies against K8.1 (a KSHV glycoprotein) by enzyme immunoassay and against viral latency antigens by immunofluorescence assay. Individuals positive by either were considered KSHV-seropositive. Titres were performed for positive subjects. We used polymerase chain reaction to quantify circulating KSHV DNA.
Results Of 467 subjects (median age 80 years), 40 were KSHV-seropositive (8.6%). Seroprevalence was 8.8% among Jews (18 of 204), similar to other religious groups, and did not differ by sex or region of birth. However, K8.1 antibody titres were higher in men than women (geometric mean titre 177 versus 35, P = 0.03) and increased with age (P = 0.02). The K8.1 titres were higher in three people from Central/Eastern Europe (1280, 1280, 320), all of whom were Jewish, than in others (geometric mean titre 39, P = 0.006). The single person with detectable circulating KSHV (457 copies/million cells) had the highest titre (5120).
Conclusions The KSHV seroprevalence was not elevated among elderly Jews, despite their known high risk for KS. However, among KSHV-seropositive individuals, K8.1 titres were highest in subgroups at greatest risk for KS (men, older individuals, people from Central/Eastern Europe) and may identify individuals with poor immune control of KSHV replication during asymptomatic infection.
Kaposi’s sarcoma-associated herpesvirus (KSHV, also known as human herpesvirus 8) plays a causal role in Kaposi’s sarcoma (KS). The human immunodeficiency virus (HIV) epidemic led to an explosive increase in KS incidence in young adults. In the absence of HIV infection, the ‘classic’ form of KS occurs in immunocompetent elderly adults, especially men, and presents as an indolent process involving the skin and subcutaneous tissue of the lower limbs. Worldwide, the incidence of classic KS is relatively high in Mediterranean Europe, which reflects a high regional prevalence of KSHV infection.1–5
An elevated risk of classic KS is also seen in elderly Jews in the US. Studies in New York and Los Angeles, in the years before the HIV epidemic, found Jews over-represented among patients with KS.6–8 One US study found that Jews and Italians had similarly elevated KS risk (odds ratios 6.6 for Jews and 5.6 for Italians, compared to other individuals).7 Likewise, elderly Jews in Israel have a high incidence of KS.9
Nonetheless, it is unclear whether the high KS risk among elderly Jews reflects a heightened prevalence of KSHV or, instead, poor immune control of asymptomatic infection. Previous studies in Israeli Jews have documented KSHV seroprevalence rates of 5–10% in the general population.10,11 Of importance, differences in the composition of the Jewish populations in the US and Israel complicate comparisons of the epidemiology of KS and KSHV in these countries. These differences can be understood in the context of the migrations of the Jewish people over the past 2000 years (termed the ‘Diaspora’).12 As a result of these movements, a large population of Jews (Ashkenazi Jews) eventually arose in Central/Eastern Europe. Another large group (Sephardic Jews) trace their origins to Spain during the period of Islamic rule. Most US Jews are of Ashkenazi descent, while Israel includes both Ashkenazi and Sephardic Jews. Differences in disease risk between Ashkenazi and Sephardic Jews might be due to environmental exposures or genetic differences (e.g. Tay-Sachs disease is common only in Ashkenazi Jews13). In Israel, Sephardic Jews have a higher risk of KS than do Ashkenazi Jews, which could be due, at least in part, to higher KSHV prevalence.9,10
No prior study has examined KSHV epidemiology among US Jews, or elderly Jews in particular, who are at risk for classic KS. We therefore performed a cross-sectional study of patients attending a New York City geriatrics clinic. A substantial proportion of the patients in this practice are Jewish, and many patients were born outside the US. We measured KSHV seroprevalence in a sample of the Jewish and non-Jewish patients. Additionally, to look for differences in immune-mediated control of asymptomatic infection, we titred KSHV antibodies and assayed for KSHV viraemia.
Methods
We studied patients (age ≥65 years) from the Coffey Geriatrics Associates practice at Mount Sinai Hospital (New York City) from February to December 2000. Patients were recruited consecutively. However, to enrich the proportion of Jewish subjects and males, we intermittently suspended enrolment of other subjects. Also, as part of a health-related lecture series for elderly adults at a neighbouring Young Men’s and Women’s Hebrew Association, we enrolled 14 additional elderly Jewish subjects during a single-day period. Following a uniform protocol, subjects answered questions regarding demographic information and medical history and provided a blood sample. Participating subjects provided informed consent. This study was approved by institutional review boards at the National Cancer Institute and Mount Sinai Hospital.
Plasma and cells were separated within 24 hours of phlebotomy and stored at –70°C. As previously described,14 we tested subjects for antibodies using two assays: an enzyme immunoassay for antibodies to K8.1, which is a KSHV structural glycoprotein; and an indirect immunofluorescence assay (IFA) for antibodies against KSHV latency associated nuclear antigen (LANA), encoded by orf 73. Individuals were considered KSHV-seropositive if they had antibodies detectable by either K8.1 EIA (optical density >1.00) or IFA. Additionally, we measured antibody titres by K8.1 EIA or IFA for individuals who were positive on these assays. Finally, for seropositive subjects, we used Taqman polymerase chain reaction (PCR) to detect and quantify KSHV DNA in peripheral blood mononuclear cells, as previously described.15
We compared KSHV seroprevalence across groups using the χ2 test (Fisher exact test when counts were small) and tested for linear trend using the Mantel-Haenszel χ2 test. We compared antibody titres between groups using the Wilcoxon rank sum test and examined the relationship between titres and age with linear regression. A 5% significance level was used in statistical tests.
Results
The study included 467 subjects (Table). Two-thirds were female. The median age was 80 years (range 65–99). By design, Jews comprised the largest religious group (43.7%), although substantial proportions of subjects were Protestant or Catholic. Most study subjects (73.9%) were born in the US. Of Hispanic subjects, 22 (52.4%) were born in Puerto Rico. Among Jews, 157 (77.0%) were born in North America, 25 (12.3%) in Central/ Eastern Europe, 14 (6.9%) in Western Europe, 1 (0.5%) in Mediterranean Europe, and 7 (3.4%) in other regions. Jews made up the majority of people born in Central/Eastern Europe (86.2%).
Forty subjects (8.6%) were KSHV-seropositive. There were no significant differences in seroprevalence across categories of sex, race, or region of birth (Table). Seroprevalence was highest in individuals ≥88 years old (Table), and a linear trend in seroprevalence with age was significant (P = 0.03).
Seroprevalence was 8.8% among Jews, which did not differ from seroprevalence in other religious groups. Fifteen of 180 Ashkenazi Jews (8.3%) were seropositive, compared with 2 of 12 Sephardic Jews (16.7%, P = 0.29). Seropositive Ashkenazi Jews were born in the US (12 subjects), Czechoslovakia (1), Hungary (1), or Russia (1). The two seropositive Sephardic Jews were born in the US and Suriname.
Only one of the 40 seropositive subjects had detectable KSHV in peripheral blood mononuclear cells (457 copies per million cells). This individual, one of two Puerto Rican subjects (9.1%) who were KSHV-seropositive, had no history of cancer or immunosuppressing conditions. He was K8.1-seropositive but IFA-seronegative.
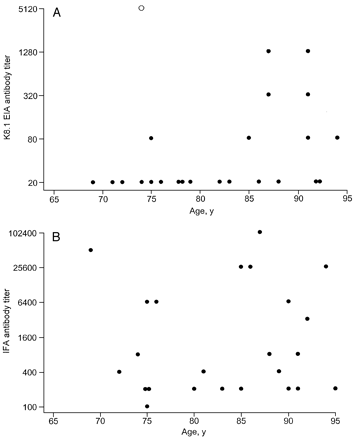
We measured K8.1 and IFA antibody titres in people with detectable antibody on these assays. The highest K8.1 antibody titre was observed in the Puerto Rican individual with viraemia (titre 5120). The K8.1 antibody titres were significantly higher in men than women (geometric mean titre 177 versus 35, P = 0.03). The K8.1 antibody titres also tended to increase with age (Figure, Panel A). This trend was most apparent after excluding the subject with viraemia (P = 0.02). The K8.1 antibody titres did not differ overall by religion (data not shown). However, K8.1 antibody titres were significantly higher in the three K8.1-seropositive subjects from Central/Eastern Europe (titres 1280, 1280, 320), all of whom were Jewish, than in other seropositive subjects (geometric mean titre 39, P = 0.006). By contrast, IFA antibody titres did not differ between men and women (geometric mean titre 872 versus 1600, P = 0.75) and did not vary by age (Figure, Panel B; P = 0.84). The IFA antibody titre also did not differ across subgroups defined by religion or place of birth (data not shown).
Discussion
We found that 8.8% of elderly Jews living in New York City were KSHV-seropositive. Two prior studies documented KSHV antibodies in 5–10% of Israeli Jews.10,11 Nonetheless, seroprevalence estimates from those two studies and ours are not directly comparable. Most subjects in the previous studies were much younger than our subjects (almost all were children or young/middle-aged adults). In one of the studies, KSHV seroprevalence was 18% in a small subgroup of individuals ≥55-years-old,10 which is higher than we observed. However, unlike our study, both Israeli studies included a large fraction of Sephardic Jews, who may more often be KSHV-seropositive than Ashkenazi Jews.10,11 The prior studies did not include non-Jews as a comparison group.
Among elderly adults in our study, we did not find especially high KSHV seroprevalence in Ashkenazi Jews or in people from Central/Eastern Europe. This was somewhat surprising because these groups appear to have heightened risk for classic KS.6–9 Furthermore, seroprevalence among our Jewish subjects was noticeably lower than seen in the Mediterranean region. For example, 20% of elderly adults from Sicily and 25% from Sardinia have KSHV antibodies,2 over twice as high as we found in US Jews, even though Italians and Ashkenazi Jews have a similarly elevated risk of classic KS.1,2,7,9 Likewise, in our study men and women had similar seroprevalence rates, although elderly men have a higher risk of classic KS than women.2,9 Taken together, these considerations imply that observed differences in KS risk across various populations are not entirely due to differences in the prevalence of KSHV infection. Instead, the risk of KS is partly determined by events that occur after infection, specifically how well KSHV infection is controlled by the immune system in asymptomatically infected people.
Further support for this idea is provided by our data on antibody titres. KSHV viraemia is uncommon in asymptomatic infection, even in the presence of immunosuppression, suggesting an absence or only a low level of viral replication.16 Notably, the single individual in our study who had detectable viraemia had a very high K8.1 antibody titre. Also, we found that to some extent, K8.1 antibody titres in various subgroups paralleled their reported KS risk, with relatively high titres seen in men, adults aged over 85 years (Figure, panel A), and people from Central/Eastern Europe. It seems plausible that high K8.1 antibody titres could reflect higher levels of KSHV replication, since the K8.1 glycoprotein is a structural component of KSHV expressed only during viral replication. In turn, poor immune control of viral replication might predispose KSHV-infected individuals to develop KS. The IFA antibody titres (which measure antibodies to LANA, a protein vital for viral latency and persistence) were not associated with KS risk in our study, although that was seen in a study of Italian blood donors.3
Combined with other information, our seroprevalence data can be used to estimate the risk for developing KS among elderly Jews with asymptomatic KSHV infection. Overall, KS incidence in the US is 1.3 per 100 000 person-years among older adults (≥65-years-old), based on data from before the 1980 onset of the HIV epidemic.17 Among US Jews, the incidence of this malignancy is roughly six times higher (i.e. 7.8 per 100 000 person-years).7,8 Given that all cases of KS occur in KSHV-infected individuals (who, based on our data, make up 8.8% of the elderly Jewish population), we can then estimate that the incidence of KS is 89 per 100 000 person-years among KSHV-infected elderly adults (i.e. 7.8/0.088, per 100 000 person-years). Similar calculations for Israeli Jews, using published data,9,10 yield a KS incidence of 36 per 100 000 person-years for KSHV-infected people ≥55-years-old. These estimates are both higher than those previously calculated for elderly KSHV-infected adults in Sicily, Sardinia, or Malta,2 which ranged from 9 to 32 per 100 000 person-years. The calculations thus illustrate that the relatively high risk for KS among elderly Jews arises largely because of a high risk for this malignancy among those who are already KSHV-infected, rather than from an especially high prevalence of KSHV infection. This component of KS risk, which we suggest reflects immune control of KSHV infection, could conceivably be determined by host genetics, age at KSHV infection, characteristics of the infecting viral strain, or other environmental exposures.
The incidence of classic KS is relatively high in Puerto Rico. Before the HIV epidemic, KS incidence among older adults was reported to be 48% greater there than in the continental US.18 In our study, KSHV seroprevalence was similar for Hispanics born in Puerto Rico and elsewhere. Importantly, one Puerto Rican individual had detectable circulating KSHV, in the absence of immunosuppression. Viraemia was present at a higher level (457 copies per million cells) than typically seen even in HIV-infected KS patients (median 20 KSHV copies per million cells).19 Additional studies of people from Puerto Rico may help explain their elevated KS risk.
The absence of a gold-standard test for asymptomatic KSHV infection poses a continuing challenge for studying the epidemiology of this infection.14 Of interest, there was only fair agreement on serostatus between our two tests, K8.1 enzyme immunoassay (EIA) and IFA. Among 40 seropositive subjects, only 8 were positive on both assays (κ = 0.30). This lack of agreement might indicate that one or both tests were inaccurate (low sensitivity or specificity). However, in a previous study of elderly adults,14 we found that our K8.1 EIA had high sensitivity (100%) and specificity (98%); the IFA also had high sensitivity (99%), although it was less specific (90%). We therefore interpreted the lack of agreement between the two tests as reflecting biological differences among subjects in their antibody response to infection, i.e. some infected individuals primarily mount an antibody response to latent infection (as measured by IFA), while some manifest an antibody response to replicating virus (as measured by K8.1 EIA). Thus, we considered people positive on either test as being infected. Future studies will be aided by a more complete understanding of the natural history of KSHV infection and identification of highly accurate serological assays. A further limitation of our study was the small size of some subgroups, such as Sephardic Jews and non-Jews from Central/Eastern Europe.
In summary, we found that KSHV seroprevalence in elderly US Jews was 8.8%, similar to that observed in other groups of older-aged adults. Antibody titres to a structural protein of KSHV (K8.1) paralleled KSHV risk and might identify individuals whose immune system controls KSHV replication poorly. Additional studies of the elderly will help elucidate the pathogenesis of classic KS.
Elderly Jews have a relatively high incidence of Kaposi’s sarcoma, but the seroprevalence of Kaposi’s sarcoma-associated herpesvirus (KSHV, the causative agent) was not elevated among Jewish patients in a New York geriatrics clinic.
Antibody titres against a structural protein of KSHV were highest in groups with known high risk for Kaposi’s sarcoma (men, the most aged, people from Central/Eastern Europe) and in an individual with KSHV viraemia, suggesting that poor immune control of KSHV infection predisposes to Kaposi’s sarcoma.
Kaposi’s sarcoma-associated herpesvirus (KSHV) seroprevalence by demographic group
| Characteristic . | N . | K8.1a-seropositive n (%) . | IFAb-seropositive n (%) . | Seropositive by K8.1 or IFA n (%) . |
|---|---|---|---|---|
| Subjects with missing values are excluded from Table. | ||||
| aA KSHV glycoprotein. | ||||
| bIndirect immunofluorescence assay. | ||||
| Sex | ||||
| Male | 157 | 7 (4.5) | 8 (5.1) | 12 (7.6) |
| Female | 310 | 17 (5.5) | 16 (5.2) | 28 (9.0) |
| P-value | 0.64 | 0.98 | 0.61 | |
| Religion | ||||
| Jewish | 204 | 12 (5.9) | 11 (5.4) | 18 (8.8) |
| Protestant | 148 | 4 (2.7) | 9 (6.1) | 12 (8.1) |
| Catholic | 90 | 6 (6.7) | 4 (4.4) | 8 (8.9) |
| Other | 22 | 1 (4.6) | 0 (0) | 1 (4.6) |
| P-value | 0.38 | 0.83 | 0.92 | |
| Race | ||||
| White | 315 | 19 (6.0) | 16 (5.1) | 28 (8.9) |
| Black | 105 | 1 (1.0) | 6 (5.7) | 7 (6.7) |
| Hispanic | 42 | 3 (7.1) | 2 (4.8) | 4 (9.5) |
| Other | 5 | 1 (20.0) | 0 (0) | 1 (20.0) |
| P-value | 0.04 | 0.96 | 0.51 | |
| Age, years | ||||
| 65–73 | 93 | 3 (3.2) | 2 (2.2) | 5 (5.4) |
| 74–78 | 108 | 7 (6.5) | 6 (5.6) | 10 (9.3) |
| 79–82 | 91 | 2 (2.2) | 2 (2.2) | 4 (4.4) |
| 83–87 | 102 | 5 (4.9) | 5 (4.9) | 8 (7.8) |
| 88–99 | 73 | 7 (9.6) | 9 (12.3) | 13 (17.8) |
| P-value for trend | 0.20 | 0.02 | 0.03 | |
| Region of birth | ||||
| Europe, Western | 19 | 0 (0) | 0 (0) | 0 (0) |
| Europe, Central/Eastern | 29 | 3 (10.3) | 1 (3.5) | 3 (10.3) |
| Europe, Mediterranean | 5 | 0 (0) | 0 (0) | 0 (0) |
| North America | 348 | 16 (4.6) | 20 (5.8) | 30 (8.6) |
| Latin America | 55 | 4 (7.3) | 2 (3.6) | 5 (9.1) |
| Africa and Asia | 11 | 1 (9.1) | 1 (9.1) | 2 (18.2) |
| P-value | 0.42 | 0.84 | 0.58 | |
| Characteristic . | N . | K8.1a-seropositive n (%) . | IFAb-seropositive n (%) . | Seropositive by K8.1 or IFA n (%) . |
|---|---|---|---|---|
| Subjects with missing values are excluded from Table. | ||||
| aA KSHV glycoprotein. | ||||
| bIndirect immunofluorescence assay. | ||||
| Sex | ||||
| Male | 157 | 7 (4.5) | 8 (5.1) | 12 (7.6) |
| Female | 310 | 17 (5.5) | 16 (5.2) | 28 (9.0) |
| P-value | 0.64 | 0.98 | 0.61 | |
| Religion | ||||
| Jewish | 204 | 12 (5.9) | 11 (5.4) | 18 (8.8) |
| Protestant | 148 | 4 (2.7) | 9 (6.1) | 12 (8.1) |
| Catholic | 90 | 6 (6.7) | 4 (4.4) | 8 (8.9) |
| Other | 22 | 1 (4.6) | 0 (0) | 1 (4.6) |
| P-value | 0.38 | 0.83 | 0.92 | |
| Race | ||||
| White | 315 | 19 (6.0) | 16 (5.1) | 28 (8.9) |
| Black | 105 | 1 (1.0) | 6 (5.7) | 7 (6.7) |
| Hispanic | 42 | 3 (7.1) | 2 (4.8) | 4 (9.5) |
| Other | 5 | 1 (20.0) | 0 (0) | 1 (20.0) |
| P-value | 0.04 | 0.96 | 0.51 | |
| Age, years | ||||
| 65–73 | 93 | 3 (3.2) | 2 (2.2) | 5 (5.4) |
| 74–78 | 108 | 7 (6.5) | 6 (5.6) | 10 (9.3) |
| 79–82 | 91 | 2 (2.2) | 2 (2.2) | 4 (4.4) |
| 83–87 | 102 | 5 (4.9) | 5 (4.9) | 8 (7.8) |
| 88–99 | 73 | 7 (9.6) | 9 (12.3) | 13 (17.8) |
| P-value for trend | 0.20 | 0.02 | 0.03 | |
| Region of birth | ||||
| Europe, Western | 19 | 0 (0) | 0 (0) | 0 (0) |
| Europe, Central/Eastern | 29 | 3 (10.3) | 1 (3.5) | 3 (10.3) |
| Europe, Mediterranean | 5 | 0 (0) | 0 (0) | 0 (0) |
| North America | 348 | 16 (4.6) | 20 (5.8) | 30 (8.6) |
| Latin America | 55 | 4 (7.3) | 2 (3.6) | 5 (9.1) |
| Africa and Asia | 11 | 1 (9.1) | 1 (9.1) | 2 (18.2) |
| P-value | 0.42 | 0.84 | 0.58 | |
Kaposi’s sarcoma-associated herpesvirus (KSHV) seroprevalence by demographic group
| Characteristic . | N . | K8.1a-seropositive n (%) . | IFAb-seropositive n (%) . | Seropositive by K8.1 or IFA n (%) . |
|---|---|---|---|---|
| Subjects with missing values are excluded from Table. | ||||
| aA KSHV glycoprotein. | ||||
| bIndirect immunofluorescence assay. | ||||
| Sex | ||||
| Male | 157 | 7 (4.5) | 8 (5.1) | 12 (7.6) |
| Female | 310 | 17 (5.5) | 16 (5.2) | 28 (9.0) |
| P-value | 0.64 | 0.98 | 0.61 | |
| Religion | ||||
| Jewish | 204 | 12 (5.9) | 11 (5.4) | 18 (8.8) |
| Protestant | 148 | 4 (2.7) | 9 (6.1) | 12 (8.1) |
| Catholic | 90 | 6 (6.7) | 4 (4.4) | 8 (8.9) |
| Other | 22 | 1 (4.6) | 0 (0) | 1 (4.6) |
| P-value | 0.38 | 0.83 | 0.92 | |
| Race | ||||
| White | 315 | 19 (6.0) | 16 (5.1) | 28 (8.9) |
| Black | 105 | 1 (1.0) | 6 (5.7) | 7 (6.7) |
| Hispanic | 42 | 3 (7.1) | 2 (4.8) | 4 (9.5) |
| Other | 5 | 1 (20.0) | 0 (0) | 1 (20.0) |
| P-value | 0.04 | 0.96 | 0.51 | |
| Age, years | ||||
| 65–73 | 93 | 3 (3.2) | 2 (2.2) | 5 (5.4) |
| 74–78 | 108 | 7 (6.5) | 6 (5.6) | 10 (9.3) |
| 79–82 | 91 | 2 (2.2) | 2 (2.2) | 4 (4.4) |
| 83–87 | 102 | 5 (4.9) | 5 (4.9) | 8 (7.8) |
| 88–99 | 73 | 7 (9.6) | 9 (12.3) | 13 (17.8) |
| P-value for trend | 0.20 | 0.02 | 0.03 | |
| Region of birth | ||||
| Europe, Western | 19 | 0 (0) | 0 (0) | 0 (0) |
| Europe, Central/Eastern | 29 | 3 (10.3) | 1 (3.5) | 3 (10.3) |
| Europe, Mediterranean | 5 | 0 (0) | 0 (0) | 0 (0) |
| North America | 348 | 16 (4.6) | 20 (5.8) | 30 (8.6) |
| Latin America | 55 | 4 (7.3) | 2 (3.6) | 5 (9.1) |
| Africa and Asia | 11 | 1 (9.1) | 1 (9.1) | 2 (18.2) |
| P-value | 0.42 | 0.84 | 0.58 | |
| Characteristic . | N . | K8.1a-seropositive n (%) . | IFAb-seropositive n (%) . | Seropositive by K8.1 or IFA n (%) . |
|---|---|---|---|---|
| Subjects with missing values are excluded from Table. | ||||
| aA KSHV glycoprotein. | ||||
| bIndirect immunofluorescence assay. | ||||
| Sex | ||||
| Male | 157 | 7 (4.5) | 8 (5.1) | 12 (7.6) |
| Female | 310 | 17 (5.5) | 16 (5.2) | 28 (9.0) |
| P-value | 0.64 | 0.98 | 0.61 | |
| Religion | ||||
| Jewish | 204 | 12 (5.9) | 11 (5.4) | 18 (8.8) |
| Protestant | 148 | 4 (2.7) | 9 (6.1) | 12 (8.1) |
| Catholic | 90 | 6 (6.7) | 4 (4.4) | 8 (8.9) |
| Other | 22 | 1 (4.6) | 0 (0) | 1 (4.6) |
| P-value | 0.38 | 0.83 | 0.92 | |
| Race | ||||
| White | 315 | 19 (6.0) | 16 (5.1) | 28 (8.9) |
| Black | 105 | 1 (1.0) | 6 (5.7) | 7 (6.7) |
| Hispanic | 42 | 3 (7.1) | 2 (4.8) | 4 (9.5) |
| Other | 5 | 1 (20.0) | 0 (0) | 1 (20.0) |
| P-value | 0.04 | 0.96 | 0.51 | |
| Age, years | ||||
| 65–73 | 93 | 3 (3.2) | 2 (2.2) | 5 (5.4) |
| 74–78 | 108 | 7 (6.5) | 6 (5.6) | 10 (9.3) |
| 79–82 | 91 | 2 (2.2) | 2 (2.2) | 4 (4.4) |
| 83–87 | 102 | 5 (4.9) | 5 (4.9) | 8 (7.8) |
| 88–99 | 73 | 7 (9.6) | 9 (12.3) | 13 (17.8) |
| P-value for trend | 0.20 | 0.02 | 0.03 | |
| Region of birth | ||||
| Europe, Western | 19 | 0 (0) | 0 (0) | 0 (0) |
| Europe, Central/Eastern | 29 | 3 (10.3) | 1 (3.5) | 3 (10.3) |
| Europe, Mediterranean | 5 | 0 (0) | 0 (0) | 0 (0) |
| North America | 348 | 16 (4.6) | 20 (5.8) | 30 (8.6) |
| Latin America | 55 | 4 (7.3) | 2 (3.6) | 5 (9.1) |
| Africa and Asia | 11 | 1 (9.1) | 1 (9.1) | 2 (18.2) |
| P-value | 0.42 | 0.84 | 0.58 | |
Kaposi’s sarcoma-associated herpesvirus (KSHV) antibody titre as a function of age
Panel A This panel depicts antibody titres, measured with an enzyme immunoassay for antibodies to the KSHV glycoprotein K8.1, as a function of age. Results are shown for the 24 individuals who had antibodies against this antigen. Titres are depicted on a log-transformed scale. The titre for the single individual with detectable circulating KSHV DNA is shown as an open circle (titre of 5120).
Panel B This panel shows antibody titres, measured using an indirect immunofluorescence assay, as a function of age. Data are presented for the 24 IFA-seropositive subjects. Titres are shown on a log-transformed scale. The subject with circulating KSHV DNA was IFA-negative and so is not depicted in this panel.
Funding: This project was funded in part by the National Cancer Institute, National Institutes of Health, under Contracts No. NO1-CO-56000 and NO1-CO-12400. The authors gratefully acknowledge the contributions of the nursing and administrative staff at the Coffey Geriatrics Associates practice, especially Siobhan Sundel and Tara Easter; patient interviewers Jay Smith and Caprice Royal; study managers Vivian Szeto and Amali Amarasinghe, and computer programmers Jeff Ancheta and Sylvia Cohn (Research Triangle Institute, Rockville, MD); and laboratory workers Vickie Marshall, Mike Walters, Andrea Stossel and Christine Gamache (Scientific Applications International Corporation, Frederick, MD).
References
Geddes M, Franceschi S, Barchielli A et al. Kaposi’s sarcoma in Italy before and after the AIDS epidemic.
Vitale F, Briffa DV, Whitby D et al. Kaposi’s sarcoma herpes virus and Kaposi’s sarcoma in the elderly populations of 3 Mediterranean islands.
Whitby D, Luppi M, Barozzi P, Boshoff C, Weiss RA, Torelli G. Human herpesvirus 8 seroprevalence in blood donors and lymphoma patients from different regions of Italy.
Calabro ML, Sheldon J, Favero A et al. Seroprevalence of Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 in several regions of Italy.
Santarelli R, de Marco R, Masala MV et al. Direct correlation between human herpesvirus-8 seroprevalence and classic Kaposi’s sarcoma incidence in Northern Sardinia.
Digiovanna JJ, Safai B. Kaposi’s sarcoma. Retrospective study of 90 cases with particular emphasis on the familial occurrence, ethnic background and prevalence of other diseases.
Brownstein MH, Shapiro L, Skolnik P. Kaposi’s sarcoma in community practice.
Ross RK, Casagrande JT, Dworsky RL, Levine A, Mack T. Kaposi’s sarcoma in Los Angeles, California.
Iscovich J, Boffetta P, Winkelmann R, Brennan P, Aziza E. Classic Kaposi’s sarcoma in Jews living in Israel, 1961–1989: a population-based incidence study.
Davidovici B, Karakis I, Bourboulia D et al. Seroepidemiology and molecular epidemiology of Kaposi’s sarcoma-associated herpesvirus among Jewish population groups in Israel.
Iscovich J, Fischbein A, Fisher-Fischbein J et al. Seroprevalence of Kaposi’s sarcoma-associated herpesvirus in healthy adults in Israel.
Scheindlin RP. A Short History of the Jewish People: From Legendary Times to Modern Statehood. Oxford: Oxford University Press, 1998.
Peterson GM, Rotter JI, Cantor RM et al. The Tay-Sachs gene in North American Jewish populations: geographic variations and origin.
Engels EA, Sinclair MD, Biggar RJ et al. Latent class analysis of human herpesvirus 8 assay performance and infection prevalence in sub-Saharan Africa and Malta.
Biggar RJ, Whitby D, Marshall V, Linhares AC, Black F. Human herpesvirus 8 in Brazilian Amerindians: a hyperendemic population with a new subtype.
Pauk J, Huang M-L, Brodie SJ et al. Mucosal shedding of human herpesvirus 8 in men.
SEER Cancer Incidence Public-Use Database, 1973–1997. [August 1999 submission]. National Cancer Institute.
Biggar RJ, Horm J, Fraumeni JF Jr, Greene MH, Goedert JJ. Incidence of Kaposi’s sarcoma and mycosis fungoides in the United States including Puerto Rico, 1973–1981.