-
PDF
- Split View
-
Views
-
Cite
Cite
Frank de Vries, Corinne de Vries, Cyrus Cooper, Bert Leufkens, Tjeerd-Pieter van Staa, Reanalysis of two studies with contrasting results on the association between statin use and fracture risk: the General Practice Research Database, International Journal of Epidemiology, Volume 35, Issue 5, October 2006, Pages 1301–1308, https://doi.org/10.1093/ije/dyl147
Close - Share Icon Share
Abstract
Background Two recent case–control studies by Meier et al. and van Staa et al. used the UK General Practice Research Database (GPRD) to examine the association between the use of statins and the risk of fractures, with different results. The objective of the present study was to examine methodological explanations for the discrepant results.
Methods We created two datasets, which mimicked the previous study designs: a ‘selected population’ (SP) case–control dataset, with fracture cases matched to controls nested within a selected cohort (Meier et al.), and an ‘entire population’ (EP) case–control dataset, with both cases and controls sampled from the total GPRD population (van Staa et al.). Cases and controls were matched by gender, age (year of birth or 5 year age bands), and general practice.
Results The study included 131 855 fracture cases. The crude odds ratio (OR) for hip fracture in statin users was 0.37 (95% CI 0.27–0.52) in the SP and 0.54 (95% CI 0.39–0.74) in the EP dataset. This difference was reduced when matching by year of birth, rather than by 5 year age bands: crude ORs were 0.58 (95% CI 0.43–0.79) and 0.61 (95% CI 0.44–0.88), respectively. In the SP dataset, 37% of the cases could be matched by year of birth, while this was achieved for 99% in the ‘EP’ dataset. The exposure time-window, the selection of confounders, and exclusion of high-risk patients also influenced results.
Conclusion Residual confounding by a matching variable and different definitions of the exposure time window explained differences in results. In case–control studies of drug use and fracture risk, broad matching criteria for age should be avoided and the selection of the time-window for exposure should be carefully considered.
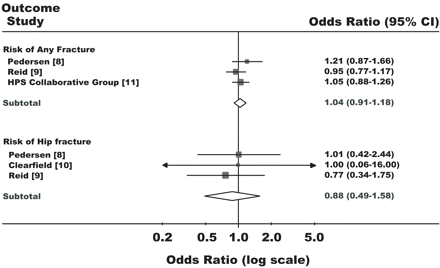
Following the finding that statins increase bone formation in rodents,1 two studies independently evaluated the association between the risk of fracture and use of statins in humans.2–4 Although in both studies medical records from the UK General Practice Research Database (GPRD) were used, different results were obtained. Meier and co-workers reported a statistically significant (P < 0.001) odds ratio (OR) of 0.12 (95% CI 0.04–0.41) for hip fracture in current statin users compared with non-users, while van Staa and co-workers found an OR of 0.59 (95% CI 0.31–1.13). In the subsequent discussion, Meier proposed that inclusion of patients at high risk of fractures might have led to biased results in the study by van Staa et al.5 In an editorial, Hennesy and Strom6 suggested that the inclusion of patients who sustained ‘unspecified fractures’, the use of different coding dictionaries and different time-window definitions for statin exposure might explain the different results. van Staa argued that varying definitions of follow-up between users and non-users could have created an imbalance in the matching of fracture cases in the study by Meier et al.3,7 None of these hypotheses have been formally tested. Furthermore, randomized controlled clinical trials (RCTs) have provided little evidence for statins as anti-osteoporotic agents (Figure 1).8–11
Effects of statins on risk of fracture in randomized controlled clinical trials
The objective of this study was to examine whether different matching procedures, time-window definitions, and exclusion criteria might explain the discrepant results of the two case–control studies by Meier et al. and van Staa et al.2,3Table 1 lists the major differences between both study designs, including those that this paper will evaluate as the reason for the different results.
| Characteristic . | Original ‘SP’ case–control study2 . | Original ‘EP’ case–control study3 . | Difference evaluated in current study? . |
|---|---|---|---|
| Study population | Users of lipid-lowering drugs, patients with a diagnosis of hyperlipidaemia and 50 000 untreated patients who were enrolled between the late eighties and September 1998 | All subjects enrolled between January 1987 and July 1999 | No, the current study was evaluated in the GPRD with data from January 1987 to April 2002 |
| Study design | Cases and controls were selected from a subset of the study population | Cases and controls were selected from the complete study population | No, information on the selection of the GPRD subset was not available |
| Age (years) | 50 through 89 at start of follow-up | 50 and older at the index date | Yes |
| Study outcome | Fractures of the femur/hip, humerus, hand, wrist or lower arm, vertebrae, clavicle, foot or malleolus or an unspecified location | Fractures of the vertebrae, clavicle, humerus, radius/ulna, carpus, hip, ankle, or foot | No, the outcome studied comprised the fracture types from the original ‘SP’ case–control study |
| Exclusion criteria at baseline | History of osteoporosis, osteomalacia, alcoholism, cancer excluding non-melanoma skin cancer and previous use of bisphosphonates | None | Yes |
| Definition current statin use | At least one statin prescription, but no other lipid lowering drugs in the 30 days before the index date | At least one statin prescription in the 6 months before the index date, regardless of other lipid lowering drugs | Yes |
| Matching variables | Age, gender, general practice, and duration of follow-up | Age, gender and general practice | Yes |
| Disease coding | ICD-8 cross-mapping | ICD-9 cross-mapping | No, ICD9 coding dictionary was used |
| Variables included in multivariate analysis | Exposure to HRT, oral corticosteroids, smoking, BMI, and the number of GP visits (‘limited adjusted model’) | All potential confounders in the ‘Method’ section of this paper (‘fully adjusted model’) including anaemia and depression, but without a history of diabetes, rheumatoid arthritis, falls, smoking, NSAID, bronchodilator and beta-blocker use | Yes, adjustment with the ‘fully adjusted model’ and the ‘limited adjusted model’ was evaluated |
| Characteristic . | Original ‘SP’ case–control study2 . | Original ‘EP’ case–control study3 . | Difference evaluated in current study? . |
|---|---|---|---|
| Study population | Users of lipid-lowering drugs, patients with a diagnosis of hyperlipidaemia and 50 000 untreated patients who were enrolled between the late eighties and September 1998 | All subjects enrolled between January 1987 and July 1999 | No, the current study was evaluated in the GPRD with data from January 1987 to April 2002 |
| Study design | Cases and controls were selected from a subset of the study population | Cases and controls were selected from the complete study population | No, information on the selection of the GPRD subset was not available |
| Age (years) | 50 through 89 at start of follow-up | 50 and older at the index date | Yes |
| Study outcome | Fractures of the femur/hip, humerus, hand, wrist or lower arm, vertebrae, clavicle, foot or malleolus or an unspecified location | Fractures of the vertebrae, clavicle, humerus, radius/ulna, carpus, hip, ankle, or foot | No, the outcome studied comprised the fracture types from the original ‘SP’ case–control study |
| Exclusion criteria at baseline | History of osteoporosis, osteomalacia, alcoholism, cancer excluding non-melanoma skin cancer and previous use of bisphosphonates | None | Yes |
| Definition current statin use | At least one statin prescription, but no other lipid lowering drugs in the 30 days before the index date | At least one statin prescription in the 6 months before the index date, regardless of other lipid lowering drugs | Yes |
| Matching variables | Age, gender, general practice, and duration of follow-up | Age, gender and general practice | Yes |
| Disease coding | ICD-8 cross-mapping | ICD-9 cross-mapping | No, ICD9 coding dictionary was used |
| Variables included in multivariate analysis | Exposure to HRT, oral corticosteroids, smoking, BMI, and the number of GP visits (‘limited adjusted model’) | All potential confounders in the ‘Method’ section of this paper (‘fully adjusted model’) including anaemia and depression, but without a history of diabetes, rheumatoid arthritis, falls, smoking, NSAID, bronchodilator and beta-blocker use | Yes, adjustment with the ‘fully adjusted model’ and the ‘limited adjusted model’ was evaluated |
| Characteristic . | Original ‘SP’ case–control study2 . | Original ‘EP’ case–control study3 . | Difference evaluated in current study? . |
|---|---|---|---|
| Study population | Users of lipid-lowering drugs, patients with a diagnosis of hyperlipidaemia and 50 000 untreated patients who were enrolled between the late eighties and September 1998 | All subjects enrolled between January 1987 and July 1999 | No, the current study was evaluated in the GPRD with data from January 1987 to April 2002 |
| Study design | Cases and controls were selected from a subset of the study population | Cases and controls were selected from the complete study population | No, information on the selection of the GPRD subset was not available |
| Age (years) | 50 through 89 at start of follow-up | 50 and older at the index date | Yes |
| Study outcome | Fractures of the femur/hip, humerus, hand, wrist or lower arm, vertebrae, clavicle, foot or malleolus or an unspecified location | Fractures of the vertebrae, clavicle, humerus, radius/ulna, carpus, hip, ankle, or foot | No, the outcome studied comprised the fracture types from the original ‘SP’ case–control study |
| Exclusion criteria at baseline | History of osteoporosis, osteomalacia, alcoholism, cancer excluding non-melanoma skin cancer and previous use of bisphosphonates | None | Yes |
| Definition current statin use | At least one statin prescription, but no other lipid lowering drugs in the 30 days before the index date | At least one statin prescription in the 6 months before the index date, regardless of other lipid lowering drugs | Yes |
| Matching variables | Age, gender, general practice, and duration of follow-up | Age, gender and general practice | Yes |
| Disease coding | ICD-8 cross-mapping | ICD-9 cross-mapping | No, ICD9 coding dictionary was used |
| Variables included in multivariate analysis | Exposure to HRT, oral corticosteroids, smoking, BMI, and the number of GP visits (‘limited adjusted model’) | All potential confounders in the ‘Method’ section of this paper (‘fully adjusted model’) including anaemia and depression, but without a history of diabetes, rheumatoid arthritis, falls, smoking, NSAID, bronchodilator and beta-blocker use | Yes, adjustment with the ‘fully adjusted model’ and the ‘limited adjusted model’ was evaluated |
| Characteristic . | Original ‘SP’ case–control study2 . | Original ‘EP’ case–control study3 . | Difference evaluated in current study? . |
|---|---|---|---|
| Study population | Users of lipid-lowering drugs, patients with a diagnosis of hyperlipidaemia and 50 000 untreated patients who were enrolled between the late eighties and September 1998 | All subjects enrolled between January 1987 and July 1999 | No, the current study was evaluated in the GPRD with data from January 1987 to April 2002 |
| Study design | Cases and controls were selected from a subset of the study population | Cases and controls were selected from the complete study population | No, information on the selection of the GPRD subset was not available |
| Age (years) | 50 through 89 at start of follow-up | 50 and older at the index date | Yes |
| Study outcome | Fractures of the femur/hip, humerus, hand, wrist or lower arm, vertebrae, clavicle, foot or malleolus or an unspecified location | Fractures of the vertebrae, clavicle, humerus, radius/ulna, carpus, hip, ankle, or foot | No, the outcome studied comprised the fracture types from the original ‘SP’ case–control study |
| Exclusion criteria at baseline | History of osteoporosis, osteomalacia, alcoholism, cancer excluding non-melanoma skin cancer and previous use of bisphosphonates | None | Yes |
| Definition current statin use | At least one statin prescription, but no other lipid lowering drugs in the 30 days before the index date | At least one statin prescription in the 6 months before the index date, regardless of other lipid lowering drugs | Yes |
| Matching variables | Age, gender, general practice, and duration of follow-up | Age, gender and general practice | Yes |
| Disease coding | ICD-8 cross-mapping | ICD-9 cross-mapping | No, ICD9 coding dictionary was used |
| Variables included in multivariate analysis | Exposure to HRT, oral corticosteroids, smoking, BMI, and the number of GP visits (‘limited adjusted model’) | All potential confounders in the ‘Method’ section of this paper (‘fully adjusted model’) including anaemia and depression, but without a history of diabetes, rheumatoid arthritis, falls, smoking, NSAID, bronchodilator and beta-blocker use | Yes, adjustment with the ‘fully adjusted model’ and the ‘limited adjusted model’ was evaluated |
Methods
We replicated the study design of Meier et al. [referred to as the ‘selected population’ (SP) case–control dataset] and the study design of van Staa et al. [‘entire population’ (EP) case–control dataset] in the GPRD with data collected from 1 January 1987 through 30 April 2002. The complete dataset included all permanently registered patients aged 50 years or older.
‘SP’ case–control dataset
The SP case–control dataset was created in three defined cohorts of patients.2 The first cohort included all patients who received at least one prescription for a statin (i.e. atorvastatin, cerivastatin, fluvastatin, pravastatin, or simvastatin), a fibrate (i.e. bezafibrate, ciprofibrate, clofibrate, fenofibrate, or gemfibrozil), or a lipid-lowering drug other than statins or fibrates (i.e. colestipol hydrochloride, cholestyramine, acipimox, or nicotinic acid). The second cohort consisted of all people with a diagnosis of hyperlipidaemia and the third cohort consisted of a random sample of 2 50 000 people who received neither a diagnosis of hyperlipidaemia nor a prescription for a lipid-lowering drug at any time. These patient numbers are proportionally similar to those analysed by Meier et al.2 Start of data collection was defined as the date of enrolment of a patient in a practice or the date of enrolment of a practice in GPRD, whichever came later. The start of follow-up for this study was defined as the date of the first prescription of a lipid-lowering drug (cohort 1) or as the start of data collection (cohort 2 and 3). Within these three cohorts, all patients with a first occurrence of a fracture were identified. The fractures were restricted to those of the femur/hip, humerus, hand, wrist, or lower arm, vertebrae, clavicle, foot, or malleolus or an unspecified location, similar to those selected by Meier et al.2 The date of the fracture was taken as the index date. From the three cohorts, up to six control patients were randomly selected for each case. Cases and controls could be selected from different cohorts. They were matched by year of birth, sex, practice, and the year of start of follow-up using the incidence-density sampling technique. Controls had to be alive and enrolled in GPRD at the index date. If no controls of similar age could be found, the age band used for matching was expanded stepwise up to a maximum of 5 years.2 If no control within the 5 year age band could be identified, the case was matched to a control with similar matching parameters except for the practice criterion. Control patients had the same index date as their matched case.
‘EP’ case–control dataset
The EP case–control dataset was created in the total GPRD population of patients aged 50 years or older, similar to the methodology used by van Staa and co-workers.3,4 Cases were permanently registered patients with a first-ever fracture after the start of data collection. Each case was matched to one control patient (patients without a history of any type of fracture) by year of birth, calendar time, sex, and general practice using the incidence sampling technique.
Exposure assessment
Users of lipid-lowering agents were classified according to single or mixed use before the index date of statins, fibrates, or other lipid-lowering drugs, according to the classification in the original SP analysis.2 As the time-window for exposure to lipid-lowering drugs was defined differently in the two original studies, two definitions were used. In the first definition (similar to that used by Meier et al.), current users were patients who were prescribed a lipid-lowering drug in the 30 days or less before the index date. The second exposure definition (similar to that used by van Staa and co-workers) defined current users as patients who were prescribed a lipid-lowering drug in the 6 months or less before the index date. Exposure duration was assessed by counting the number of statin prescriptions, as used in the original SP study design.2 Time since first statin use was determined by calculating the period between index date and first prescription.
Statistical analysis
Crude ORs of fracture in statin users compared with never-users and 95% confidence intervals (CI) were estimated using conditional logistic regression. A history of diabetes mellitus, rheumatoid arthritis, congestive heart failure, seizures, thalassaemia, sickle cell disease, pernicious anaemia, dementia, psychotic disorder, stroke, and chronic obstructive pulmonary disease and a history of hyperthyroidism or falls 1 year before the index date were considered as potential confounding variables. Prescribing in the 6 months prior to index date for anticonvulsants, methotrexate, hormone replacement therapy (HRT), thiazide diuretics, beta-blockers, anxiolytics/hypnotics, antipsychotics, antidepressants, anti-Parkinson drugs, systemic and inhaled corticosteroids, bronchodilators, and a minimum of 3 prescriptions of non-steroidal anti-inflammatory drugs (NSAIDs) were also included in the regression analysis. The last recorded smoking status and body mass index (BMI) before the index date were also included (some practices do not enter these data since it is not part of required data collection). Adjustment for these variables is referred to as the ‘fully adjusted model’. In order to replicate the adjustments in the original analysis by Meier et al.,2 the number of general practitioner (GP) visits before the index date was also calculated. Final adjusted models were fitted using backward elimination. The Mantel–Haenszel estimator of combined ORs of randomized clinical trials in Figure 1 was calculated according to the method described by Hauck.12 Spline regression lines were calculated using the GPLOT procedure of SAS version 8.2 (SAS Institute Inc., Cary, NC, USA).
Results
Totally 1 31 855 patients sustained one or more fractures, and 1 26 028 patients received one or more prescriptions for lipid-lowering drugs in the GPRD population of patients aged 50 years or older. Table 2 shows baseline characteristics in the two case–control datasets. In the SP case–control dataset, only 37% of the cases had been matched to controls by year of birth, while this was achieved for 99% of patients in the EP case–control dataset.
Characteristics of cases and controls in the ‘SP’ and ‘EP’ case–control datasets
| . | ‘SP’ case–control design . | . | . | . | . | ‘EP’ case–control design . | . | . | . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Cases . | . | Controls . | . | Crude . | Cases . | . | Controls . | . | Crude . | ||||||||||
| Characteristic | (n = 17 948) | (%) | (n = 95 468) | (%) | OR (95% CI) | (n = 1 31 838) | (%) | (n = 1 31 838) | (%) | OR (95% CI) | ||||||||||
| Mean age (years) | 72.0 | 71.1 | 71.6 | 71.6 | ||||||||||||||||
| Degree of matching | ||||||||||||||||||||
| By same year of birth | 35 650 | (37%) | 1 30 291 | (99%) | ||||||||||||||||
| Control 1 through 5 years older | 26 970 | (28%) | 541 | (0%) | ||||||||||||||||
| Control 1 through 5 years younger | 32 848 | (34%) | 1033 | (1%) | ||||||||||||||||
| Number of women | 13 726 | (76%) | 72 274 | (76%) | 98 762 | (75%) | 98 762 | (75%) | ||||||||||||
| Mean duration of follow-up (years) | 3.5 | 3.5 | 4.0 | 4.0 | ||||||||||||||||
| BMI | ||||||||||||||||||||
| <20 | 689 | (4%) | 2730 | (3%) | 1.21 (1.10–1.32) | 5610 | (4%) | 4324 | (3%) | 1.21 (1.16–1.26) | ||||||||||
| 20–25 | 4228 | (24%) | 21 072 | (22%) | 1.00 | 30 330 | (23%) | 28 244 | (21%) | 1.00 | ||||||||||
| 25–29.9 | 3823 | (21%) | 20 784 | (22%) | 0.92 (0.88–0.97) | 24 668 | (19%) | 25 451 | (19%) | 0.90 (0.88–0.92) | ||||||||||
| ≥30 | 1544 | (9%) | 8943 | (9%) | 0.86 (0.81–0.92) | 9701 | (7%) | 10 772 | (8%) | 0.84 (0.81–0.86) | ||||||||||
| Unknown | 7664 | (43%) | 41 939 | (44%) | 0.90 (0.85–0.94) | 61 529 | (47%) | 63 047 | (48%) | 0.89 (0.87–0.91) | ||||||||||
| Smoking status | ||||||||||||||||||||
| Current smoker | 2019 | (11%) | 10 091 | (11%) | 1.08 (1.02–1.14) | 15 333 | (12%) | 13 907 | (11%) | 1.10 (1.07–1.13) | ||||||||||
| Ex smoker | 1272 | (7%) | 6111 | (6%) | 1.12 (1.05–1.20) | 7763 | (6%) | 7132 | (5%) | 1.09 (1.05–1.13) | ||||||||||
| Non-smoker | 8344 | (46%) | 43 699 | (46%) | 1.00 | 58 766 | (45%) | 58 158 | (44%) | 1.00 | ||||||||||
| Unknown | 6313 | (35%) | 35 567 | (37%) | 0.91 (0.87–0.95) | 49 976 | (38%) | 52 641 | (40%) | 0.91 (0.89–0.93) | ||||||||||
| Diseases before index date | ||||||||||||||||||||
| Heart failure | 781 | (4%) | 3157 | (3%) | 1.23 (1.13–1.34) | 5986 | (5%) | 4663 | (4%) | 1.32 (1.27–1.37) | ||||||||||
| Stroke | 473 | (3%) | 1711 | (2%) | 1.41 (1.27–1.57) | 3563 | (3%) | 2414 | (2%) | 1.50 (1.43–1.58) | ||||||||||
| Diabetes mellitus | 306 | (2%) | 1613 | (2%) | 1.00 (0.88–1.13) | 2320 | (2%) | 2333 | (2%) | 0.99 (0.94–1.05) | ||||||||||
| Drug use 182 days before index date | ||||||||||||||||||||
| Thiazide diuretics | 2498 | (14%) | 14 121 | (15%) | 0.92 (0.88–0.96) | 16 445 | (12%) | 17 511 | (13%) | 0.93 (0.91–0.95) | ||||||||||
| Systemic corticosteroids | 1202 | (7%) | 4109 | (4%) | 1.59 (1.48–1.70) | 8231 | (6%) | 5216 | (4%) | 1.63 (1.57–1.69) | ||||||||||
| HRT | 610 | (3%) | 4338 | (5%) | 0.74 (0.67–0.81) | 5046 | (4%) | 6301 | (5%) | 0.76 (0.73–0.79) | ||||||||||
| Statin exposure only | ||||||||||||||||||||
| Never exposed to lipid-lowering drugs | 14 762 | (82%) | 79 475 | (83%) | 1.00 | 1 28 572 | (98%) | 1 28 588 | (98%) | 1.00 | ||||||||||
| Use of statins 30 days before | 951 | (5%) | 5659 | (6%) | 0.82 (0.74–0.90) | 899 | (1%) | 1031 | (1%) | 0.87 (0.79–0.95) | ||||||||||
| Use of statins 6 months before | 1596 | (9%) | 8688 | (9%) | 0.92 (0.85–1.00) | 1503 | (1%) | 1577 | (1%) | 0.95 (0.89–1.02) | ||||||||||
| Hip fracture | 2470 | 12 223 | 19 366 | 19 366 | ||||||||||||||||
| Never exposed to lipid-lowering drugs | 2217 | (90%) | 10 749 | (88%) | 1.00 | 19 113 | (99%) | 19 048 | (98%) | 1.00 | ||||||||||
| Use of statins 30 days before | 67 | (3%) | 542 | (4%) | 0.37 (0.27–0.52) | 58 | (0%) | 106 | (0%) | 0.54 (0.39–0.74) | ||||||||||
| Use of statins 6 months before | 122 | (5%) | 827 | (7%) | 0.47 (0.35–0.63) | 111 | (0%) | 163 | (1%) | 0.67 (0.52–0.85) | ||||||||||
| Vertebral fracture | 1077 | 5665 | 7606 | 7606 | ||||||||||||||||
| Never exposed to lipid-lowering drugs | 857 | (80%) | 4678 | (83%) | 1.00 | 7393 | (97%) | 7404 | (97%) | 1.00 | ||||||||||
| Use of statins 30 days before | 55 | (5%) | 395 | (7%) | 0.62 (0.42–0.92) | 47 | (1%) | 65 | (1%) | 0.72 (0.49–1.06) | ||||||||||
| Use of statins 6 months before | 105 | (10%) | 972 | (17%) | 0.81 (0.58–1.14) | 89 | (1%) | 103 | (1%) | 0.87 (0.64–1.16) | ||||||||||
| . | ‘SP’ case–control design . | . | . | . | . | ‘EP’ case–control design . | . | . | . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Cases . | . | Controls . | . | Crude . | Cases . | . | Controls . | . | Crude . | ||||||||||
| Characteristic | (n = 17 948) | (%) | (n = 95 468) | (%) | OR (95% CI) | (n = 1 31 838) | (%) | (n = 1 31 838) | (%) | OR (95% CI) | ||||||||||
| Mean age (years) | 72.0 | 71.1 | 71.6 | 71.6 | ||||||||||||||||
| Degree of matching | ||||||||||||||||||||
| By same year of birth | 35 650 | (37%) | 1 30 291 | (99%) | ||||||||||||||||
| Control 1 through 5 years older | 26 970 | (28%) | 541 | (0%) | ||||||||||||||||
| Control 1 through 5 years younger | 32 848 | (34%) | 1033 | (1%) | ||||||||||||||||
| Number of women | 13 726 | (76%) | 72 274 | (76%) | 98 762 | (75%) | 98 762 | (75%) | ||||||||||||
| Mean duration of follow-up (years) | 3.5 | 3.5 | 4.0 | 4.0 | ||||||||||||||||
| BMI | ||||||||||||||||||||
| <20 | 689 | (4%) | 2730 | (3%) | 1.21 (1.10–1.32) | 5610 | (4%) | 4324 | (3%) | 1.21 (1.16–1.26) | ||||||||||
| 20–25 | 4228 | (24%) | 21 072 | (22%) | 1.00 | 30 330 | (23%) | 28 244 | (21%) | 1.00 | ||||||||||
| 25–29.9 | 3823 | (21%) | 20 784 | (22%) | 0.92 (0.88–0.97) | 24 668 | (19%) | 25 451 | (19%) | 0.90 (0.88–0.92) | ||||||||||
| ≥30 | 1544 | (9%) | 8943 | (9%) | 0.86 (0.81–0.92) | 9701 | (7%) | 10 772 | (8%) | 0.84 (0.81–0.86) | ||||||||||
| Unknown | 7664 | (43%) | 41 939 | (44%) | 0.90 (0.85–0.94) | 61 529 | (47%) | 63 047 | (48%) | 0.89 (0.87–0.91) | ||||||||||
| Smoking status | ||||||||||||||||||||
| Current smoker | 2019 | (11%) | 10 091 | (11%) | 1.08 (1.02–1.14) | 15 333 | (12%) | 13 907 | (11%) | 1.10 (1.07–1.13) | ||||||||||
| Ex smoker | 1272 | (7%) | 6111 | (6%) | 1.12 (1.05–1.20) | 7763 | (6%) | 7132 | (5%) | 1.09 (1.05–1.13) | ||||||||||
| Non-smoker | 8344 | (46%) | 43 699 | (46%) | 1.00 | 58 766 | (45%) | 58 158 | (44%) | 1.00 | ||||||||||
| Unknown | 6313 | (35%) | 35 567 | (37%) | 0.91 (0.87–0.95) | 49 976 | (38%) | 52 641 | (40%) | 0.91 (0.89–0.93) | ||||||||||
| Diseases before index date | ||||||||||||||||||||
| Heart failure | 781 | (4%) | 3157 | (3%) | 1.23 (1.13–1.34) | 5986 | (5%) | 4663 | (4%) | 1.32 (1.27–1.37) | ||||||||||
| Stroke | 473 | (3%) | 1711 | (2%) | 1.41 (1.27–1.57) | 3563 | (3%) | 2414 | (2%) | 1.50 (1.43–1.58) | ||||||||||
| Diabetes mellitus | 306 | (2%) | 1613 | (2%) | 1.00 (0.88–1.13) | 2320 | (2%) | 2333 | (2%) | 0.99 (0.94–1.05) | ||||||||||
| Drug use 182 days before index date | ||||||||||||||||||||
| Thiazide diuretics | 2498 | (14%) | 14 121 | (15%) | 0.92 (0.88–0.96) | 16 445 | (12%) | 17 511 | (13%) | 0.93 (0.91–0.95) | ||||||||||
| Systemic corticosteroids | 1202 | (7%) | 4109 | (4%) | 1.59 (1.48–1.70) | 8231 | (6%) | 5216 | (4%) | 1.63 (1.57–1.69) | ||||||||||
| HRT | 610 | (3%) | 4338 | (5%) | 0.74 (0.67–0.81) | 5046 | (4%) | 6301 | (5%) | 0.76 (0.73–0.79) | ||||||||||
| Statin exposure only | ||||||||||||||||||||
| Never exposed to lipid-lowering drugs | 14 762 | (82%) | 79 475 | (83%) | 1.00 | 1 28 572 | (98%) | 1 28 588 | (98%) | 1.00 | ||||||||||
| Use of statins 30 days before | 951 | (5%) | 5659 | (6%) | 0.82 (0.74–0.90) | 899 | (1%) | 1031 | (1%) | 0.87 (0.79–0.95) | ||||||||||
| Use of statins 6 months before | 1596 | (9%) | 8688 | (9%) | 0.92 (0.85–1.00) | 1503 | (1%) | 1577 | (1%) | 0.95 (0.89–1.02) | ||||||||||
| Hip fracture | 2470 | 12 223 | 19 366 | 19 366 | ||||||||||||||||
| Never exposed to lipid-lowering drugs | 2217 | (90%) | 10 749 | (88%) | 1.00 | 19 113 | (99%) | 19 048 | (98%) | 1.00 | ||||||||||
| Use of statins 30 days before | 67 | (3%) | 542 | (4%) | 0.37 (0.27–0.52) | 58 | (0%) | 106 | (0%) | 0.54 (0.39–0.74) | ||||||||||
| Use of statins 6 months before | 122 | (5%) | 827 | (7%) | 0.47 (0.35–0.63) | 111 | (0%) | 163 | (1%) | 0.67 (0.52–0.85) | ||||||||||
| Vertebral fracture | 1077 | 5665 | 7606 | 7606 | ||||||||||||||||
| Never exposed to lipid-lowering drugs | 857 | (80%) | 4678 | (83%) | 1.00 | 7393 | (97%) | 7404 | (97%) | 1.00 | ||||||||||
| Use of statins 30 days before | 55 | (5%) | 395 | (7%) | 0.62 (0.42–0.92) | 47 | (1%) | 65 | (1%) | 0.72 (0.49–1.06) | ||||||||||
| Use of statins 6 months before | 105 | (10%) | 972 | (17%) | 0.81 (0.58–1.14) | 89 | (1%) | 103 | (1%) | 0.87 (0.64–1.16) | ||||||||||
Characteristics of cases and controls in the ‘SP’ and ‘EP’ case–control datasets
| . | ‘SP’ case–control design . | . | . | . | . | ‘EP’ case–control design . | . | . | . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Cases . | . | Controls . | . | Crude . | Cases . | . | Controls . | . | Crude . | ||||||||||
| Characteristic | (n = 17 948) | (%) | (n = 95 468) | (%) | OR (95% CI) | (n = 1 31 838) | (%) | (n = 1 31 838) | (%) | OR (95% CI) | ||||||||||
| Mean age (years) | 72.0 | 71.1 | 71.6 | 71.6 | ||||||||||||||||
| Degree of matching | ||||||||||||||||||||
| By same year of birth | 35 650 | (37%) | 1 30 291 | (99%) | ||||||||||||||||
| Control 1 through 5 years older | 26 970 | (28%) | 541 | (0%) | ||||||||||||||||
| Control 1 through 5 years younger | 32 848 | (34%) | 1033 | (1%) | ||||||||||||||||
| Number of women | 13 726 | (76%) | 72 274 | (76%) | 98 762 | (75%) | 98 762 | (75%) | ||||||||||||
| Mean duration of follow-up (years) | 3.5 | 3.5 | 4.0 | 4.0 | ||||||||||||||||
| BMI | ||||||||||||||||||||
| <20 | 689 | (4%) | 2730 | (3%) | 1.21 (1.10–1.32) | 5610 | (4%) | 4324 | (3%) | 1.21 (1.16–1.26) | ||||||||||
| 20–25 | 4228 | (24%) | 21 072 | (22%) | 1.00 | 30 330 | (23%) | 28 244 | (21%) | 1.00 | ||||||||||
| 25–29.9 | 3823 | (21%) | 20 784 | (22%) | 0.92 (0.88–0.97) | 24 668 | (19%) | 25 451 | (19%) | 0.90 (0.88–0.92) | ||||||||||
| ≥30 | 1544 | (9%) | 8943 | (9%) | 0.86 (0.81–0.92) | 9701 | (7%) | 10 772 | (8%) | 0.84 (0.81–0.86) | ||||||||||
| Unknown | 7664 | (43%) | 41 939 | (44%) | 0.90 (0.85–0.94) | 61 529 | (47%) | 63 047 | (48%) | 0.89 (0.87–0.91) | ||||||||||
| Smoking status | ||||||||||||||||||||
| Current smoker | 2019 | (11%) | 10 091 | (11%) | 1.08 (1.02–1.14) | 15 333 | (12%) | 13 907 | (11%) | 1.10 (1.07–1.13) | ||||||||||
| Ex smoker | 1272 | (7%) | 6111 | (6%) | 1.12 (1.05–1.20) | 7763 | (6%) | 7132 | (5%) | 1.09 (1.05–1.13) | ||||||||||
| Non-smoker | 8344 | (46%) | 43 699 | (46%) | 1.00 | 58 766 | (45%) | 58 158 | (44%) | 1.00 | ||||||||||
| Unknown | 6313 | (35%) | 35 567 | (37%) | 0.91 (0.87–0.95) | 49 976 | (38%) | 52 641 | (40%) | 0.91 (0.89–0.93) | ||||||||||
| Diseases before index date | ||||||||||||||||||||
| Heart failure | 781 | (4%) | 3157 | (3%) | 1.23 (1.13–1.34) | 5986 | (5%) | 4663 | (4%) | 1.32 (1.27–1.37) | ||||||||||
| Stroke | 473 | (3%) | 1711 | (2%) | 1.41 (1.27–1.57) | 3563 | (3%) | 2414 | (2%) | 1.50 (1.43–1.58) | ||||||||||
| Diabetes mellitus | 306 | (2%) | 1613 | (2%) | 1.00 (0.88–1.13) | 2320 | (2%) | 2333 | (2%) | 0.99 (0.94–1.05) | ||||||||||
| Drug use 182 days before index date | ||||||||||||||||||||
| Thiazide diuretics | 2498 | (14%) | 14 121 | (15%) | 0.92 (0.88–0.96) | 16 445 | (12%) | 17 511 | (13%) | 0.93 (0.91–0.95) | ||||||||||
| Systemic corticosteroids | 1202 | (7%) | 4109 | (4%) | 1.59 (1.48–1.70) | 8231 | (6%) | 5216 | (4%) | 1.63 (1.57–1.69) | ||||||||||
| HRT | 610 | (3%) | 4338 | (5%) | 0.74 (0.67–0.81) | 5046 | (4%) | 6301 | (5%) | 0.76 (0.73–0.79) | ||||||||||
| Statin exposure only | ||||||||||||||||||||
| Never exposed to lipid-lowering drugs | 14 762 | (82%) | 79 475 | (83%) | 1.00 | 1 28 572 | (98%) | 1 28 588 | (98%) | 1.00 | ||||||||||
| Use of statins 30 days before | 951 | (5%) | 5659 | (6%) | 0.82 (0.74–0.90) | 899 | (1%) | 1031 | (1%) | 0.87 (0.79–0.95) | ||||||||||
| Use of statins 6 months before | 1596 | (9%) | 8688 | (9%) | 0.92 (0.85–1.00) | 1503 | (1%) | 1577 | (1%) | 0.95 (0.89–1.02) | ||||||||||
| Hip fracture | 2470 | 12 223 | 19 366 | 19 366 | ||||||||||||||||
| Never exposed to lipid-lowering drugs | 2217 | (90%) | 10 749 | (88%) | 1.00 | 19 113 | (99%) | 19 048 | (98%) | 1.00 | ||||||||||
| Use of statins 30 days before | 67 | (3%) | 542 | (4%) | 0.37 (0.27–0.52) | 58 | (0%) | 106 | (0%) | 0.54 (0.39–0.74) | ||||||||||
| Use of statins 6 months before | 122 | (5%) | 827 | (7%) | 0.47 (0.35–0.63) | 111 | (0%) | 163 | (1%) | 0.67 (0.52–0.85) | ||||||||||
| Vertebral fracture | 1077 | 5665 | 7606 | 7606 | ||||||||||||||||
| Never exposed to lipid-lowering drugs | 857 | (80%) | 4678 | (83%) | 1.00 | 7393 | (97%) | 7404 | (97%) | 1.00 | ||||||||||
| Use of statins 30 days before | 55 | (5%) | 395 | (7%) | 0.62 (0.42–0.92) | 47 | (1%) | 65 | (1%) | 0.72 (0.49–1.06) | ||||||||||
| Use of statins 6 months before | 105 | (10%) | 972 | (17%) | 0.81 (0.58–1.14) | 89 | (1%) | 103 | (1%) | 0.87 (0.64–1.16) | ||||||||||
| . | ‘SP’ case–control design . | . | . | . | . | ‘EP’ case–control design . | . | . | . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Cases . | . | Controls . | . | Crude . | Cases . | . | Controls . | . | Crude . | ||||||||||
| Characteristic | (n = 17 948) | (%) | (n = 95 468) | (%) | OR (95% CI) | (n = 1 31 838) | (%) | (n = 1 31 838) | (%) | OR (95% CI) | ||||||||||
| Mean age (years) | 72.0 | 71.1 | 71.6 | 71.6 | ||||||||||||||||
| Degree of matching | ||||||||||||||||||||
| By same year of birth | 35 650 | (37%) | 1 30 291 | (99%) | ||||||||||||||||
| Control 1 through 5 years older | 26 970 | (28%) | 541 | (0%) | ||||||||||||||||
| Control 1 through 5 years younger | 32 848 | (34%) | 1033 | (1%) | ||||||||||||||||
| Number of women | 13 726 | (76%) | 72 274 | (76%) | 98 762 | (75%) | 98 762 | (75%) | ||||||||||||
| Mean duration of follow-up (years) | 3.5 | 3.5 | 4.0 | 4.0 | ||||||||||||||||
| BMI | ||||||||||||||||||||
| <20 | 689 | (4%) | 2730 | (3%) | 1.21 (1.10–1.32) | 5610 | (4%) | 4324 | (3%) | 1.21 (1.16–1.26) | ||||||||||
| 20–25 | 4228 | (24%) | 21 072 | (22%) | 1.00 | 30 330 | (23%) | 28 244 | (21%) | 1.00 | ||||||||||
| 25–29.9 | 3823 | (21%) | 20 784 | (22%) | 0.92 (0.88–0.97) | 24 668 | (19%) | 25 451 | (19%) | 0.90 (0.88–0.92) | ||||||||||
| ≥30 | 1544 | (9%) | 8943 | (9%) | 0.86 (0.81–0.92) | 9701 | (7%) | 10 772 | (8%) | 0.84 (0.81–0.86) | ||||||||||
| Unknown | 7664 | (43%) | 41 939 | (44%) | 0.90 (0.85–0.94) | 61 529 | (47%) | 63 047 | (48%) | 0.89 (0.87–0.91) | ||||||||||
| Smoking status | ||||||||||||||||||||
| Current smoker | 2019 | (11%) | 10 091 | (11%) | 1.08 (1.02–1.14) | 15 333 | (12%) | 13 907 | (11%) | 1.10 (1.07–1.13) | ||||||||||
| Ex smoker | 1272 | (7%) | 6111 | (6%) | 1.12 (1.05–1.20) | 7763 | (6%) | 7132 | (5%) | 1.09 (1.05–1.13) | ||||||||||
| Non-smoker | 8344 | (46%) | 43 699 | (46%) | 1.00 | 58 766 | (45%) | 58 158 | (44%) | 1.00 | ||||||||||
| Unknown | 6313 | (35%) | 35 567 | (37%) | 0.91 (0.87–0.95) | 49 976 | (38%) | 52 641 | (40%) | 0.91 (0.89–0.93) | ||||||||||
| Diseases before index date | ||||||||||||||||||||
| Heart failure | 781 | (4%) | 3157 | (3%) | 1.23 (1.13–1.34) | 5986 | (5%) | 4663 | (4%) | 1.32 (1.27–1.37) | ||||||||||
| Stroke | 473 | (3%) | 1711 | (2%) | 1.41 (1.27–1.57) | 3563 | (3%) | 2414 | (2%) | 1.50 (1.43–1.58) | ||||||||||
| Diabetes mellitus | 306 | (2%) | 1613 | (2%) | 1.00 (0.88–1.13) | 2320 | (2%) | 2333 | (2%) | 0.99 (0.94–1.05) | ||||||||||
| Drug use 182 days before index date | ||||||||||||||||||||
| Thiazide diuretics | 2498 | (14%) | 14 121 | (15%) | 0.92 (0.88–0.96) | 16 445 | (12%) | 17 511 | (13%) | 0.93 (0.91–0.95) | ||||||||||
| Systemic corticosteroids | 1202 | (7%) | 4109 | (4%) | 1.59 (1.48–1.70) | 8231 | (6%) | 5216 | (4%) | 1.63 (1.57–1.69) | ||||||||||
| HRT | 610 | (3%) | 4338 | (5%) | 0.74 (0.67–0.81) | 5046 | (4%) | 6301 | (5%) | 0.76 (0.73–0.79) | ||||||||||
| Statin exposure only | ||||||||||||||||||||
| Never exposed to lipid-lowering drugs | 14 762 | (82%) | 79 475 | (83%) | 1.00 | 1 28 572 | (98%) | 1 28 588 | (98%) | 1.00 | ||||||||||
| Use of statins 30 days before | 951 | (5%) | 5659 | (6%) | 0.82 (0.74–0.90) | 899 | (1%) | 1031 | (1%) | 0.87 (0.79–0.95) | ||||||||||
| Use of statins 6 months before | 1596 | (9%) | 8688 | (9%) | 0.92 (0.85–1.00) | 1503 | (1%) | 1577 | (1%) | 0.95 (0.89–1.02) | ||||||||||
| Hip fracture | 2470 | 12 223 | 19 366 | 19 366 | ||||||||||||||||
| Never exposed to lipid-lowering drugs | 2217 | (90%) | 10 749 | (88%) | 1.00 | 19 113 | (99%) | 19 048 | (98%) | 1.00 | ||||||||||
| Use of statins 30 days before | 67 | (3%) | 542 | (4%) | 0.37 (0.27–0.52) | 58 | (0%) | 106 | (0%) | 0.54 (0.39–0.74) | ||||||||||
| Use of statins 6 months before | 122 | (5%) | 827 | (7%) | 0.47 (0.35–0.63) | 111 | (0%) | 163 | (1%) | 0.67 (0.52–0.85) | ||||||||||
| Vertebral fracture | 1077 | 5665 | 7606 | 7606 | ||||||||||||||||
| Never exposed to lipid-lowering drugs | 857 | (80%) | 4678 | (83%) | 1.00 | 7393 | (97%) | 7404 | (97%) | 1.00 | ||||||||||
| Use of statins 30 days before | 55 | (5%) | 395 | (7%) | 0.62 (0.42–0.92) | 47 | (1%) | 65 | (1%) | 0.72 (0.49–1.06) | ||||||||||
| Use of statins 6 months before | 105 | (10%) | 972 | (17%) | 0.81 (0.58–1.14) | 89 | (1%) | 103 | (1%) | 0.87 (0.64–1.16) | ||||||||||
The risks of fracture among those who had received one or more statin prescriptions in the 6 months before were generally lower with the SP case–control dataset, compared with the EP case–control dataset (Table 3). This was particularly obvious for patients who sustained a hip fracture, yielding an OR of 0.48 (95% CI 0.36–0.64) in the SP dataset, and 0.60 (95% CI 0.46–0.78) in the EP dataset (when adjusting for the variables originally used by Meier et al.). Adjustment with a larger set of potential confounders reduced the magnitude of effect [OR for risk of hip fracture of 0.60 (95% CI 0.45–0.81) in the SP dataset and 0.72 (95% CI 0.54–0.95) in the EP dataset].
Risk of fracture and current statin use in the original and new case–control datasets
| . | Adjusted OR (95% CI) . | . | Adjusted OR (95% CI), according to similar confounders as used by Meier et al.2,a . | . | Adjusted OR (95% CI), according to the ‘fully adjusted model’ described in the Methods section . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure beforeStatin only . | Original ‘SP’ dataset2,b . | Original‘EP’ dataset3,c . | Updated ‘SP’ dataset . | Updated ‘EP’ dataset . | Updated ‘SP’ dataset . | Updated ‘EP’ dataset . | ||||||
| Never exposed to lipid-lowering drugs | 1.00 | 1.00d | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Any fracture | ||||||||||||
| 30 days before | 0.55 (0.44–0.69) | 0.82 (0.74–0.91) | 0.74 (0.68–0.82) | 0.85 (0.77–0.94) | 0.92 (0.83–1.01) | |||||||
| 6 months before | 1.01 (0.88–1.16) | 0.93 (0.85–1.01) | 0.82 (0.76–0.89) | 0.96 (0.88–1.04) | 1.01 (0.94–1.09) | |||||||
| Hip fracture | ||||||||||||
| 30 days before | 0.12 (0.04–0.41) | 0.38 (0.27–0.53) | 0.48 (0.34–0.68) | 0.50 (0.36–0.68) | 0.54 (0.38–0.78) | |||||||
| 6 months before | 0.59 (0.31–1.13) | 0.48 (0.36–0.64) | 0.60 (0.46–0.78) | 0.60 (0.45–0.81) | 0.72 (0.54–0.95) | |||||||
| Vertebral fracture | ||||||||||||
| 30 days before | 0.14 (0.02–0.88) | 0.52 (0.34–0.80) | 0.59 (0.38–0.92) | 0.63 (0.41–0.95) | 0.68 (0.44–1.05) | |||||||
| 6 months before | 1.15 (0.62–2.14) | 0.70 (0.49–1.01) | 0.63 (0.45–0.89) | 0.83 (0.57–1.19) | 0.82 (0.59–1.14) | |||||||
| . | Adjusted OR (95% CI) . | . | Adjusted OR (95% CI), according to similar confounders as used by Meier et al.2,a . | . | Adjusted OR (95% CI), according to the ‘fully adjusted model’ described in the Methods section . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure beforeStatin only . | Original ‘SP’ dataset2,b . | Original‘EP’ dataset3,c . | Updated ‘SP’ dataset . | Updated ‘EP’ dataset . | Updated ‘SP’ dataset . | Updated ‘EP’ dataset . | ||||||
| Never exposed to lipid-lowering drugs | 1.00 | 1.00d | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Any fracture | ||||||||||||
| 30 days before | 0.55 (0.44–0.69) | 0.82 (0.74–0.91) | 0.74 (0.68–0.82) | 0.85 (0.77–0.94) | 0.92 (0.83–1.01) | |||||||
| 6 months before | 1.01 (0.88–1.16) | 0.93 (0.85–1.01) | 0.82 (0.76–0.89) | 0.96 (0.88–1.04) | 1.01 (0.94–1.09) | |||||||
| Hip fracture | ||||||||||||
| 30 days before | 0.12 (0.04–0.41) | 0.38 (0.27–0.53) | 0.48 (0.34–0.68) | 0.50 (0.36–0.68) | 0.54 (0.38–0.78) | |||||||
| 6 months before | 0.59 (0.31–1.13) | 0.48 (0.36–0.64) | 0.60 (0.46–0.78) | 0.60 (0.45–0.81) | 0.72 (0.54–0.95) | |||||||
| Vertebral fracture | ||||||||||||
| 30 days before | 0.14 (0.02–0.88) | 0.52 (0.34–0.80) | 0.59 (0.38–0.92) | 0.63 (0.41–0.95) | 0.68 (0.44–1.05) | |||||||
| 6 months before | 1.15 (0.62–2.14) | 0.70 (0.49–1.01) | 0.63 (0.45–0.89) | 0.83 (0.57–1.19) | 0.82 (0.59–1.14) | |||||||
Adjusted for the criteria in the original design by Meier, i.e. BMI, smoking, number of GP visits 1 year before, use of oral corticosteroids, and HRT 6 months before the index date.
Patients with a history of osteoporosis, osteomalacia, alcoholism, cancer (excluding non-melanoma skin cancer), and bisphosphonate use prior to start of follow-up were excluded.
Adjusted for potential confounders as listed in the Methods section, including anaemia and depression, but without a history of diabetes mellitus, rheumatoid arthritis, falls, smoking, use of bronchodilators, beta-blockers, and NSAIDS.
Never exposed to statins.
Risk of fracture and current statin use in the original and new case–control datasets
| . | Adjusted OR (95% CI) . | . | Adjusted OR (95% CI), according to similar confounders as used by Meier et al.2,a . | . | Adjusted OR (95% CI), according to the ‘fully adjusted model’ described in the Methods section . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure beforeStatin only . | Original ‘SP’ dataset2,b . | Original‘EP’ dataset3,c . | Updated ‘SP’ dataset . | Updated ‘EP’ dataset . | Updated ‘SP’ dataset . | Updated ‘EP’ dataset . | ||||||
| Never exposed to lipid-lowering drugs | 1.00 | 1.00d | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Any fracture | ||||||||||||
| 30 days before | 0.55 (0.44–0.69) | 0.82 (0.74–0.91) | 0.74 (0.68–0.82) | 0.85 (0.77–0.94) | 0.92 (0.83–1.01) | |||||||
| 6 months before | 1.01 (0.88–1.16) | 0.93 (0.85–1.01) | 0.82 (0.76–0.89) | 0.96 (0.88–1.04) | 1.01 (0.94–1.09) | |||||||
| Hip fracture | ||||||||||||
| 30 days before | 0.12 (0.04–0.41) | 0.38 (0.27–0.53) | 0.48 (0.34–0.68) | 0.50 (0.36–0.68) | 0.54 (0.38–0.78) | |||||||
| 6 months before | 0.59 (0.31–1.13) | 0.48 (0.36–0.64) | 0.60 (0.46–0.78) | 0.60 (0.45–0.81) | 0.72 (0.54–0.95) | |||||||
| Vertebral fracture | ||||||||||||
| 30 days before | 0.14 (0.02–0.88) | 0.52 (0.34–0.80) | 0.59 (0.38–0.92) | 0.63 (0.41–0.95) | 0.68 (0.44–1.05) | |||||||
| 6 months before | 1.15 (0.62–2.14) | 0.70 (0.49–1.01) | 0.63 (0.45–0.89) | 0.83 (0.57–1.19) | 0.82 (0.59–1.14) | |||||||
| . | Adjusted OR (95% CI) . | . | Adjusted OR (95% CI), according to similar confounders as used by Meier et al.2,a . | . | Adjusted OR (95% CI), according to the ‘fully adjusted model’ described in the Methods section . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure beforeStatin only . | Original ‘SP’ dataset2,b . | Original‘EP’ dataset3,c . | Updated ‘SP’ dataset . | Updated ‘EP’ dataset . | Updated ‘SP’ dataset . | Updated ‘EP’ dataset . | ||||||
| Never exposed to lipid-lowering drugs | 1.00 | 1.00d | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Any fracture | ||||||||||||
| 30 days before | 0.55 (0.44–0.69) | 0.82 (0.74–0.91) | 0.74 (0.68–0.82) | 0.85 (0.77–0.94) | 0.92 (0.83–1.01) | |||||||
| 6 months before | 1.01 (0.88–1.16) | 0.93 (0.85–1.01) | 0.82 (0.76–0.89) | 0.96 (0.88–1.04) | 1.01 (0.94–1.09) | |||||||
| Hip fracture | ||||||||||||
| 30 days before | 0.12 (0.04–0.41) | 0.38 (0.27–0.53) | 0.48 (0.34–0.68) | 0.50 (0.36–0.68) | 0.54 (0.38–0.78) | |||||||
| 6 months before | 0.59 (0.31–1.13) | 0.48 (0.36–0.64) | 0.60 (0.46–0.78) | 0.60 (0.45–0.81) | 0.72 (0.54–0.95) | |||||||
| Vertebral fracture | ||||||||||||
| 30 days before | 0.14 (0.02–0.88) | 0.52 (0.34–0.80) | 0.59 (0.38–0.92) | 0.63 (0.41–0.95) | 0.68 (0.44–1.05) | |||||||
| 6 months before | 1.15 (0.62–2.14) | 0.70 (0.49–1.01) | 0.63 (0.45–0.89) | 0.83 (0.57–1.19) | 0.82 (0.59–1.14) | |||||||
Adjusted for the criteria in the original design by Meier, i.e. BMI, smoking, number of GP visits 1 year before, use of oral corticosteroids, and HRT 6 months before the index date.
Patients with a history of osteoporosis, osteomalacia, alcoholism, cancer (excluding non-melanoma skin cancer), and bisphosphonate use prior to start of follow-up were excluded.
Adjusted for potential confounders as listed in the Methods section, including anaemia and depression, but without a history of diabetes mellitus, rheumatoid arthritis, falls, smoking, use of bronchodilators, beta-blockers, and NSAIDS.
Never exposed to statins.
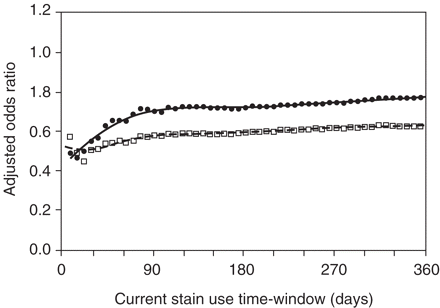
The use of shorter time-windows for measuring current statin use reduced the ORs for fracture. The fully adjusted OR for hip fracture was 0.49 (95% CI 0.24–1.00) in the EP case–control dataset when defining exposure on the basis of statin prescribing in the 1 week before the index date, compared with an OR of 0.78 (95% CI 0.60–1.02) when using a time-window of 2 years (Figure 2). In both study designs, 60% of the case patients and 65% of the control patients who were exposed to statins in the 6 months before the index date were also exposed to statins in the 30 days before the index date.
Spline regression plot of hip fracture risk and length (days) of the current use of statins time-window. Squares and dashed line: ‘SP’ dataset; Dots and solid line: ‘EP’ dataset. The current use time-window was 52 times extended stepwise by 7 days, starting at 7 days before the index date and ending 364 days before the index date
Table 4 shows the effects of changes in matching procedures and patient selection on the risk of hip fracture in statin users. Matching by exact year of birth, instead of using a 5 year age band, decreased the differences between the two designs and moved ORs towards 1. Exclusion of patients at high risk of fracture had different effects on ORs: the magnitude of the effect in the SP dataset [adjusted OR for hip fracture of 0.44 (95% CI 0.24–0.79)] increased whereas the opposite was the case in the EP dataset [OR 0.67 (95% CI 0.46–0.98)]. In the original SP case–control dataset, start of follow-up was defined differently between users and non-users of statins and, consequently, the amount of follow-up time used to determine high-risk status differed between users and non-users of statins (means of 1.7 and 4 years, respectively). The inclusion of femur fractures with unspecified location of fracture, the exclusion of patients who were 90 years and older, or matching by duration of follow-up in the SP case–control dataset did not substantially change results.
Effect of changes in matching procedures and patient selection on risk of hip fracture and statin use
| ‘SP’ dataset | ‘EP’ dataset | |||
| Analysis (current statin use time-window) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | ||
| Original analyses2,3 | ||||
| 30 days prior | 0.12 (0.04–0.41) | |||
| 6 months prior | 0.59 (0.31–1.13) | |||
| Fully adjusted model | ||||
| 30 days prior | 0.50 (0.36–0.68) | 0.54 (0.38–0.78) | ||
| 6 months prior | 0.60 (0.45–0.81) | 0.72 (0.54–0.95) | ||
| Exclusion of high-risk patients according to the original Meier analysisa | ||||
| 30 days prior | 0.44 (0.24–0.79) | 0.67 (0.46–0.98) | ||
| 6 months prior | 0.55 (0.32–0.96) | 0.84 (0.62–1.13) | ||
| Matching by same year of birth instead of an up to 5 year expanding age band | ||||
| 30 days prior | 0.72 (0.53–0.99) | 0.67 (0.47–0.97) | ||
| 6 months prior | 0.82 (0.63–1.05) | 0.77 (0.58–1.02) | ||
| Matching by gender, age, and practice but without duration of follow-up | ||||
| 30 days prior | 0.51 (0.39–0.68) | |||
| 6 months prior | 0.60 (0.48–0.75) | |||
| ‘SP’ dataset | ‘EP’ dataset | |||
| Analysis (current statin use time-window) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | ||
| Original analyses2,3 | ||||
| 30 days prior | 0.12 (0.04–0.41) | |||
| 6 months prior | 0.59 (0.31–1.13) | |||
| Fully adjusted model | ||||
| 30 days prior | 0.50 (0.36–0.68) | 0.54 (0.38–0.78) | ||
| 6 months prior | 0.60 (0.45–0.81) | 0.72 (0.54–0.95) | ||
| Exclusion of high-risk patients according to the original Meier analysisa | ||||
| 30 days prior | 0.44 (0.24–0.79) | 0.67 (0.46–0.98) | ||
| 6 months prior | 0.55 (0.32–0.96) | 0.84 (0.62–1.13) | ||
| Matching by same year of birth instead of an up to 5 year expanding age band | ||||
| 30 days prior | 0.72 (0.53–0.99) | 0.67 (0.47–0.97) | ||
| 6 months prior | 0.82 (0.63–1.05) | 0.77 (0.58–1.02) | ||
| Matching by gender, age, and practice but without duration of follow-up | ||||
| 30 days prior | 0.51 (0.39–0.68) | |||
| 6 months prior | 0.60 (0.48–0.75) | |||
Patients with a history of osteoporosis, osteomalacia, alcoholism, cancer (excluding non-melanoma skin cancer), and bisphosphonate use before start of follow-up (‘SP’ dataset) or before the left censoring date (‘EP’ dataset) were excluded.
Effect of changes in matching procedures and patient selection on risk of hip fracture and statin use
| ‘SP’ dataset | ‘EP’ dataset | |||
| Analysis (current statin use time-window) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | ||
| Original analyses2,3 | ||||
| 30 days prior | 0.12 (0.04–0.41) | |||
| 6 months prior | 0.59 (0.31–1.13) | |||
| Fully adjusted model | ||||
| 30 days prior | 0.50 (0.36–0.68) | 0.54 (0.38–0.78) | ||
| 6 months prior | 0.60 (0.45–0.81) | 0.72 (0.54–0.95) | ||
| Exclusion of high-risk patients according to the original Meier analysisa | ||||
| 30 days prior | 0.44 (0.24–0.79) | 0.67 (0.46–0.98) | ||
| 6 months prior | 0.55 (0.32–0.96) | 0.84 (0.62–1.13) | ||
| Matching by same year of birth instead of an up to 5 year expanding age band | ||||
| 30 days prior | 0.72 (0.53–0.99) | 0.67 (0.47–0.97) | ||
| 6 months prior | 0.82 (0.63–1.05) | 0.77 (0.58–1.02) | ||
| Matching by gender, age, and practice but without duration of follow-up | ||||
| 30 days prior | 0.51 (0.39–0.68) | |||
| 6 months prior | 0.60 (0.48–0.75) | |||
| ‘SP’ dataset | ‘EP’ dataset | |||
| Analysis (current statin use time-window) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | ||
| Original analyses2,3 | ||||
| 30 days prior | 0.12 (0.04–0.41) | |||
| 6 months prior | 0.59 (0.31–1.13) | |||
| Fully adjusted model | ||||
| 30 days prior | 0.50 (0.36–0.68) | 0.54 (0.38–0.78) | ||
| 6 months prior | 0.60 (0.45–0.81) | 0.72 (0.54–0.95) | ||
| Exclusion of high-risk patients according to the original Meier analysisa | ||||
| 30 days prior | 0.44 (0.24–0.79) | 0.67 (0.46–0.98) | ||
| 6 months prior | 0.55 (0.32–0.96) | 0.84 (0.62–1.13) | ||
| Matching by same year of birth instead of an up to 5 year expanding age band | ||||
| 30 days prior | 0.72 (0.53–0.99) | 0.67 (0.47–0.97) | ||
| 6 months prior | 0.82 (0.63–1.05) | 0.77 (0.58–1.02) | ||
| Matching by gender, age, and practice but without duration of follow-up | ||||
| 30 days prior | 0.51 (0.39–0.68) | |||
| 6 months prior | 0.60 (0.48–0.75) | |||
Patients with a history of osteoporosis, osteomalacia, alcoholism, cancer (excluding non-melanoma skin cancer), and bisphosphonate use before start of follow-up (‘SP’ dataset) or before the left censoring date (‘EP’ dataset) were excluded.
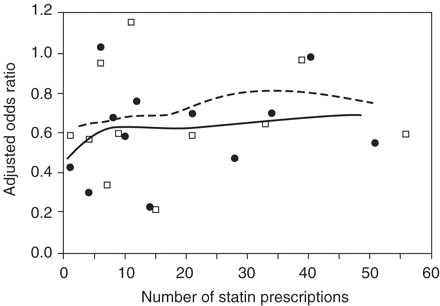
Figure 3 shows the association between the number of statin prescriptions before the index date and the adjusted OR for hip fracture in both study designs. Among current users, the risk of hip fracture was reduced after only one statin prescription (30 days of treatment) and remained stable with increasing numbers of prescriptions.
Spline regression plot of the number of statin prescriptions and risk of hip fracture among patients who used statins 30 days prior to index date. Squares and dashed line: ‘SP’ dataset; Dots and solid line: ‘EP’ dataset
Discussion
We examined methodological reasons for the discrepant results of two previous case–control studies that examined the association between use of statins and risk of fracture in the same database.2,3 We found that the age-band used for matching cases and controls, the selection of potential confounders, the exclusion of high-risk patients, and different definitions for exposure time-windows led to differences in results between the SP and the EP case–control study designs.
We found that results changed substantively with the choice of the exposure time-window. The two case–control studies applied different time-windows for exposure: Meier assessed the statin prescribing in the 30 days prior to the index date, while van Staa used a 6 month period.2,3 The exposure time-window should cover the time-period during which the drug can cause or prevent the outcome, which is related to the lag-time of effect after start of treatment and cessation of effect after treatment discontinuation. The length and timing of this exposure time-window can influence the estimates of exposure risks.13,14 It seems unlikely that statins' effect on the bone occur within 30 days of start of use. In vitro, statins protect bone through a mechanism similar to aminobisphosphonates.15,16 Two large randomized controlled clinical trials reported reductions in hip fracture risk only after 6–12 months of bisphosphonate use.17,18 This suggests that a longer time-window is appropriate in the evaluation of the effects of statins on the bone.
Both studies matched fracture cases to controls by age and used a matching procedure that expanded the difference in age in a stepwise manner, if no control was found. We found that the SP and EP case–control studies yielded different results when using the broader matching for age, but this difference disappeared when matching by year of birth. The SP case–control dataset based on the broad age matching criteria included hip fracture cases that were on average 2 years older than their matched controls in the SP dataset. In contrast there was no difference in age between cases and controls in the EP dataset. The SP case–control study obtained controls from three selected cohorts, which included a much smaller number of patients compared with the total GPRD population used in the EP case–control study. Age is a strong risk factor for fracture risk, and residual confounding may, thus, explain part of the risk reduction observed in the SP case–control study.
In laboratory studies, Mundy et al. found positive effects of statins on the expression of bone morphogenetic protein 2 (BMP-2) and bone growth.1 Following this, several epidemiological studies were conducted. Pooling the results of these observational studies, Bauer et al. reported a summary OR for hip fracture risk in statin users of 0.43 (95% CI 0.25–0.75).19 However, the pooling of the results of randomized clinical trials did not provide strong evidence for a protective effect of statins for any type of fracture (Figure 1). Our study included the largest number of statin users compared with previous research, and we were able to conduct a detailed analysis of the association between duration of statin use and risk of fracture. A decreased risk of hip fracture was found already after one statin prescription and there was no relationship between duration of statin use and size of fracture risk reduction. It seems unlikely that this reduction in risk within 30 days of statin treatment is caused by pharmacological effects of statins. Although Mundy reported an increased bone formation in rodents already after 5 weeks of simvastatin administration,1 this seems implausible in humans given the limited number of bone remodelling sites.
The reduced fracture risk observed in this study might, thus, be due to unknown confounders. It might be related to confounding by socioeconomic status and the ‘healthy drug user’ effect, biases that have been proposed as an explanation for different results of clinical trials and observational research in the study of HRT and coronary heart disease.20–23 Although few studies have examined the association between socioeconomic status and hip fracture risk, a large population-based case–control study suggests that low socioeconomic status is associated with increased risk of hip fracture.24 In addition, in a large cohort study, lower socioeconomic status appeared to be inversely associated with statin use.25 Healthy user bias is likely to have occurred because long-term statin users tend to be healthier and physically more active than non-users.26 Unfortunately, these hypotheses cannot be tested directly in the GPRD, as information on socioeconomic status or ‘healthy drug users’ was not available in GPRD at the time of data collection. However, as of 2006, the GPRD will be using a trusted third party to enable record linkage to other NHS datasets. Such linkage is planned for small-area deprivation indices. Inadequate adjustment for socioeconomic status and healthy user bias are likely explanations for the discrepant findings between observational studies and randomized controlled trials in the study of statin use and fracture risk, but this should be examined in future studies.
The objective of this study was not to exactly replicate the results of the two studies but to test various hypotheses that could explain the discrepant results. For this purpose, we used a single dataset with similar definitions for drug exposures and diagnoses. Our study designs were not identical to those employed by Meier and van Staa, as we did not have access to all computer programs and coding dictionaries. Moreover, restoring the original datasets using the same date ranges was virtually impossible. After July 1999, several general practices have contributed additional data from the period 1987 through 1999, which were not available at the time Meier and van Staa did their analyses. Thus, our study was conducted in a larger and more recent GPRD dataset. As the use of statins has increased dramatically in recent years, the characteristics of statin users may have changed over time. Another limitation was that there was incomplete recording for BMI and smoking, as these data are not part of compulsory data collection in GPRD.
Our results have important methodological implications. Residual confounding by a matching variable may occur when a case–control study is nested within a relatively small cohort, especially when multiple matching variables are used. In case–control studies of drug use and fracture risk, broad matching criteria for age should be avoided and the selection of the time-window for exposure should be carefully considered.
In case–control studies in selected populations, expanding age-band algorithms should be avoided as much as possible when matching by year of birth in healthcare databases.
When an expanding age-band algorithm in the matching procedure is necessary for some reason, sensitivity analysis is highly recommended.
Selection of the time-window for exposure should also be carefully considered, as it may change the risk estimates.
Costs of travel and accommodation for F de Vries were reimbursed by the Netherlands Organization for Health Research and Development (Grant 13800910.82), and the Netherlands Organization for Scientific Research (Grant 2004/03573/CPI).
References
Mundy G, Garrett R, Harris S et al. Stimulation of bone formation in vitro and in rodents by statins.
Meier CR, Schlienger RG, Kraenzlin ME et al. HMG-CoA reductase inhibitors and the risk of fractures.
van Staa TP, Wegman S, de Vries F et al. Use of statins and risk of fractures.
Reid IR, Hague W, Emberson J et al. Effect of pravastatin on frequency of fracture in the LIPID study: secondary analysis of a randomised controlled trial.
Clearfield M, Downs JR, Weis S et al. Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS): efficacy and tolerability of long-term treatment with lovastatin in women.
Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomized placebo-controlled trial.
van Staa TP, Abenhaim L, Leufkens H. A study of the effects of exposure misclassification due to the time-window design in pharmacoepidemiologic studies.
McMahon AD, Evans JM, McGilchrist MM et al. Drug exposure risk windows and unexposed comparator groups for cohort studies in pharmacoepidemiology.
van Beek E, Pieterman E, Cohen L et al. Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates.
Reszka AA, Halasy-Nagy J, Rodan GA. Nitrogen-bisphosphonates block retinoblastoma phosphorylation and cell growth by inhibiting the cholesterol biosynthetic pathway in a keratinocyte model for esophageal irritation.
Black DM, Cummings SR, Karpf DB et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group.
Ensrud KE, Barrett-Connor EL, Schwartz A et al. Fracture Intervention Trial Long-Term Extension Research Group. Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial long-term extension.
Bauer DC, Mundy GR, Jamal SA et al. Use of statins and fracture: results of 4 prospective studies and cumulative meta-analysis of observational studies and controlled trials.
Petitti D. Commentary: Hormone replacement therapy and coronary heart disease: four lessons.
Ray WA, Daugherty JR, Griffin MR. Lipid-lowering agents and the risk of hip fracture in a Medicaid population.
Ray WA. Evaluating medication effects outside of clinical trials: new-user designs.
Humphrey LL, Chan BK, Sox HC. Postmenopausal hormone replacement therapy and the primary prevention of cardiovascular disease.
Farahmand BY, Persson PG, Michaelsson K et al. Socioeconomic status, marital status and hip fracture risk: a population-based case-control study.
Ko DT, Mamdani M, Alter DA. Lipid-lowering therapy with statins in high-risk elderly patients: the treatment-risk paradox.