-
PDF
- Split View
-
Views
-
Cite
Cite
Vikram Patel, Sulochana Pednekar, Helen Weiss, Merlyn Rodrigues, Preetam Barros, Bernice Nayak, Vandana Tanksale, Beryl West, Prasad Nevrekar, Betty R Kirkwood, David Mabey, Why do women complain of vaginal discharge? A population survey of infectious and pyschosocial risk factors in a South Asian community, International Journal of Epidemiology, Volume 34, Issue 4, August 2005, Pages 853–862, https://doi.org/10.1093/ije/dyi072
Close - Share Icon Share
Abstract
Background Vaginal discharge is a common complaint, particularly among women in Asia. Although presumed to be caused by reproductive tract infections (RTIs), the association between the complaint and the presence of RTIs is weak. This study aimed to investigate the risk factors of the complaint of vaginal discharge.
Methods We conducted a community-based survey of 3000 women aged 18–50 years, randomly sampled from a population in Goa, India. Women who gave informed consent were invited to participate in a structured interview, which elicited data on the primary outcome (the experience of current abnormal vaginal discharge) and psychosocial exposures: gender adversity; symptoms of somatoform disorders; and common mental disorders (CMD). All women were required to provide vaginal and/or urine samples for diagnosis of RTIs using gold standard laboratory tests. Risk factors were analysed using logistic regression with the binary outcome of the complaint of vaginal discharge.
Results Of the 2494 women (83%) who agreed to participate, 14.5% complained of having an abnormal vaginal discharge. Stress was the most common causal attribution for the complaint. The final multivariate model found that high scores for CMD (OR 2.16, 1.4–3.2) and somatoform disorders (6.23, 4.0–9.7) and the use of an intrauterine contraceptive device (1.86, 1.0–3.4) were independently associated with the complaint. Low literacy (0.54, 0.4–0.8) and age >40 years (0.29, 0.2–0.4) were associated with a reduced risk. RTI were not associated with the complaint (1.24, 0.9–1.6).
Conclusions Psychosocial factors have the strongest association with the complaint of vaginal discharge. Syndromic management algorithms need refinement so that women with complaints that are non-infectious in aetiology are offered psychosocial interventions.
The complaint of abnormal vaginal discharge is very common, particularly in South Asia where about a quarter of all adult women report this complaint.1 The complaint is associated with considerable disability, health seeking, and associated costs.1–3 The World Health Organization has recommended syndromic management, in which women complaining of discharge are treated for some or all of the five common reproductive tract infections (RTIs): Chlamydia trachomatis infection, gonorrhoea, and trichomoniasis, which are sexually transmitted infections (STIs) and bacterial vaginosis (BV) and candidiasis, which result from disturbances in the normal bacterial flora of the vagina.4 However, recent evidence suggests that the association between the complaint of vaginal discharge and the presence of RTIs is weak. Thus, studies in clinical settings in Bangladesh and India reported that only 30 and 60% of women complaining of vaginal discharge had a laboratory confirmed RTI.5,6 As a result, the syndromic approach for the treatment of vaginal discharge leads to inappropriate treatment in a high proportion of cases.7 The economic cost of syndromic management per true case of RTI treated in South Asia ranged from $3.61 to $4.25, while the social cost of incorrectly labelling a woman as suffering from an STI is inestimable.5,6,8 Despite the poor correlation between RTIs and vaginal discharge, the syndromic approach remains the cornerstone of intervention programmes for women's reproductive health, mainly due to lack of evidence supporting alternative aetiologies for the complaint.
Common mental disorders (CMD) such as depressive and anxiety disorders and somatoform disorders, which are characterized by medically unexplained physical symptoms, are among the most common health problems in primary care settings.9,10 There is a substantial literature from the West demonstrating a strong relationship between gynaecological symptoms and these mental disorders.11–14 A study in India reported higher rates of depression in women attending a gynaecological clinic compared with women attending a general medical clinic.15 There is evidence from clinical and community studies linking the complaint of vaginal discharge with mental disorders in India.16,17 It has been hypothesized that the complaint of vaginal discharge may represent a culturally shaped ‘bodily idiom of distress’, in which concerns about loss of genital secretions reflect wider issues of social stress.16,1819 The implications of such an association are enormous for they would suggest that, in addition to the focus on infectious aetiologies, reproductive health programmes would need to consider psychosocial interventions for women with the complaint of vaginal discharge. However, to the best of our knowledge, there are no studies combining methodologies to assess both infectious and psychosocial aetiologies for gynaecological complaints from a developing country. The aim of the study, in this paper, is to quantify the extent to which psychosocial risk factors are associated with the complaint of vaginal discharge.
Methods
Setting
The study was located in the state of Goa on India's west coast. Goa has a population of 1.4 million.20
Sample
The study population was selected from women living in the catchment covered by the Aldona Primary Health Centre (PHC) area of north Goa. The sampling frame consisted of the 8595 women recorded as aged 18–45 years (the reproductive age range) in the Family Health Register. The lower age limit of 18 years was chosen for logistic reasons, since we would need consent from parents to recruit women younger than 18 years. A simple random sample of 3000 women was selected from the sampling frame. Since the Family Registers varied in date (between 1 and 4 years), we allowed for the fact that some women recorded as aged 45 would be older, and considered women aged up to 50 years eligible for recruitment. Other inclusion criteria were: expected residence in the area for the next 12 months; speaking one of the study languages; not suffering from cognitive impairment which would make giving informed consent difficult; and not being pregnant. If a selected woman did not meet these criteria, then she was replaced with another woman from the household, or the neighbouring home, who met the eligibility criteria.
Recruitment procedure
Recruitment took place from November 2001 to May 2003. The two mandatory requirements for participation were a face-to-face interview with a trained researcher, and the collection of vaginal or urine specimens for the diagnosis of RTIs. For women who consented to a gynaecological speculum examination (n = 1398), two high vaginal swabs, for polymerase chain reaction (PCR), and two vaginal swabs (for smears and culture) were collected. For unmarried women, and women who refused the offer of a gynaecological examination, first-void urine specimens were collected in lieu of the high vaginal swabs, and self-administered vaginal swabs for smears and culture. The use of self-administered swabs was shown to be feasible in an earlier study in Goa, which also reported a high level of concordance between self-administered and gynaecological collected specimens for the detection of RTI.21
Interview
The study employed a semi-structured interview, which was a composite of questions eliciting data on different aspects of the woman's personal and health history. The composite interview was piloted with 100 women attending gynaecological outpatient clinics in the two hospitals in north Goa. Informed consent was obtained from these subjects. The final version of the interview consisted of the following sections.
Personal data
Age, education, religion, and marital status from all subjects including those who refused to participate. Economic indicators were: type of housing, access to safe drinking water and a toilet, household composition and income, employment status, indebtedness, the experience of hunger in the previous 3 months, and the perception of the financial situation of the family. These questions were derived from interviews used in the NFHS-II22 and a study of poverty and mental illness.23 All women who had had a sexual relationship in the past were asked about pregnancies and their outcome and the use of contraceptives.
Psychosocial exposures
Two types of psychosocial exposures were measured, i.e. those measuring gender disadvantage and social support and those measuring mental health. Questions on gender disadvantage and social support covered four domains. The first was the experience of verbal, physical, and sexual violence by the spouse in the previous year and concerns about her partner's extramarital relationships and substance abuse. The second inquired about the autonomy the woman had to make decisions regarding visiting her mother/friend's home, seeing a doctor, keeping money aside for personal use, and having time to do things for herself. The responses were added to generate an autonomy score. The third inquired about the level of engagement, in the past 3 months, with four activities, i.e. religious activities, participation in a community/voluntary group, social outings to meet friends/relatives, and having friends/relatives visit her. The addition of ratings of these four items generated a social integration score. The final domain consisted of five items regarding social support from family when faced with different situations (good news, a personal problem, needing to borrow a small amount of money, feeling low, and becoming ill). These were added to generate a Family Support Score. Questions on these risk factors were derived from the NFHS-II interview22 and an interview used in a study of gender disadvantage and post-natal depression in Goa.24
Two measures were used for mental health. The scale for somatic symptoms was used to measure somatic symptoms, which are features of somatoform disorders. The scale, which consists of questions regarding the experience of 20 common somatic symptoms in the previous 2 weeks, has been used in India.25 Four categories of symptoms are inquired about: pain-related symptoms such as headache and body ache; sensory symptoms such as hot/cold sensations and tingling; non-specific symptoms such as tiredness and tremors; and biological function symptoms such as poor sleep and constipation. Each symptom is rated on a Likert scale of 0–2 of increasing severity; the final score is a summation of the 20 item scores. The second measure was the revised clinical interview schedule (CISR), a structured interview for the measurement and diagnosis of CMD in community and primary care settings.26 The Konkani language version of the CISR used in the present study has been used in previous studies in Goa.27 The interview consists of 14 sections, each covering specific symptoms such as anxiety, depression, irritability, obsessions, compulsions, and panic. The sum of the section scores generates a total score (range 0–57), which is a measure of non-psychotic psychiatric morbidity.
Outcome
The primary outcome measure was the complaint of abnormal vaginal discharge experienced in the previous 3 months. Women who had experienced this complaint were asked about the perceived cause, characteristics of discharge and help-seeking behaviour and whether they were experiencing it at the time of the interview. In addition, four other types of morbidity were inquired about in the previous 3 months: itching in the genital area, sores or blisters in the genital area, pain in the lower abdomen (excluding menstrual pain), and pain or burning during urination.28
Laboratory data
RTIs were diagnosed in a single laboratory, using the following tests: for chlamydial and gonococcal infection, PCR using the Roche Amplicor System (Roche Molecular Systems, Alameda, CA, USA) with internal controls, according the manufacturer's instructions; for Trichomonas vaginalis infection, culture using the InPouch TV Culture Kit (Biomed Diagnostic, San Jose, CA, USA) incubated at 37°C for up to 5 days and examined daily for motile trichomonads; for BV, the reading of gram-stained slides based on Nugent's score,29 and for candidiasis, the reading of gram-stained slides using a rating of the density of yeast cells seen per high power field. Slides were read by trained laboratory technologists. These tests are the most sensitive and specific tests available for the diagnosis of RTIs.30 The laboratory participated in the Quality Control for Molecular Diagnostics (UK) annual quality control tests for C. trachomatis and Neisseria gonorrhoeae, and in external quality assurance for BV.
Ethical considerations
The study proposal received ethical approval from the ethical committee of the London School of Hygiene & Tropical Medicine and from the Independent Ethics Commission, Mumbai (India). All women received copies of their laboratory reports and were offered free care by the study gynaecologists and mental health professionals based on the findings of the data collection; for example, subjects who were severely depressed were offered psychiatric consultation and antidepressants; all RTIs were treated with antibiotics on the basis of the results of the laboratory diagnostic tests.
Analysis
Logistic regression analyses were carried out with the complaint of abnormal vaginal discharge as the outcome. Potential risk factors were considered in groups: socioeconomic factors; obstetric factors/contraception usage and RTIs; and psychosocial factors subdivided into gender disadvantage and social support, and mental health. First, the bivariate association of each socioeconomic factor with the vaginal discharge was investigated; all factors whose association reached significance at P < 0.1 were included in a multivariate model. All factors, which remained significantly associated with the outcome (P < 0.1), in this model were retained. Next the association between each risk factor in the other groups and the complaint of vaginal discharge was assessed by adding the single risk factor into the multivariate model in order to adjust for the socioeconomic confounders. RTI risk factors were evaluated in four ways: each infection separately, any RTI, any STI, and the number of infections. The number of missing values for specific RTI diagnoses ranged from 6 (0.2%) for trichomoniasis to 71 (2.8%) for C. trachomatis infection. The primary reasons for missing values for BV and candidiasis were inadequate smears (for example 57/62 for both) and for C. trachomatis and gonorrhoea were inhibition (for example, 48/71 missing values for the former). For the latter three estimates, missing values for specific infections were assumed as being negative results. The role of RTIs as risk factors were also examined separately for the subgroup of women who had not sought medical treatment for the complaint. Continuous outcomes, such as the CISR, somatoform disorder symptom and autonomy scores were converted to categories (for e.g. tertiles), based on the distribution of scores. Next a composite multivariate model covering all domains was formed; this consisted of:
the subset of socioeconomic factors in the first multivariate model,
any psychosocial and obstetric/contraceptive factors for which the P-value adjusting, for the socioeconomic factors was <0.1,
any RTI on an a priori basis.
The final multivariate logistic regression model was reached by dropping factors, other than RTI, one at a time until all remaining factors were significant at the P < 0.1 level.
Results
Of the 3000 randomly selected women, 2494 consented to participate in the study (83.1%). The commonest reasons for refusal were that the woman did not have time to participate (52.4%) or that a family member had not given permission for her to participate (18.8%). Of those who participated, 957 (38.4%) were subjects who replaced a randomly selected woman, the commonest reason being the selected woman was no longer resident (41.7%) or was unlikely to be resident for the duration of the study (4.7%), and errors in the records of the Family Health Register (39.8%). Women who consented differed significantly from those who refused. Women who refused were more likely to be ethnic Goans (9.9% vs 1.6%, P < 0.001), unmarried (41.4% vs 26.7%, P < 0.001), younger in age (mean 31.3 years vs 32.3 years, P = 0.01) and to have completed more years of education (10.2 years vs 8.4 years, P < 0.001).
Prevalence and characteristics of gynaecological symptoms
Abnormal vaginal discharge was reported by 361 women (14.5%; 95% CI 13.1–15.9). The majority of women with the complaint (93%) were experiencing it at the time of the interview. 218 (60%) women had another coexisting gynaecological complaint. Table 1 shows that each of these other complaints was strongly associated with the complaint of abnormal vaginal discharge (P < 0.001).
Prevalence and association of current gynaecological symptoms with the complaint of abnormal vaginal discharge in the past 3 months (n = 2494)
| Complaint in the past 3 months . | Overall prevalence (%; 95% CI) . | Prevalence in women reporting vaginal discharge (n = 361) . | OR (95% CI) . |
|---|---|---|---|
| Itching in genital area | 366 (14.7; 13.3–16.1) | 143 (39.6%) | 5.62 (4.3–7.2) |
| Sores/blisters in genital area | 91 (3.6; 2.9–4.4) | 46 (12.7%) | 6.77 (4.4–10.4) |
| Pain in lower abdomen (not menstrual) | 346 (13.9;12.5–15.3) | 109 (30.2%) | 3.46 (2.7–4.5) |
| Pain/burning while passing urine | 234 (9.4; 8.2–10.6) | 71 (19.7%) | 2.96 (2.2–4.0) |
| Complaint in the past 3 months . | Overall prevalence (%; 95% CI) . | Prevalence in women reporting vaginal discharge (n = 361) . | OR (95% CI) . |
|---|---|---|---|
| Itching in genital area | 366 (14.7; 13.3–16.1) | 143 (39.6%) | 5.62 (4.3–7.2) |
| Sores/blisters in genital area | 91 (3.6; 2.9–4.4) | 46 (12.7%) | 6.77 (4.4–10.4) |
| Pain in lower abdomen (not menstrual) | 346 (13.9;12.5–15.3) | 109 (30.2%) | 3.46 (2.7–4.5) |
| Pain/burning while passing urine | 234 (9.4; 8.2–10.6) | 71 (19.7%) | 2.96 (2.2–4.0) |
Prevalence and association of current gynaecological symptoms with the complaint of abnormal vaginal discharge in the past 3 months (n = 2494)
| Complaint in the past 3 months . | Overall prevalence (%; 95% CI) . | Prevalence in women reporting vaginal discharge (n = 361) . | OR (95% CI) . |
|---|---|---|---|
| Itching in genital area | 366 (14.7; 13.3–16.1) | 143 (39.6%) | 5.62 (4.3–7.2) |
| Sores/blisters in genital area | 91 (3.6; 2.9–4.4) | 46 (12.7%) | 6.77 (4.4–10.4) |
| Pain in lower abdomen (not menstrual) | 346 (13.9;12.5–15.3) | 109 (30.2%) | 3.46 (2.7–4.5) |
| Pain/burning while passing urine | 234 (9.4; 8.2–10.6) | 71 (19.7%) | 2.96 (2.2–4.0) |
| Complaint in the past 3 months . | Overall prevalence (%; 95% CI) . | Prevalence in women reporting vaginal discharge (n = 361) . | OR (95% CI) . |
|---|---|---|---|
| Itching in genital area | 366 (14.7; 13.3–16.1) | 143 (39.6%) | 5.62 (4.3–7.2) |
| Sores/blisters in genital area | 91 (3.6; 2.9–4.4) | 46 (12.7%) | 6.77 (4.4–10.4) |
| Pain in lower abdomen (not menstrual) | 346 (13.9;12.5–15.3) | 109 (30.2%) | 3.46 (2.7–4.5) |
| Pain/burning while passing urine | 234 (9.4; 8.2–10.6) | 71 (19.7%) | 2.96 (2.2–4.0) |
Women who were experiencing abnormal vaginal discharge were asked closed questions regarding the perceived cause of the discharge. The commonest causal models were stress and emotional factors (36.6%), excess heat in the body (35.2%), and infection (30.5%). The commonest colour of the vaginal discharge was white (82.5%) followed by yellow (14.1%). More than half the women (53.2%) reported their discharge to be odourless. Discharge was reported to be present only on certain days of the menstrual cycle by 56.8% of women; the commonest occasion was premenstrual (61.8%). More than one-third of the women (38.2%) reported that they had to change their underwear at least once a day because of the volume of discharge. A variety of methods were used to control the discharge, the commonest being consulting a doctor (50.4%), seeking advice from a relative (40.2%), taking a biomedical medicine (37.4%), and taking a tonic (26.4%).
Associations of the complaint of vaginal discharge with socioeconomic risk factors
The socioeconomic characteristics of the study population are presented in Table 2, together with their association with the complaint of vaginal discharge. The majority of women were Hindu (74.6%); most of the remainder were Christian (22.2%). Women who were unable to read or write constitute 14.3%. The majority of women were homemakers (66.7%), one-fifth (20.9%) were paid employees, and the remainder were students, or were seasonally or self-employed. The average household income per month, based on the average income in the previous 3 months, was Rs 4575 (∼100 US$), or a mean per capita income per month of Rs 988 (∼21 US$). Forty percentage of homes had a toilet and 44.4% had piped water in the home, but more than one-third of the women (36.1%) had no toilet facility of any kind. One-third of the households (33.3%) were currently in debt and although severe economic difficulties (as reflected by the experience of hunger in the recent 3 months) were uncommon (5.2%), nearly one-third of the women (31.9%) reported difficulty in making ends meet. The association of socioeconomic characteristics with the complaint of vaginal discharge is presented in Table 2.
Crude and adjusted estimates of the association of socioeconomic risk factors with the complaint of abnormal vaginal discharge (n = 2494)
| Factor . | Proportion in sample (%) . | Prevalence of vaginal discharge (%) . | Univariate OR (95% CI) . | Adjusted OR (95% CI)a . | ||||
|---|---|---|---|---|---|---|---|---|
| Sociodemographic factors | ||||||||
| Age | ||||||||
| 18–24 | 20.2 | 17.7 | 1 | 1 | ||||
| 25–29 | 18.4 | 18.9 | 1.09 (0.8–1.5) | 1.12 (0.8–1.6) | ||||
| 30–34 | 20.0 | 16.4 | 0.92 (0.7–1.3) | 0.96 (0.7–1.3) | ||||
| 35–39 | 18.9 | 11.6 | 0.61 (0.4–0.9) | 0.67(0.5–1.0) | ||||
| 40–50 | 22.4 | 8.6 | 0.44 (0.3–0.6)b | 0.52(0.4–0.8) | ||||
| Subject type | ||||||||
| Random | 61.6 | 12.4 | 1 | 1 | ||||
| Replacement | 38.4 | 17.8 | 1.53 (1.2–1.9)b | 1.40 (1.1–1.8) | ||||
| Marital status | ||||||||
| Married | 70.2 | 13.6 | 1 | – | ||||
| Single | 26.7 | 17.1 | 1.31 (1.0–1.7)b | – | ||||
| Divorced/widowed/separated | 3.2 | 11.4 | 0.82 (0.4–1.7) | – | ||||
| Language | ||||||||
| Konkani | 83.2 | 14.8 | 1 | – | ||||
| English | 13.7 | 12.5 | 0.83 (0.6–1.2) | – | ||||
| Other | 3.1 | 15.6 | 1.07 (0.6–2.0) | – | ||||
| Education (years) | ||||||||
| None | 9.7 | 10.4 | 1 | – | ||||
| 1–9 | 39.9 | 15.7 | 1.61 (1.0–2.5)b | – | ||||
| 10–14 | 39.7 | 14.8 | 1.49 (0.9–2.3) | – | ||||
| 15–23 | 10.8 | 12.6 | 1.25 (0.7–2.2) | – | ||||
| Literate | ||||||||
| Yes (read and write) | 85.7 | 15.2 | 1 | 1 | ||||
| No | 14.3 | 10.1 | 0.63 (0.4–0.9)b | 0.62 (0.4–0.9) | ||||
| Ethnicity | ||||||||
| Goan | 90.1 | 14.6 | 1 | – | ||||
| Other | 9.9 | 13.8 | 0.94 (0.6–1.4) | – | ||||
| Religion | ||||||||
| Hindu | 74.6 | 14.5 | 1 | – | ||||
| Christian | 22.2 | 13.9 | 0.95 (0.7–1.3) | – | ||||
| Muslim | 3.2 | 17.3 | 1.23 (0.7–2.2) | – | ||||
| Occupation | ||||||||
| Homemaker | 66.7 | 14.2 | 1 | – | ||||
| Employed | 20.9 | 15.2 | 1.08 (0.8–1.4) | – | ||||
| Other | 12.4 | 4.8 | 1.05 (0.7–1.5) | – | ||||
| Household composition and income | ||||||||
| Household size | ||||||||
| 1–3 | 16.7 | 11.8 | 1 | |||||
| 4–5 | 53.7 | 14.6 | 1.29 (0.9–1.8) | – | ||||
| 6–9 | 26.0 | 15.3 | 1.35 (0.9–2.0) | – | ||||
| 10–17 | 3.6 | 19.1 | 1.77 (1.0–3.3)b | – | ||||
| Number of children in household | ||||||||
| None | 26.4 | 13.4 | 1 | – | ||||
| 1 | 24.9 | 14.5 | 1.10 (0.8–1.5) | – | ||||
| 2 | 30.3 | 15.2 | 1.16 (0.9–1.6) | – | ||||
| >3 | 18.4 | 14.8 | 1.13 (0.8–1.6) | – | ||||
| Housing | ||||||||
| Own home | 89.1 | 14.2 | 1 | – | ||||
| Other | 10.9 | 16.4 | 1.18 (0.8–1.7) | – | ||||
| No. bedrooms | ||||||||
| 1 | 43.3 | 15.1 | 1 | – | ||||
| 2 | 38.9 | 13.7 | 0.89 (0.7–1.1) | – | ||||
| >3 | 17.7 | 14.7 | 0.97 (0.7–1.3) | – | ||||
| Toilet access | ||||||||
| In house | 40.6 | 12.4 | 1 | 1 | ||||
| Outside house | 59.4 | 15.8 | 1.32 (1.0–1.6)b | 1.29 (1.0–1.6) | ||||
| Tap water in house | ||||||||
| Yes | 44.4 | 14.7 | 1 | – | ||||
| No | 55.6 | 14.3 | 0.96 (0.8–1.2) | – | ||||
| Economic factors | ||||||||
| Per capita income (monthly) | ||||||||
| <2000 | 34.4 | 15.4 | 1 | – | ||||
| 2000–2999 | 17.4 | 13.0 | 0.82 (0.6–1.2) | – | ||||
| 3000–4999 | 24.6 | 14.7 | 0.94 (0.7–1.3) | – | ||||
| 5000–9999 | 16.0 | 15.1 | 0.98 (0.7–1.4) | – | ||||
| >10 000 | 7.6 | 12.8 | 0.80 (0.5–1.2) | – | ||||
| Family in debt | ||||||||
| Yes | 33.3 | 14.6 | 1 | – | ||||
| No | 64.7 | 14.5 | 0.99 (0.8–1.3) | – | ||||
| Don't know | 2.0 | 11.8 | 0.78 (0.3–1.9) | – | ||||
| Hunger in the past 3 months | ||||||||
| No | 94.8 | 14.1 | 1 | 1 | ||||
| Yes | 5.2 | 20.8 | 1.59 (1.0–2.5)b | 1.77 (1.1–2.8) | ||||
| Perception of making ends meet | ||||||||
| Yes | 68.1 | 14.0 | 1 | – | ||||
| No | 31.9 | 15.5 | 1.12 | (0.9–1.4) | ||||
| Factor . | Proportion in sample (%) . | Prevalence of vaginal discharge (%) . | Univariate OR (95% CI) . | Adjusted OR (95% CI)a . | ||||
|---|---|---|---|---|---|---|---|---|
| Sociodemographic factors | ||||||||
| Age | ||||||||
| 18–24 | 20.2 | 17.7 | 1 | 1 | ||||
| 25–29 | 18.4 | 18.9 | 1.09 (0.8–1.5) | 1.12 (0.8–1.6) | ||||
| 30–34 | 20.0 | 16.4 | 0.92 (0.7–1.3) | 0.96 (0.7–1.3) | ||||
| 35–39 | 18.9 | 11.6 | 0.61 (0.4–0.9) | 0.67(0.5–1.0) | ||||
| 40–50 | 22.4 | 8.6 | 0.44 (0.3–0.6)b | 0.52(0.4–0.8) | ||||
| Subject type | ||||||||
| Random | 61.6 | 12.4 | 1 | 1 | ||||
| Replacement | 38.4 | 17.8 | 1.53 (1.2–1.9)b | 1.40 (1.1–1.8) | ||||
| Marital status | ||||||||
| Married | 70.2 | 13.6 | 1 | – | ||||
| Single | 26.7 | 17.1 | 1.31 (1.0–1.7)b | – | ||||
| Divorced/widowed/separated | 3.2 | 11.4 | 0.82 (0.4–1.7) | – | ||||
| Language | ||||||||
| Konkani | 83.2 | 14.8 | 1 | – | ||||
| English | 13.7 | 12.5 | 0.83 (0.6–1.2) | – | ||||
| Other | 3.1 | 15.6 | 1.07 (0.6–2.0) | – | ||||
| Education (years) | ||||||||
| None | 9.7 | 10.4 | 1 | – | ||||
| 1–9 | 39.9 | 15.7 | 1.61 (1.0–2.5)b | – | ||||
| 10–14 | 39.7 | 14.8 | 1.49 (0.9–2.3) | – | ||||
| 15–23 | 10.8 | 12.6 | 1.25 (0.7–2.2) | – | ||||
| Literate | ||||||||
| Yes (read and write) | 85.7 | 15.2 | 1 | 1 | ||||
| No | 14.3 | 10.1 | 0.63 (0.4–0.9)b | 0.62 (0.4–0.9) | ||||
| Ethnicity | ||||||||
| Goan | 90.1 | 14.6 | 1 | – | ||||
| Other | 9.9 | 13.8 | 0.94 (0.6–1.4) | – | ||||
| Religion | ||||||||
| Hindu | 74.6 | 14.5 | 1 | – | ||||
| Christian | 22.2 | 13.9 | 0.95 (0.7–1.3) | – | ||||
| Muslim | 3.2 | 17.3 | 1.23 (0.7–2.2) | – | ||||
| Occupation | ||||||||
| Homemaker | 66.7 | 14.2 | 1 | – | ||||
| Employed | 20.9 | 15.2 | 1.08 (0.8–1.4) | – | ||||
| Other | 12.4 | 4.8 | 1.05 (0.7–1.5) | – | ||||
| Household composition and income | ||||||||
| Household size | ||||||||
| 1–3 | 16.7 | 11.8 | 1 | |||||
| 4–5 | 53.7 | 14.6 | 1.29 (0.9–1.8) | – | ||||
| 6–9 | 26.0 | 15.3 | 1.35 (0.9–2.0) | – | ||||
| 10–17 | 3.6 | 19.1 | 1.77 (1.0–3.3)b | – | ||||
| Number of children in household | ||||||||
| None | 26.4 | 13.4 | 1 | – | ||||
| 1 | 24.9 | 14.5 | 1.10 (0.8–1.5) | – | ||||
| 2 | 30.3 | 15.2 | 1.16 (0.9–1.6) | – | ||||
| >3 | 18.4 | 14.8 | 1.13 (0.8–1.6) | – | ||||
| Housing | ||||||||
| Own home | 89.1 | 14.2 | 1 | – | ||||
| Other | 10.9 | 16.4 | 1.18 (0.8–1.7) | – | ||||
| No. bedrooms | ||||||||
| 1 | 43.3 | 15.1 | 1 | – | ||||
| 2 | 38.9 | 13.7 | 0.89 (0.7–1.1) | – | ||||
| >3 | 17.7 | 14.7 | 0.97 (0.7–1.3) | – | ||||
| Toilet access | ||||||||
| In house | 40.6 | 12.4 | 1 | 1 | ||||
| Outside house | 59.4 | 15.8 | 1.32 (1.0–1.6)b | 1.29 (1.0–1.6) | ||||
| Tap water in house | ||||||||
| Yes | 44.4 | 14.7 | 1 | – | ||||
| No | 55.6 | 14.3 | 0.96 (0.8–1.2) | – | ||||
| Economic factors | ||||||||
| Per capita income (monthly) | ||||||||
| <2000 | 34.4 | 15.4 | 1 | – | ||||
| 2000–2999 | 17.4 | 13.0 | 0.82 (0.6–1.2) | – | ||||
| 3000–4999 | 24.6 | 14.7 | 0.94 (0.7–1.3) | – | ||||
| 5000–9999 | 16.0 | 15.1 | 0.98 (0.7–1.4) | – | ||||
| >10 000 | 7.6 | 12.8 | 0.80 (0.5–1.2) | – | ||||
| Family in debt | ||||||||
| Yes | 33.3 | 14.6 | 1 | – | ||||
| No | 64.7 | 14.5 | 0.99 (0.8–1.3) | – | ||||
| Don't know | 2.0 | 11.8 | 0.78 (0.3–1.9) | – | ||||
| Hunger in the past 3 months | ||||||||
| No | 94.8 | 14.1 | 1 | 1 | ||||
| Yes | 5.2 | 20.8 | 1.59 (1.0–2.5)b | 1.77 (1.1–2.8) | ||||
| Perception of making ends meet | ||||||||
| Yes | 68.1 | 14.0 | 1 | – | ||||
| No | 31.9 | 15.5 | 1.12 | (0.9–1.4) | ||||
Adjustments are for all variables whose association in the multivariate model was P < 0.1; estimates are presented only for variables whose adjusted estimates reached P < 0.1.
Variables whose univariate association reached P < 0.1.
Crude and adjusted estimates of the association of socioeconomic risk factors with the complaint of abnormal vaginal discharge (n = 2494)
| Factor . | Proportion in sample (%) . | Prevalence of vaginal discharge (%) . | Univariate OR (95% CI) . | Adjusted OR (95% CI)a . | ||||
|---|---|---|---|---|---|---|---|---|
| Sociodemographic factors | ||||||||
| Age | ||||||||
| 18–24 | 20.2 | 17.7 | 1 | 1 | ||||
| 25–29 | 18.4 | 18.9 | 1.09 (0.8–1.5) | 1.12 (0.8–1.6) | ||||
| 30–34 | 20.0 | 16.4 | 0.92 (0.7–1.3) | 0.96 (0.7–1.3) | ||||
| 35–39 | 18.9 | 11.6 | 0.61 (0.4–0.9) | 0.67(0.5–1.0) | ||||
| 40–50 | 22.4 | 8.6 | 0.44 (0.3–0.6)b | 0.52(0.4–0.8) | ||||
| Subject type | ||||||||
| Random | 61.6 | 12.4 | 1 | 1 | ||||
| Replacement | 38.4 | 17.8 | 1.53 (1.2–1.9)b | 1.40 (1.1–1.8) | ||||
| Marital status | ||||||||
| Married | 70.2 | 13.6 | 1 | – | ||||
| Single | 26.7 | 17.1 | 1.31 (1.0–1.7)b | – | ||||
| Divorced/widowed/separated | 3.2 | 11.4 | 0.82 (0.4–1.7) | – | ||||
| Language | ||||||||
| Konkani | 83.2 | 14.8 | 1 | – | ||||
| English | 13.7 | 12.5 | 0.83 (0.6–1.2) | – | ||||
| Other | 3.1 | 15.6 | 1.07 (0.6–2.0) | – | ||||
| Education (years) | ||||||||
| None | 9.7 | 10.4 | 1 | – | ||||
| 1–9 | 39.9 | 15.7 | 1.61 (1.0–2.5)b | – | ||||
| 10–14 | 39.7 | 14.8 | 1.49 (0.9–2.3) | – | ||||
| 15–23 | 10.8 | 12.6 | 1.25 (0.7–2.2) | – | ||||
| Literate | ||||||||
| Yes (read and write) | 85.7 | 15.2 | 1 | 1 | ||||
| No | 14.3 | 10.1 | 0.63 (0.4–0.9)b | 0.62 (0.4–0.9) | ||||
| Ethnicity | ||||||||
| Goan | 90.1 | 14.6 | 1 | – | ||||
| Other | 9.9 | 13.8 | 0.94 (0.6–1.4) | – | ||||
| Religion | ||||||||
| Hindu | 74.6 | 14.5 | 1 | – | ||||
| Christian | 22.2 | 13.9 | 0.95 (0.7–1.3) | – | ||||
| Muslim | 3.2 | 17.3 | 1.23 (0.7–2.2) | – | ||||
| Occupation | ||||||||
| Homemaker | 66.7 | 14.2 | 1 | – | ||||
| Employed | 20.9 | 15.2 | 1.08 (0.8–1.4) | – | ||||
| Other | 12.4 | 4.8 | 1.05 (0.7–1.5) | – | ||||
| Household composition and income | ||||||||
| Household size | ||||||||
| 1–3 | 16.7 | 11.8 | 1 | |||||
| 4–5 | 53.7 | 14.6 | 1.29 (0.9–1.8) | – | ||||
| 6–9 | 26.0 | 15.3 | 1.35 (0.9–2.0) | – | ||||
| 10–17 | 3.6 | 19.1 | 1.77 (1.0–3.3)b | – | ||||
| Number of children in household | ||||||||
| None | 26.4 | 13.4 | 1 | – | ||||
| 1 | 24.9 | 14.5 | 1.10 (0.8–1.5) | – | ||||
| 2 | 30.3 | 15.2 | 1.16 (0.9–1.6) | – | ||||
| >3 | 18.4 | 14.8 | 1.13 (0.8–1.6) | – | ||||
| Housing | ||||||||
| Own home | 89.1 | 14.2 | 1 | – | ||||
| Other | 10.9 | 16.4 | 1.18 (0.8–1.7) | – | ||||
| No. bedrooms | ||||||||
| 1 | 43.3 | 15.1 | 1 | – | ||||
| 2 | 38.9 | 13.7 | 0.89 (0.7–1.1) | – | ||||
| >3 | 17.7 | 14.7 | 0.97 (0.7–1.3) | – | ||||
| Toilet access | ||||||||
| In house | 40.6 | 12.4 | 1 | 1 | ||||
| Outside house | 59.4 | 15.8 | 1.32 (1.0–1.6)b | 1.29 (1.0–1.6) | ||||
| Tap water in house | ||||||||
| Yes | 44.4 | 14.7 | 1 | – | ||||
| No | 55.6 | 14.3 | 0.96 (0.8–1.2) | – | ||||
| Economic factors | ||||||||
| Per capita income (monthly) | ||||||||
| <2000 | 34.4 | 15.4 | 1 | – | ||||
| 2000–2999 | 17.4 | 13.0 | 0.82 (0.6–1.2) | – | ||||
| 3000–4999 | 24.6 | 14.7 | 0.94 (0.7–1.3) | – | ||||
| 5000–9999 | 16.0 | 15.1 | 0.98 (0.7–1.4) | – | ||||
| >10 000 | 7.6 | 12.8 | 0.80 (0.5–1.2) | – | ||||
| Family in debt | ||||||||
| Yes | 33.3 | 14.6 | 1 | – | ||||
| No | 64.7 | 14.5 | 0.99 (0.8–1.3) | – | ||||
| Don't know | 2.0 | 11.8 | 0.78 (0.3–1.9) | – | ||||
| Hunger in the past 3 months | ||||||||
| No | 94.8 | 14.1 | 1 | 1 | ||||
| Yes | 5.2 | 20.8 | 1.59 (1.0–2.5)b | 1.77 (1.1–2.8) | ||||
| Perception of making ends meet | ||||||||
| Yes | 68.1 | 14.0 | 1 | – | ||||
| No | 31.9 | 15.5 | 1.12 | (0.9–1.4) | ||||
| Factor . | Proportion in sample (%) . | Prevalence of vaginal discharge (%) . | Univariate OR (95% CI) . | Adjusted OR (95% CI)a . | ||||
|---|---|---|---|---|---|---|---|---|
| Sociodemographic factors | ||||||||
| Age | ||||||||
| 18–24 | 20.2 | 17.7 | 1 | 1 | ||||
| 25–29 | 18.4 | 18.9 | 1.09 (0.8–1.5) | 1.12 (0.8–1.6) | ||||
| 30–34 | 20.0 | 16.4 | 0.92 (0.7–1.3) | 0.96 (0.7–1.3) | ||||
| 35–39 | 18.9 | 11.6 | 0.61 (0.4–0.9) | 0.67(0.5–1.0) | ||||
| 40–50 | 22.4 | 8.6 | 0.44 (0.3–0.6)b | 0.52(0.4–0.8) | ||||
| Subject type | ||||||||
| Random | 61.6 | 12.4 | 1 | 1 | ||||
| Replacement | 38.4 | 17.8 | 1.53 (1.2–1.9)b | 1.40 (1.1–1.8) | ||||
| Marital status | ||||||||
| Married | 70.2 | 13.6 | 1 | – | ||||
| Single | 26.7 | 17.1 | 1.31 (1.0–1.7)b | – | ||||
| Divorced/widowed/separated | 3.2 | 11.4 | 0.82 (0.4–1.7) | – | ||||
| Language | ||||||||
| Konkani | 83.2 | 14.8 | 1 | – | ||||
| English | 13.7 | 12.5 | 0.83 (0.6–1.2) | – | ||||
| Other | 3.1 | 15.6 | 1.07 (0.6–2.0) | – | ||||
| Education (years) | ||||||||
| None | 9.7 | 10.4 | 1 | – | ||||
| 1–9 | 39.9 | 15.7 | 1.61 (1.0–2.5)b | – | ||||
| 10–14 | 39.7 | 14.8 | 1.49 (0.9–2.3) | – | ||||
| 15–23 | 10.8 | 12.6 | 1.25 (0.7–2.2) | – | ||||
| Literate | ||||||||
| Yes (read and write) | 85.7 | 15.2 | 1 | 1 | ||||
| No | 14.3 | 10.1 | 0.63 (0.4–0.9)b | 0.62 (0.4–0.9) | ||||
| Ethnicity | ||||||||
| Goan | 90.1 | 14.6 | 1 | – | ||||
| Other | 9.9 | 13.8 | 0.94 (0.6–1.4) | – | ||||
| Religion | ||||||||
| Hindu | 74.6 | 14.5 | 1 | – | ||||
| Christian | 22.2 | 13.9 | 0.95 (0.7–1.3) | – | ||||
| Muslim | 3.2 | 17.3 | 1.23 (0.7–2.2) | – | ||||
| Occupation | ||||||||
| Homemaker | 66.7 | 14.2 | 1 | – | ||||
| Employed | 20.9 | 15.2 | 1.08 (0.8–1.4) | – | ||||
| Other | 12.4 | 4.8 | 1.05 (0.7–1.5) | – | ||||
| Household composition and income | ||||||||
| Household size | ||||||||
| 1–3 | 16.7 | 11.8 | 1 | |||||
| 4–5 | 53.7 | 14.6 | 1.29 (0.9–1.8) | – | ||||
| 6–9 | 26.0 | 15.3 | 1.35 (0.9–2.0) | – | ||||
| 10–17 | 3.6 | 19.1 | 1.77 (1.0–3.3)b | – | ||||
| Number of children in household | ||||||||
| None | 26.4 | 13.4 | 1 | – | ||||
| 1 | 24.9 | 14.5 | 1.10 (0.8–1.5) | – | ||||
| 2 | 30.3 | 15.2 | 1.16 (0.9–1.6) | – | ||||
| >3 | 18.4 | 14.8 | 1.13 (0.8–1.6) | – | ||||
| Housing | ||||||||
| Own home | 89.1 | 14.2 | 1 | – | ||||
| Other | 10.9 | 16.4 | 1.18 (0.8–1.7) | – | ||||
| No. bedrooms | ||||||||
| 1 | 43.3 | 15.1 | 1 | – | ||||
| 2 | 38.9 | 13.7 | 0.89 (0.7–1.1) | – | ||||
| >3 | 17.7 | 14.7 | 0.97 (0.7–1.3) | – | ||||
| Toilet access | ||||||||
| In house | 40.6 | 12.4 | 1 | 1 | ||||
| Outside house | 59.4 | 15.8 | 1.32 (1.0–1.6)b | 1.29 (1.0–1.6) | ||||
| Tap water in house | ||||||||
| Yes | 44.4 | 14.7 | 1 | – | ||||
| No | 55.6 | 14.3 | 0.96 (0.8–1.2) | – | ||||
| Economic factors | ||||||||
| Per capita income (monthly) | ||||||||
| <2000 | 34.4 | 15.4 | 1 | – | ||||
| 2000–2999 | 17.4 | 13.0 | 0.82 (0.6–1.2) | – | ||||
| 3000–4999 | 24.6 | 14.7 | 0.94 (0.7–1.3) | – | ||||
| 5000–9999 | 16.0 | 15.1 | 0.98 (0.7–1.4) | – | ||||
| >10 000 | 7.6 | 12.8 | 0.80 (0.5–1.2) | – | ||||
| Family in debt | ||||||||
| Yes | 33.3 | 14.6 | 1 | – | ||||
| No | 64.7 | 14.5 | 0.99 (0.8–1.3) | – | ||||
| Don't know | 2.0 | 11.8 | 0.78 (0.3–1.9) | – | ||||
| Hunger in the past 3 months | ||||||||
| No | 94.8 | 14.1 | 1 | 1 | ||||
| Yes | 5.2 | 20.8 | 1.59 (1.0–2.5)b | 1.77 (1.1–2.8) | ||||
| Perception of making ends meet | ||||||||
| Yes | 68.1 | 14.0 | 1 | – | ||||
| No | 31.9 | 15.5 | 1.12 | (0.9–1.4) | ||||
Adjustments are for all variables whose association in the multivariate model was P < 0.1; estimates are presented only for variables whose adjusted estimates reached P < 0.1.
Variables whose univariate association reached P < 0.1.
Univariate analyses identified that a number of socioeconomic variables were associated with the complaint of vaginal discharge. A multivariate logistic regression model identified a subset of key factors, which remained significantly associated with vaginal discharge after adjustment for the other factors. These were: younger age, being a replacement subject, being literate, not having a toilet in the house, and having experienced hunger in the previous 3 months. An analysis of the association of risk factors associated with marital history was carried out for married women. The risk factors were: age of marriage; duration of marriage; husband's literacy, education and employment; period of time the husband had been away from the marital home in the previous 90 days; and any extramarital relationship of the respondent in the previous 12 months. None of these risk factors were associated with the complaint after adjustment for socioeconomic risk factors.
Associations of the complaint of vaginal discharge with other factors
Logistic regression analyses identified a number of psychosocial, obstetric, infectious, and contraception related variables individually associated (P < 0.1) with vaginal discharge, after adjusting for socioeconomic factors (Table 3). These were verbal abuse, sexual violence, and concerns regarding the husband's extramarital relationships (among married women); low social integration and autonomy scores; high CMD and somatoform disorder symptom scores; ever having had a pregnancy; the use of an IUCD; any RTI and the number of RTIs. The presence of an RTI was not associated with whether the woman had sought medical care for her complaint or had reported frequent change of underwear as a consequence of the discharge. We did not find a significant association between the complaint of vaginal discharge and RTI/STI in the subgroup of women who were married.
Adjusted estimates of association of psychosocial, reproductive, and infectious risk factors with the complaint of abnormal vaginal discharge (n = 2494 unless otherwise stated)
| Variable . | Proportion in in sample (%) . | Prevalence of complaint of vaginal discharge (%) . | Adjusted OR (95% CI)a . | |||
|---|---|---|---|---|---|---|
| Gender disadvantage and social support | ||||||
| Husband verbal abuse (n = 1750)b | ||||||
| No | 85.2 | 12.9 | 1 | |||
| Yes | 14.8 | 17.0 | 1.38 (1.0–2.0) | |||
| Husband sexual abuse (n = 1750)b | ||||||
| No | 96.3 | 13.2 | 1 | |||
| Yes | 3.7 | 21.9 | 1.86 (1.0–3.5) | |||
| Concerns about husband's extramarital affair (n = 1750)b | ||||||
| No | 98.6 | 13.2 | 1 | |||
| Yes | 1.4 | 33.3 | 3.45 (1.4–8.4) | |||
| Social integration score | ||||||
| High | 31.5 | 12.6 | 1 | |||
| Medium | 32.8 | 15.3 | 1.23 (0.9–1.6) | |||
| Low | 35.7 | 15.4 | 1.21 (0.9–1.6) | |||
| Autonomy Score | ||||||
| High | 33.4 | 13.8 | 1 | |||
| Medium | 42.6 | 12.8 | 0.84 (0.6–1.1) | |||
| Low | 24.1 | 18.3 | 1.23 (0.9–1.7) | |||
| Mental health | ||||||
| CIS-R score | ||||||
| 0 | 59.8 | 9.2 | 1 | |||
| 1–2 | 11.5 | 13.3 | 1.58 (1.1–2.3) | |||
| 3–4 | 9.5 | 19.4 | 2.64 (1.8–3.8) | |||
| 5–8 | 9.3 | 23.8 | 3.46 (2.4–5.0) | |||
| >8 | 9.9 | 33.3 | 5.61 (4.0–7.8) | |||
| Somatoform symptom score | ||||||
| 0–1 | 30.7 | 5.9 | 1 | |||
| 2–3 | 19.8 | 8.9 | 1.67 (1.1–2.6) | |||
| 4–7 | 29.3 | 16.3 | 3.53 (2.4–5.1) | |||
| 8+ | 20.2 | 30.4 | 9.02 (6.2–13.1) | |||
| Obstetric and infectious factors | ||||||
| Ever Pregnant | ||||||
| No | 30.7 | 16.3 | 1 | |||
| Yes | 69.3 | 13.7 | 1.26 (0.9–1.8) | |||
| Use of IUCD (n = 1750) | ||||||
| No | 95.8 | 13.1 | 1 | |||
| Yes | 4.2 | 24.7 | 1.88 (1.1–3.3) | |||
| Chlamydiac (n = 2423) | ||||||
| Absent | 98.7 | 14.5 | 1 | |||
| Present | 1.3 | 12.5 | 1.03 (0.3–2.9) | |||
| Gonorrhoeac (n = 2425) | ||||||
| Absent | 98.1 | 14.4 | 1 | |||
| Present | 1.9 | 17.0 | 1.26 (0.6–2.8) | |||
| BVc (n = 2432) | ||||||
| Absent | 82.2 | 14.2 | 1 | |||
| Present | 17.8 | 16.4 | 1.24 (0.9–1.6) | |||
| Trichomoniasisc (n = 2488) | ||||||
| Absent | 98.8 | 14.6 | 1 | |||
| Present | 1.2 | 10.3 | 0.69 (0.2–2.3) | |||
| Candidiasisc (n = 2488) | ||||||
| Absent | 91.5 | 14.4 | 1 | |||
| Present | 8.5 | 16.9 | 1.24 (0.8–1.8) | |||
| Any RTI | ||||||
| No | 73.1 | 13.8 | 1 | |||
| Yes | 26.9 | 16.2 | 1.25 (1.0–1.6) | |||
| Any STIc | ||||||
| No | 95.9 | 14.6 | 1 | |||
| Yes | 4.1 | 11.9 | 0.83 (0.4–1.6) | |||
| Number of RTI | ||||||
| 0 | 73.1 | 13.8 | 1 | |||
| 1 | 24.2 | 16.4 | 1.25 (1.0–1.6) | |||
| ≥2 | 2.7 | 14.7 | 1.21 (0.6–2.4) | |||
| Variable . | Proportion in in sample (%) . | Prevalence of complaint of vaginal discharge (%) . | Adjusted OR (95% CI)a . | |||
|---|---|---|---|---|---|---|
| Gender disadvantage and social support | ||||||
| Husband verbal abuse (n = 1750)b | ||||||
| No | 85.2 | 12.9 | 1 | |||
| Yes | 14.8 | 17.0 | 1.38 (1.0–2.0) | |||
| Husband sexual abuse (n = 1750)b | ||||||
| No | 96.3 | 13.2 | 1 | |||
| Yes | 3.7 | 21.9 | 1.86 (1.0–3.5) | |||
| Concerns about husband's extramarital affair (n = 1750)b | ||||||
| No | 98.6 | 13.2 | 1 | |||
| Yes | 1.4 | 33.3 | 3.45 (1.4–8.4) | |||
| Social integration score | ||||||
| High | 31.5 | 12.6 | 1 | |||
| Medium | 32.8 | 15.3 | 1.23 (0.9–1.6) | |||
| Low | 35.7 | 15.4 | 1.21 (0.9–1.6) | |||
| Autonomy Score | ||||||
| High | 33.4 | 13.8 | 1 | |||
| Medium | 42.6 | 12.8 | 0.84 (0.6–1.1) | |||
| Low | 24.1 | 18.3 | 1.23 (0.9–1.7) | |||
| Mental health | ||||||
| CIS-R score | ||||||
| 0 | 59.8 | 9.2 | 1 | |||
| 1–2 | 11.5 | 13.3 | 1.58 (1.1–2.3) | |||
| 3–4 | 9.5 | 19.4 | 2.64 (1.8–3.8) | |||
| 5–8 | 9.3 | 23.8 | 3.46 (2.4–5.0) | |||
| >8 | 9.9 | 33.3 | 5.61 (4.0–7.8) | |||
| Somatoform symptom score | ||||||
| 0–1 | 30.7 | 5.9 | 1 | |||
| 2–3 | 19.8 | 8.9 | 1.67 (1.1–2.6) | |||
| 4–7 | 29.3 | 16.3 | 3.53 (2.4–5.1) | |||
| 8+ | 20.2 | 30.4 | 9.02 (6.2–13.1) | |||
| Obstetric and infectious factors | ||||||
| Ever Pregnant | ||||||
| No | 30.7 | 16.3 | 1 | |||
| Yes | 69.3 | 13.7 | 1.26 (0.9–1.8) | |||
| Use of IUCD (n = 1750) | ||||||
| No | 95.8 | 13.1 | 1 | |||
| Yes | 4.2 | 24.7 | 1.88 (1.1–3.3) | |||
| Chlamydiac (n = 2423) | ||||||
| Absent | 98.7 | 14.5 | 1 | |||
| Present | 1.3 | 12.5 | 1.03 (0.3–2.9) | |||
| Gonorrhoeac (n = 2425) | ||||||
| Absent | 98.1 | 14.4 | 1 | |||
| Present | 1.9 | 17.0 | 1.26 (0.6–2.8) | |||
| BVc (n = 2432) | ||||||
| Absent | 82.2 | 14.2 | 1 | |||
| Present | 17.8 | 16.4 | 1.24 (0.9–1.6) | |||
| Trichomoniasisc (n = 2488) | ||||||
| Absent | 98.8 | 14.6 | 1 | |||
| Present | 1.2 | 10.3 | 0.69 (0.2–2.3) | |||
| Candidiasisc (n = 2488) | ||||||
| Absent | 91.5 | 14.4 | 1 | |||
| Present | 8.5 | 16.9 | 1.24 (0.8–1.8) | |||
| Any RTI | ||||||
| No | 73.1 | 13.8 | 1 | |||
| Yes | 26.9 | 16.2 | 1.25 (1.0–1.6) | |||
| Any STIc | ||||||
| No | 95.9 | 14.6 | 1 | |||
| Yes | 4.1 | 11.9 | 0.83 (0.4–1.6) | |||
| Number of RTI | ||||||
| 0 | 73.1 | 13.8 | 1 | |||
| 1 | 24.2 | 16.4 | 1.25 (1.0–1.6) | |||
| ≥2 | 2.7 | 14.7 | 1.21 (0.6–2.4) | |||
Denominator is 2494 unless otherwise stated. Adjusted for age, literacy, hunger, toilet facilities in the home, and subject type.
Married women only.
Variables for which P > 0.1.
Adjusted estimates of association of psychosocial, reproductive, and infectious risk factors with the complaint of abnormal vaginal discharge (n = 2494 unless otherwise stated)
| Variable . | Proportion in in sample (%) . | Prevalence of complaint of vaginal discharge (%) . | Adjusted OR (95% CI)a . | |||
|---|---|---|---|---|---|---|
| Gender disadvantage and social support | ||||||
| Husband verbal abuse (n = 1750)b | ||||||
| No | 85.2 | 12.9 | 1 | |||
| Yes | 14.8 | 17.0 | 1.38 (1.0–2.0) | |||
| Husband sexual abuse (n = 1750)b | ||||||
| No | 96.3 | 13.2 | 1 | |||
| Yes | 3.7 | 21.9 | 1.86 (1.0–3.5) | |||
| Concerns about husband's extramarital affair (n = 1750)b | ||||||
| No | 98.6 | 13.2 | 1 | |||
| Yes | 1.4 | 33.3 | 3.45 (1.4–8.4) | |||
| Social integration score | ||||||
| High | 31.5 | 12.6 | 1 | |||
| Medium | 32.8 | 15.3 | 1.23 (0.9–1.6) | |||
| Low | 35.7 | 15.4 | 1.21 (0.9–1.6) | |||
| Autonomy Score | ||||||
| High | 33.4 | 13.8 | 1 | |||
| Medium | 42.6 | 12.8 | 0.84 (0.6–1.1) | |||
| Low | 24.1 | 18.3 | 1.23 (0.9–1.7) | |||
| Mental health | ||||||
| CIS-R score | ||||||
| 0 | 59.8 | 9.2 | 1 | |||
| 1–2 | 11.5 | 13.3 | 1.58 (1.1–2.3) | |||
| 3–4 | 9.5 | 19.4 | 2.64 (1.8–3.8) | |||
| 5–8 | 9.3 | 23.8 | 3.46 (2.4–5.0) | |||
| >8 | 9.9 | 33.3 | 5.61 (4.0–7.8) | |||
| Somatoform symptom score | ||||||
| 0–1 | 30.7 | 5.9 | 1 | |||
| 2–3 | 19.8 | 8.9 | 1.67 (1.1–2.6) | |||
| 4–7 | 29.3 | 16.3 | 3.53 (2.4–5.1) | |||
| 8+ | 20.2 | 30.4 | 9.02 (6.2–13.1) | |||
| Obstetric and infectious factors | ||||||
| Ever Pregnant | ||||||
| No | 30.7 | 16.3 | 1 | |||
| Yes | 69.3 | 13.7 | 1.26 (0.9–1.8) | |||
| Use of IUCD (n = 1750) | ||||||
| No | 95.8 | 13.1 | 1 | |||
| Yes | 4.2 | 24.7 | 1.88 (1.1–3.3) | |||
| Chlamydiac (n = 2423) | ||||||
| Absent | 98.7 | 14.5 | 1 | |||
| Present | 1.3 | 12.5 | 1.03 (0.3–2.9) | |||
| Gonorrhoeac (n = 2425) | ||||||
| Absent | 98.1 | 14.4 | 1 | |||
| Present | 1.9 | 17.0 | 1.26 (0.6–2.8) | |||
| BVc (n = 2432) | ||||||
| Absent | 82.2 | 14.2 | 1 | |||
| Present | 17.8 | 16.4 | 1.24 (0.9–1.6) | |||
| Trichomoniasisc (n = 2488) | ||||||
| Absent | 98.8 | 14.6 | 1 | |||
| Present | 1.2 | 10.3 | 0.69 (0.2–2.3) | |||
| Candidiasisc (n = 2488) | ||||||
| Absent | 91.5 | 14.4 | 1 | |||
| Present | 8.5 | 16.9 | 1.24 (0.8–1.8) | |||
| Any RTI | ||||||
| No | 73.1 | 13.8 | 1 | |||
| Yes | 26.9 | 16.2 | 1.25 (1.0–1.6) | |||
| Any STIc | ||||||
| No | 95.9 | 14.6 | 1 | |||
| Yes | 4.1 | 11.9 | 0.83 (0.4–1.6) | |||
| Number of RTI | ||||||
| 0 | 73.1 | 13.8 | 1 | |||
| 1 | 24.2 | 16.4 | 1.25 (1.0–1.6) | |||
| ≥2 | 2.7 | 14.7 | 1.21 (0.6–2.4) | |||
| Variable . | Proportion in in sample (%) . | Prevalence of complaint of vaginal discharge (%) . | Adjusted OR (95% CI)a . | |||
|---|---|---|---|---|---|---|
| Gender disadvantage and social support | ||||||
| Husband verbal abuse (n = 1750)b | ||||||
| No | 85.2 | 12.9 | 1 | |||
| Yes | 14.8 | 17.0 | 1.38 (1.0–2.0) | |||
| Husband sexual abuse (n = 1750)b | ||||||
| No | 96.3 | 13.2 | 1 | |||
| Yes | 3.7 | 21.9 | 1.86 (1.0–3.5) | |||
| Concerns about husband's extramarital affair (n = 1750)b | ||||||
| No | 98.6 | 13.2 | 1 | |||
| Yes | 1.4 | 33.3 | 3.45 (1.4–8.4) | |||
| Social integration score | ||||||
| High | 31.5 | 12.6 | 1 | |||
| Medium | 32.8 | 15.3 | 1.23 (0.9–1.6) | |||
| Low | 35.7 | 15.4 | 1.21 (0.9–1.6) | |||
| Autonomy Score | ||||||
| High | 33.4 | 13.8 | 1 | |||
| Medium | 42.6 | 12.8 | 0.84 (0.6–1.1) | |||
| Low | 24.1 | 18.3 | 1.23 (0.9–1.7) | |||
| Mental health | ||||||
| CIS-R score | ||||||
| 0 | 59.8 | 9.2 | 1 | |||
| 1–2 | 11.5 | 13.3 | 1.58 (1.1–2.3) | |||
| 3–4 | 9.5 | 19.4 | 2.64 (1.8–3.8) | |||
| 5–8 | 9.3 | 23.8 | 3.46 (2.4–5.0) | |||
| >8 | 9.9 | 33.3 | 5.61 (4.0–7.8) | |||
| Somatoform symptom score | ||||||
| 0–1 | 30.7 | 5.9 | 1 | |||
| 2–3 | 19.8 | 8.9 | 1.67 (1.1–2.6) | |||
| 4–7 | 29.3 | 16.3 | 3.53 (2.4–5.1) | |||
| 8+ | 20.2 | 30.4 | 9.02 (6.2–13.1) | |||
| Obstetric and infectious factors | ||||||
| Ever Pregnant | ||||||
| No | 30.7 | 16.3 | 1 | |||
| Yes | 69.3 | 13.7 | 1.26 (0.9–1.8) | |||
| Use of IUCD (n = 1750) | ||||||
| No | 95.8 | 13.1 | 1 | |||
| Yes | 4.2 | 24.7 | 1.88 (1.1–3.3) | |||
| Chlamydiac (n = 2423) | ||||||
| Absent | 98.7 | 14.5 | 1 | |||
| Present | 1.3 | 12.5 | 1.03 (0.3–2.9) | |||
| Gonorrhoeac (n = 2425) | ||||||
| Absent | 98.1 | 14.4 | 1 | |||
| Present | 1.9 | 17.0 | 1.26 (0.6–2.8) | |||
| BVc (n = 2432) | ||||||
| Absent | 82.2 | 14.2 | 1 | |||
| Present | 17.8 | 16.4 | 1.24 (0.9–1.6) | |||
| Trichomoniasisc (n = 2488) | ||||||
| Absent | 98.8 | 14.6 | 1 | |||
| Present | 1.2 | 10.3 | 0.69 (0.2–2.3) | |||
| Candidiasisc (n = 2488) | ||||||
| Absent | 91.5 | 14.4 | 1 | |||
| Present | 8.5 | 16.9 | 1.24 (0.8–1.8) | |||
| Any RTI | ||||||
| No | 73.1 | 13.8 | 1 | |||
| Yes | 26.9 | 16.2 | 1.25 (1.0–1.6) | |||
| Any STIc | ||||||
| No | 95.9 | 14.6 | 1 | |||
| Yes | 4.1 | 11.9 | 0.83 (0.4–1.6) | |||
| Number of RTI | ||||||
| 0 | 73.1 | 13.8 | 1 | |||
| 1 | 24.2 | 16.4 | 1.25 (1.0–1.6) | |||
| ≥2 | 2.7 | 14.7 | 1.21 (0.6–2.4) | |||
Denominator is 2494 unless otherwise stated. Adjusted for age, literacy, hunger, toilet facilities in the home, and subject type.
Married women only.
Variables for which P > 0.1.
All variables significantly associated with vaginal discharge in Table 3 were considered for inclusion into the final model, presented in Table 4. Poor mental health, as indicated by higher CISR scores and somatoform disorder symptom scores, younger age, higher literacy, and the use of an IUCD were independently associated with the complaint.
Final multivariate logistic regression model of risk factors for the complaint of abnormal vaginal discharge (n = 2494)
| Risk factor . | Odds ratio . | 95% Confidence interval . | Two-tailed P . | |||
|---|---|---|---|---|---|---|
| CIS-R score (reference score 0) | ||||||
| 1–2 | 0.97 | 0.6–1.5 | ||||
| 3–4 | 1.40 | 0.9–2.1 | ||||
| 5–8 | 1.62 | 1.1–2.4 | ||||
| >8 | 2.16 | 1.4–3.2 | <0.001 | |||
| Somatoform symptom score (reference score <2) | ||||||
| 2–3 | 1.59 | 1.0–2.5 | ||||
| 4–7 | 3.03 | 2.0–4.5 | ||||
| 8+ | 6.23 | 4.0–9.7 | <0.001 | |||
| Not literate | 0.54 | 0.4–0.8 | 0.002 | |||
| Any RTI | 1.24 | 0.9–1.6 | 0.1 | |||
| Use of IUCD | 1.86 | 1.0–3.4 | 0.04 | |||
| Age (ref group age 18–24) | ||||||
| 25–29 | 0.84 | 0.6–1.2 | ||||
| 30–34 | 0.63 | 0.4–0.9 | ||||
| 35–39 | 0.43 | 0.3–0.6 | ||||
| 40–50 | 0.29 | 0.2–0.4 | <0.001 | |||
| Replacement subject | 1.30 | 1.0–1.6 | 0.035 | |||
| Risk factor . | Odds ratio . | 95% Confidence interval . | Two-tailed P . | |||
|---|---|---|---|---|---|---|
| CIS-R score (reference score 0) | ||||||
| 1–2 | 0.97 | 0.6–1.5 | ||||
| 3–4 | 1.40 | 0.9–2.1 | ||||
| 5–8 | 1.62 | 1.1–2.4 | ||||
| >8 | 2.16 | 1.4–3.2 | <0.001 | |||
| Somatoform symptom score (reference score <2) | ||||||
| 2–3 | 1.59 | 1.0–2.5 | ||||
| 4–7 | 3.03 | 2.0–4.5 | ||||
| 8+ | 6.23 | 4.0–9.7 | <0.001 | |||
| Not literate | 0.54 | 0.4–0.8 | 0.002 | |||
| Any RTI | 1.24 | 0.9–1.6 | 0.1 | |||
| Use of IUCD | 1.86 | 1.0–3.4 | 0.04 | |||
| Age (ref group age 18–24) | ||||||
| 25–29 | 0.84 | 0.6–1.2 | ||||
| 30–34 | 0.63 | 0.4–0.9 | ||||
| 35–39 | 0.43 | 0.3–0.6 | ||||
| 40–50 | 0.29 | 0.2–0.4 | <0.001 | |||
| Replacement subject | 1.30 | 1.0–1.6 | 0.035 | |||
Final multivariate logistic regression model of risk factors for the complaint of abnormal vaginal discharge (n = 2494)
| Risk factor . | Odds ratio . | 95% Confidence interval . | Two-tailed P . | |||
|---|---|---|---|---|---|---|
| CIS-R score (reference score 0) | ||||||
| 1–2 | 0.97 | 0.6–1.5 | ||||
| 3–4 | 1.40 | 0.9–2.1 | ||||
| 5–8 | 1.62 | 1.1–2.4 | ||||
| >8 | 2.16 | 1.4–3.2 | <0.001 | |||
| Somatoform symptom score (reference score <2) | ||||||
| 2–3 | 1.59 | 1.0–2.5 | ||||
| 4–7 | 3.03 | 2.0–4.5 | ||||
| 8+ | 6.23 | 4.0–9.7 | <0.001 | |||
| Not literate | 0.54 | 0.4–0.8 | 0.002 | |||
| Any RTI | 1.24 | 0.9–1.6 | 0.1 | |||
| Use of IUCD | 1.86 | 1.0–3.4 | 0.04 | |||
| Age (ref group age 18–24) | ||||||
| 25–29 | 0.84 | 0.6–1.2 | ||||
| 30–34 | 0.63 | 0.4–0.9 | ||||
| 35–39 | 0.43 | 0.3–0.6 | ||||
| 40–50 | 0.29 | 0.2–0.4 | <0.001 | |||
| Replacement subject | 1.30 | 1.0–1.6 | 0.035 | |||
| Risk factor . | Odds ratio . | 95% Confidence interval . | Two-tailed P . | |||
|---|---|---|---|---|---|---|
| CIS-R score (reference score 0) | ||||||
| 1–2 | 0.97 | 0.6–1.5 | ||||
| 3–4 | 1.40 | 0.9–2.1 | ||||
| 5–8 | 1.62 | 1.1–2.4 | ||||
| >8 | 2.16 | 1.4–3.2 | <0.001 | |||
| Somatoform symptom score (reference score <2) | ||||||
| 2–3 | 1.59 | 1.0–2.5 | ||||
| 4–7 | 3.03 | 2.0–4.5 | ||||
| 8+ | 6.23 | 4.0–9.7 | <0.001 | |||
| Not literate | 0.54 | 0.4–0.8 | 0.002 | |||
| Any RTI | 1.24 | 0.9–1.6 | 0.1 | |||
| Use of IUCD | 1.86 | 1.0–3.4 | 0.04 | |||
| Age (ref group age 18–24) | ||||||
| 25–29 | 0.84 | 0.6–1.2 | ||||
| 30–34 | 0.63 | 0.4–0.9 | ||||
| 35–39 | 0.43 | 0.3–0.6 | ||||
| 40–50 | 0.29 | 0.2–0.4 | <0.001 | |||
| Replacement subject | 1.30 | 1.0–1.6 | 0.035 | |||
Discussion
This study aimed to describe the infectious and psychosocial risk factors for the complaint of abnormal vaginal discharge in a community sample in South Asia. The major finding of our study is that psychosocial factors, notably the presence of a CMD or symptoms of somatoform disorders, are the factors most strongly associated with the complaint of vaginal discharge; there was little association of the complaint with RTI.
We found that, as others have reported,1,22 the complaint of abnormal vaginal discharge was commonly reported. The rate was lower than in some previous studies,22 perhaps because of the more specific question used, which qualified the objective characteristic of an abnormal discharge on the basis of three criteria (quantity, odour, and colour). Most women with the complaint also had another concurrent gynaecological symptom suggesting that the complaint is likely to be one of a cluster of symptoms as part of a gynaecological syndrome. The complaint was associated with a high degree of help seeking; half the women had consulted a medical practitioner and more than one-third had taken a biomedical medication. Younger women, who were literate with no access to a toilet in the home and women who were experiencing severe economic difficulties, were more likely to report the complaint. A number of psychosocial variables were associated with the complaint, notably experiences of gender disadvantage, CMD and the experience of symptoms of somatoform disorders. RTIs as a composite exposure were weakly associated with the complaint, but STIs, number of infections, and specific RTIs were not. The final multivariate model found that CMD; somatoform disorder symptoms; younger age; the use of an intrauterine contraceptive device, and literacy were independently associated with the complaint. RTIs were not associated with the complaint, nor were they associated with the complaint in the subgroup of women who had not taken medical treatment.
The principal limitation of this study is related to a selection bias of the study population, as those who refused were significantly different from those who participated; one of the consistent independent risk factors for vaginal discharge was younger age and younger women were more likely not to participate. This selection bias may have underestimated the prevalence of the complaint reported in our study and may affect the generalizability of our findings. Bias during sampling was also reflected in the finding that being a replacement subject was an independent risk factor for the complaint of abnormal vaginal discharge. However, being a community-based study, our findings are more likely to be generalizable than treatment setting studies where barriers to care may impose unknown selection biases. We were able to achieve high participation rates and ensure a high standard of quality control of gold-standard laboratory and epidemiological measures. We did not study the role of other potential causes of vaginal discharge such as the use of tampons or the use of douching because they were very uncommon in the local community. Viral STIs such as herpes simplex, and other bacterial STIs such as syphilis, were not measured because they are not considered causes of vaginal discharge as defined in the syndromic approach to the management of STIs.4
We conclude that the complaint of vaginal discharge is associated with multiple risk factors of which psychosocial factors are the strongest. Since our study is a cross-sectional survey, we cannot make definitive interpretations about the direction of causation. However, the fact that we found a dose–response relationship between mental health risk factors and the complaint, and that women themselves attributed their complaint to stress suggested the possibility of a causal role for psychosocial factors. Vaginal discharge may be interpreted as a somatic equivalent, at least in South Asian women, of the myriad range of medically unexplained symptoms, such as chronic pelvic pain, chronic backache, and irritable bowel syndrome.9,31 Typically, these symptoms are strongly associated with each other and with CMD.32 Both these characteristics were also found to be applicable to the complaint of vaginal discharge.
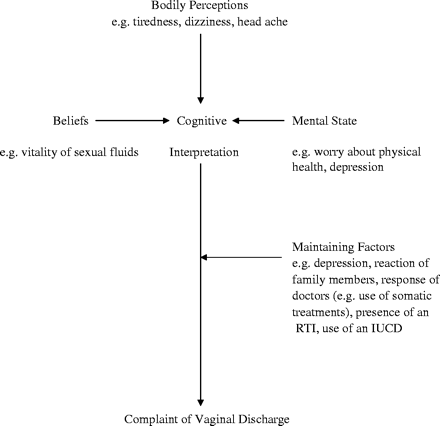
The aetiological model proposed for medically unexplained symptoms9 has been adapted to interpret the findings of this study (Figure 1 here). Medically unexplained symptoms may be explained in terms of the way bodily perceptions are processed; symptom perception is in part determined by environmental, emotional, and cognitive characteristics, such as specific cognitive illness schemes.33 Thus, the choice of symptoms may be influenced by a variety of factors; in the case of the complaint of vaginal discharge, cultural factors are likely to be important as a determinant of a cognitive illness scheme. In Asian men, semen is imbued with significance as sources of vitality and strength. The loss of semen through means such as masturbation has been associated with the well-described psychosomatic syndrome, the dhat syndrome,8,19,34 the syndrome is associated with weakness and pain symptoms. Narrative and clinical evidence from India suggest that vaginal discharge may represent the female equivalent of this syndrome.35,36
Thus, the risk factors for the complaint of abnormal vaginal discharge may vary according to the cultural setting of the study. In the South Asian setting, our study confirms that the algorithms for the syndromic management of vaginal discharge4 need refinement so that women with complaints that are non-infectious in aetiology are offered psychosocial interventions. Alternative approaches are needed for the management of common gynaecological complaints and RTIs in women, recognizing that there is a clinical consonance in providing care for both at the same point of delivery in the health care system. Such care should include both psychosocial interventions that target factors such as beliefs about illness, depression and somatic preoccupations, as well as accurate diagnostic tests for identification and specific treatment of RTIs, to achieve the twin goals of RTI control and symptom alleviation. The accurate identification and treatment of RTIs may lead to both RTI control and to some reduction in symptoms, while effective targeting of psychosocial aetiologies may lead to a significant alleviation of symptoms, reduced health care costs and disability associated with the symptom. In the absence of diagnostic tests, we recommend screening of all women with the complaint of vaginal discharge for psychosocial difficulties and providing appropriate care for such difficulties, simultaneously with the syndromic approach for the treatment of RTIs.
The complaint of abnormal vaginal discharge is common and is the target for the syndromic approach to the treatment of RTIs. However, the association between laboratory confirmed RTIs and the complaint of vaginal discharge is weak.
Psychosocial risk factors, notably common mental disorders and somatoform disorders, are strongly associated with the complaint of abnormal vaginal discharge in a South Asian community sample.
Vaginal discharge may be interpreted as the culturally determined equivalent of the range of medically unexplained symptoms, such as chronic pelvic pain, chronic backache, and irritable bowel syndrome.
The management of common gynaecological complaints should include psychosocial interventions that target factors such as beliefs about illness, depression and somatic preoccupations.
This study was funded by a Wellcome Trust Career Development Fellowship in Clinical Tropical Medicine to V.P.. We are grateful to the Directorate of Health Services, Government of Goa, which has collaborated with the project from its inception. We are grateful to Professor Priya Abraham, Dr Rosanna Peeling and Dr Suzette Menezes for their advice on laboratory aspects of the study; Dr Arvind Salelkar for his support to the study in the DHS; Dr Sheela Gupte and Dr Heiner Grosskurth for their clinical advice and supervision; Professor Glyn Lewis and Professor Mohan Isaac for their advice on psychiatric aspects of the study. We are grateful to Tamara Hurst and Fiona Marquet in London, and Anil Pandey and Ulka Lotlikar in India for their administrative support to the project. We are grateful to Matthew Hotopf for his comments on an earlier draft of this paper. Finally, we acknowledge the contribution of the research team of the Stree Arogya Shodh Project and all the women who so enthusiastically participated in this study.
Conflict of interest and role of authors: None of the authors have declared any conflict of interest.
References
Koenig M, Jejeebhoy S, Singh S, Sridhar S. Investigating women's gynaecological morbidity in India: not just another KAP survey.
Bhatia JC, Cleland J. Self-reported symptoms of gynecological morbidity and their treatment in South India.
Bhatia JC, Cleland J. Health seeking behaviour of women and costs incurred: an analysis of prospective data. In: Pachauri S, Subramaniam S (eds). Implementing a Reproductive Health Agenda in India: The Beginning. New Delhi: Population Council,
Vishwanath S, Talwar V, Prasad R, Coyaji K, Elias CJ, de Zoysa I. Syndromic management of vaginal discharge among women in a reproductive health clinic in India.
Hawkes S, Morison L, Foster S et al. Managing reproductive tract infections in women in low-income, low-prevalence situations: an evaluation of syndromic management in Matlab, Bangladesh.
Bogaerts J, Ahmed J, Akhter N, Begum N, Van Ranst M, Verhaegen J. Sexually transmitted infections in a basic healthcare clinic in Dhaka, Bangladesh: syndromic management for cervicitis is not justified.
Trollope-Kumar K. Symptoms of reproductive-tract infection—not all that they seem to be.
Mayou R, Farmer A. ABC of psychological medicine: functional somatic symptoms and syndromes.
Patel V, Abas M, Broadhead J, Todd C, Reeler AP. Depression in developing countries: lessons from Zimbabwe.
Ballinger CB, Smith AH, Hobbs PR. Factors associated with psychiatric morbidity in women: a general practice survey.
Hodgkiss AD, Sufraz R, Watson JP. Psychiatric morbidity and illness behaviour in women with chronic pelvic pain.
Byrne P. Psychiatric morbidity in a gynaecology clinic: an epidemiological survey.
Gath D, Osborn M, Bungay G et al. Psychiatric disorder and gynaecological symptoms in middle aged women: a community survey.
Agarwal P, Malik S, Padubidri V. A study of psychiatric morbidity in a gynaecology outpatient clinic.
Chaturvedi S, Chandra P, Sudarshan C, Isaac MK. A popular hidden illness among women related to vaginal discharge.
Prasad J, Abraham S, Akila B, Joseph A, Jacob KS. Symptoms related to the reproductive tract and mental health among women in rural Southern India.
Trollope-Kumar K. Cultural and biomedical meanings of the complaint of leukorrhea in South Asian women.
Patel V, Oomman NM. Mental health matters too: gynaecological morbidity and depression in South Asia.
Tanksale V, Sahasrabhojanee M, Patel V, Nevrekar P, Menezes S, Mabey D. The reliability of a structured examination protocol and self-administered vaginal swabs: a pilot study of gynecological out-patients in Goa, India.
International Institute for Population Sciences. National Family Health Survey-2, 1998–99: India. Mumbai: IIPS,
Patel V, Pereira J, Coutinho L, Fernandes R, Fernandes J, Mann A. Poverty, psychological disorder & disability in primary care attenders in Goa, India.
Patel V, Rodrigues M, De Souza N. Gender, poverty & post-natal depression: a cohort study from Goa, India.
Lewis G, Pelosi A, Araya R, Dunn G. Measuring psychiatric disorder in the community: a standardized assessment for use by lay interviewers.
Patel V, Pereira J, Mann A. Somatic and psychological models of common mental disorders in India.
Cleland J, Harlow S. The value of the imperfect: the contribution of interview surveys to the study of gynaecological ill-health. In: Jejeebhoy S, Koenig M, Elias C (eds). Reproductive Tract Infections and other Gynaecological Disorders: A Multidisciplinary Research Approach. Cambridge: Cambridge University Press,
Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardised method of Gram stain interpretation.
Meehan M, Wawer MJ, Serwadda D, Gray RH, Quinn T. Laboratory methods for the diagnosis of reproductive tract infections and selected condition in population-based studies. In: Jejeebhoy S, Koenig M, Elias C (eds). Reproductive Tract Infections and Other Gynecological Disorders: A Multidisciplinary Approach. Cambridge: Cambridge University Press,
Wessely S, Nimnuan C, Sharpe M. Functional somatic syndromes: one or many?
Simon G, Gater R, Kisely S, Piccinelli M. Somatic symptoms of distress: an international primary care study.
van Vliet KP, Everaerd W, van Zuuren FJ et al. Symptom perception: psychological correlates of symptom reporting and illness behaviour of women with medically unexplained gynecological symptoms.
Sumathipala A, Siribaddana SH, Bhugra D. Culture-bound syndromes: the story of dhat syndrome.
Bang R, Bang A. Women's perceptions of white vaginal discharge: ethnographic data from rural Maharashtra. In: Gittelsohn J, Bentley ME, Pelto PJ et al. (eds). Listening to Women Talk About Their Health: Issues & Evidence from India. New Delhi: Ford Foundation,