-
PDF
- Split View
-
Views
-
Cite
Cite
Melinda K. Munos, Christa L Fischer Walker, Robert E Black, The effect of oral rehydration solution and recommended home fluids on diarrhoea mortality, International Journal of Epidemiology, Volume 39, Issue suppl_1, April 2010, Pages i75–i87, https://doi.org/10.1093/ije/dyq025
Close - Share Icon Share
Abstract
Background Most diarrhoeal deaths can be prevented through the prevention and treatment of dehydration. Oral rehydration solution (ORS) and recommended home fluids (RHFs) have been recommended since 1970s and 1980s to prevent and treat diarrhoeal dehydration. We sought to estimate the effects of these interventions on diarrhoea mortality in children aged <5 years.
Methods We conducted a systematic review to identify studies evaluating the efficacy and effectiveness of ORS and RHFs and abstracted study characteristics and outcome measures into standardized tables. We categorized the evidence by intervention and outcome, conducted meta-analyses for all outcomes with two or more data points and graded the quality of the evidence supporting each outcome. The CHERG Rules for Evidence Review were used to estimate the effectiveness of ORS and RHFs against diarrhoea mortality.
Results We identified 205 papers for abstraction, of which 157 were included in the meta-analyses of ORS outcomes and 12 were included in the meta-analyses of RHF outcomes. We estimated that ORS may prevent 93% of diarrhoea deaths.
Conclusions ORS is effective against diarrhoea mortality in home, community and facility settings; however, there is insufficient evidence to estimate the effectiveness of RHFs against diarrhoea mortality.
Background
Diarrhoeal diseases are a leading cause of mortality in children aged <5 years, accounting for 1.7 million deaths annually.1 Because the immediate cause of death in most cases is dehydration, these deaths are almost entirely preventable if dehydration is prevented or treated. Until 1970s, intravenous (IV) infusion of fluids and electrolytes was the treatment of choice for diarrhoeal dehydration, but was expensive and impractical to use in low-resource settings. In 1960s and 1970s, physicians working in South Asia developed a simple oral rehydration solution (ORS) containing glucose and electrolytes that could be used to prevent and treat dehydration due to diarrhoea of any aetiology and in patients of all ages.2–8
At the time ORS was developed, placebo-controlled trials would have been unethical given the efficacy of IV therapy, and to our knowledge none exists. The randomized controlled trials (RCTs) of ORS conducted to confirm its efficacy instead used a comparator such as IV therapy or alternative formulations of ORS. Similarly, studies conducted in community settings to assess the effectiveness of ORS did not actively withhold ORS from the comparison area but instead evaluated the effectiveness of promotional campaigns or alternative delivery systems compared with routine health system delivery.
In 1970s, the World Health Organization (WHO) recommended that an ORS formulation with total osmolarity 311 mmol/l be used for prevention and treatment of diarrhoeal dehydration. However, alternative formulations continued to be investigated in an attempt to develop an ORS formulation that would decrease stool output or have other clinical benefits. These efforts led, in 2004, WHO to recommend low osmolarity ORS (with total osmolarity of 245 mmol/l and reduced levels of glucose and sodium), which was associated with reduced need for unscheduled IV therapy, decreased stool output and less vomiting when compared with the original formulation.9,10
As countries launched diarrhoeal disease control programmes to roll out ORS, they faced difficulties in ensuring access and achieving high coverage levels, in part due to inadequate manufacturing capacity. In an effort to improve provision of fluids in early diarrhoea episodes to prevent the development of dehydration, diarrhoeal disease control programmes promoted the use of additional fluids and home-made solutions such as rice water and sugar salt solution [collectively referred to as recommended home fluids (RHFs)].11,12 RHFs were eventually incorporated into the WHO recommendations for prevention of dehydration. Unfortunately, over the years, programmes have combined ORS and RHFs into a general and poorly defined oral rehydration therapy (ORT) category in which the respective roles of ORS and RHFs are not well delineated.
Recent Cochrane reviews have estimated the efficacy of ORS compared with IV therapy, and reduced osmolarity ORS compared with original ORS, against treatment failure.10,13 Additionally, in 1998, a Cochrane review examined the effect of rice-based, compared with glucose-based, ORS on stool output and duration of diarrhea.14 However, these reviews focused on RCTs of ORS conducted in hospitals or clinical settings and did not examine mortality as an outcome or the broader category of RHFs as an intervention. To our knowledge, there are no systematic reviews or corresponding meta-analyses assessing the effect of ORS or RHFs on diarrhoea-specific mortality. We present evidence from systematic reviews and meta-analyses drawing upon data from community- and facility-based studies to estimate the effectiveness of ORS, and, separately, RHFs on diarrhoea morbidity and mortality in children aged <5 years. We then correlate the effectiveness estimates with achieved coverage levels to generate an estimate of the effect of each intervention on cause-specific mortality.
Methods
Per CHERG Guidelines, we systematically reviewed published literature from PubMed, the Cochrane Libraries, and the WHO Regional Databases to identify studies examining the effect of oral rehydration strategies on diarrhoea morbidity and mortality in children aged <5 years. Search terms included combinations of diarrhea, dysentery, rotavirus, fluid therapy, oral rehydration solution, oral rehydration therapy, recommended home fluid and sugar salt solution. We limited the search to studies that included children aged <5 years and studies in English, French, Spanish, Portuguese and Italian.
Inclusion/exclusion criteria and definitions
For the purposes of this review, we defined and reviewed three categories of oral rehydration strategies: ORS, RHFs and ORT. We defined ORS as an electrolyte solution containing sodium, chloride, potassium, bicarbonate or citrate and glucose or another form of sugar or starch. Formulations containing small amounts of other minerals, such as magnesium, were included in this category, but solutions containing amino acids such as glycine or alanine were excluded. We also excluded solutions containing zinc, because it was not possible to separate the effects of ORS and zinc on diarrhoea morbidity. Both reduced osmolarity ORS (total osmolarity ≤250 mmol/l, per Hahn10) and higher osmolarity ORS (up to 370 mmol/l) were included in our review.
For RHFs, we included all possible home fluid alternatives in our review, including sugar–salt solution, cereal–salt solution, rice–water solution and additional fluids such as plain water, juice, tea or rice water. If the study intervention was promotion or provision of both ORS and RHF in the same area and the effects and coverage estimates of the two interventions could not be separated, we categorized it as ORT. Such studies were generally large-scale programme evaluations.
We included quasi-experimental, pre/post, observational and randomized and cluster-randomized trials reporting any of the following outcomes for children aged <5 years: all-cause or diarrhoea-specific mortality, diarrhoea hospitalizations, referrals to hospitals or health centres for diarrhoea treatment, or treatment failure. Studies in developed countries were excluded unless conducted in a low-resource setting, such as native American reservations. Treatment failure was generally defined as the need for unscheduled IV therapy, but we accepted other definitions provided that they reflected failure of the therapy to produce or maintain clinical improvement in the subjects’ dehydration state. Studies that defined treatment failure in terms of stool volume or duration of diarrhoea were excluded.
Abstraction
We abstracted all studies meeting our acceptance criteria into standardized tables, categorized by outcome, study design and type of oral rehydration strategy. Abstracted variables included study identifiers and context, design and limitations, population, characteristics of the intervention and control and outcome measures. Based on these data, we graded each study according to the CHERG adaptation of the GRADE technique.15 Scores were decreased by half a grade for each design limitation; observational studies, including pre/post studies, received a very low grade.
Analysis
We summarized the evidence for ORS and RHFs by outcome in a separate table and graded the quality of evidence for each outcome, decreasing the score for observational study designs, heterogeneity of study outcomes or lack of generalizability of the study populations or interventions. We did not produce a summary table for ORT because our goal was to generate estimates of the individual rather than joint effectiveness of ORS and RHFs, as each has different roles in diarrhoea management. For each outcome with more than one study, we conducted both fixed and random effects meta-analyses.
Mortality
For diarrhoea-specific mortality, we included randomized, cluster-randomized and quasi-experimental studies in the meta-analysis. Observational studies (other than quasi-experimental studies) that did not control for confounding were excluded, as were case–control studies and those that did not provide an adequate estimate of coverage in the intervention arm. We defined coverage as the proportion of diarrhoea episodes in children aged <5 years for which the child received ORS or RHFs. We excluded studies in which a comparator or alternative delivery system was used in the comparison arm, because the resulting relative risk did not provide meaningful information on the effect of ORS or RHFs on diarrhoea morbidity or mortality. For ORS and, separately, RHFs, we reported the pooled relative reduction in diarrhoea-specific mortality and 95% confidence interval (CI). In the case of heterogeneity, we reported the DerSimonian–Laird pooled relative reduction and 95% CI.
Treatment failure
Observational studies and RCTs of ORS or RHFs assessing treatment failure, including those that used a comparator, were included in the meta-analysis. For included studies, instead of a relative risk, we analysed the absolute treatment failure rate for each therapy that met our inclusion criteria. We conducted separate and combined meta-analyses for RCTs and observational studies and reported the Mantel–Haenszel pooled failure rate and 95% CI, or the DerSimonian–Laird pooled failure rate and 95% CI if there was evidence of heterogeneity for each.
Hospitalization/referral
The clinical guidelines and processes for hospitalization and referral in the studies we identified were variables and often not well described. Moreover, this outcome can be confounded by differences in care-seeking or referral practices between study arms, particularly in quasi-experimental studies, and the studies included in our review did not adequately address or adjust for this possibility. Given these considerations, a meta-analysis was not appropriate for this outcome, and none was conducted.
Overall effectiveness estimate
Applying the CHERG Rules for Evidence Review,15 for each outcome, we considered the quality of the evidence, number of events and generalizability of the study population and outcome to diarrhoea-specific mortality to estimate the effects of ORS and RHFs on diarrhoea mortality in children aged <5 years.
Results
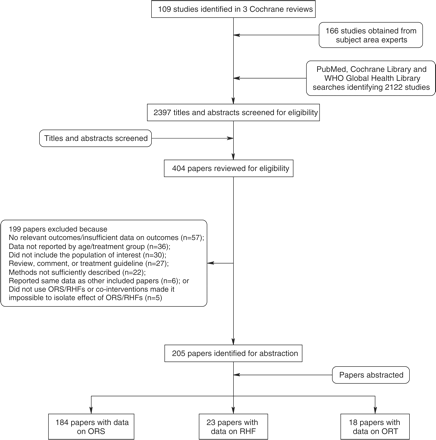
Our searches identified 2397 titles; after screening titles and abstracts, we reviewed 404 papers for our inclusion/exclusion criteria and outcomes of interest (Figure 1). We abstracted 205 papers into our final database: 184 reporting data on ORS, 23 on RHFs and 18 on ORT as defined above. For the outcomes of diarrhoea and all-cause mortality, we excluded many papers in our final database from the meta-analysis because they used observational study designs and did not control for confounding, did not report an adequate coverage indicator, had no relevant comparison group or used a case–control design. These studies are shown in Supplementary tables 1 and 2, but were not included in the meta-analyses or summary tables (Supplementary tables 1 and 2).
ORS
We identified 21 papers reporting diarrhoea-specific mortality, 3 reporting all-cause mortality, 20 reporting hospitalization/referral and 155 reporting treatment failure that met our inclusion/exclusion criteria (Supplementary table 1). Of these, three papers reporting diarrhoea mortality and 153 reporting treatment failure were included in the meta-analyses. Table 1 presents the summary characteristics of these studies and meta-analysis results by outcome.
Quality assessment of trials of ORS
| No. of studies . | Quality assessmenta . | Summary of findings . | Comments . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Directness . | No. of events . | Effect . | ||||
| Design . | Limitations . | Consistency . | Generalizability to population of interest . | Generalizability to intervention of interest . | Intervention . | Control . | Relative reduction (95% CI) . | ||
| Diarrhoea mortality rate: low outcome-specific quality | |||||||||
| Three18–20 | Quasi experimental (−1) | No adjustment for confounding variables (−0.5); ORS used in control arms in most cases, but at a lower rate than in intervention arms | Consistent benefit from all studies | India, Bangladesh and Burma (−0.5) | No studies used reduced osmolarity ORS | 27/11696 child-years | 41/5295 child years | 69% (51–80%) | Fixed effect meta-analysis |
| Treatment failure: moderate outcome-specific quality | |||||||||
| 153 total | RCTs and observational | No true control arm; most studies are hospital based; many observational (−0.5) | Heterogeneity from meta-analysis (−0.5) | South/Southeast Asia, Eastern Mediterranean, Latin America, North/South/East/West Africa, Eastern Europe and Apache reservations in USA | About 26 used low osmolarity ORS About 26 used rice- or cereal-based ORS | 1283/18 084 episodes | NA | 0.2%* (0.1–0.2%) | *Pooled failure rate Random effects meta-analysis |
| 10421−124 | RCTs | No true control arm; most studies hospital based | Heterogeneity from meta-analysis (−0.5) | South/Southeast Asia, Eastern Mediterranean, Latin America and North/West/East Africa | About 22 used low osmolarity ORS (total osmolarity ≤ 250 mmol/L); approximately 26 used rice- or cereal-based ORS | 734/9449 episodes | NA | 0.2%* (0.1–0.2%) | *Pooled failure rate Random effects meta-analysis |
| 49125–172 (DEC Ashley et al., Unpublished, 1980.) | Observational | No adjustment for confounding | Heterogeneity from meta-analysis (−0.5) | Latin America, South/Southeast Asia, Eastern Mediterranean, North/South/East/ West Africa, Eastern Europe and Apache reservations in USA | About four studies used low-osmolarity ORS | 549/8635 episodes | NA | 0.3%* (0.2–0.4%) | *Pooled failure rate Random effects meta-analysis |
| No. of studies . | Quality assessmenta . | Summary of findings . | Comments . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Directness . | No. of events . | Effect . | ||||
| Design . | Limitations . | Consistency . | Generalizability to population of interest . | Generalizability to intervention of interest . | Intervention . | Control . | Relative reduction (95% CI) . | ||
| Diarrhoea mortality rate: low outcome-specific quality | |||||||||
| Three18–20 | Quasi experimental (−1) | No adjustment for confounding variables (−0.5); ORS used in control arms in most cases, but at a lower rate than in intervention arms | Consistent benefit from all studies | India, Bangladesh and Burma (−0.5) | No studies used reduced osmolarity ORS | 27/11696 child-years | 41/5295 child years | 69% (51–80%) | Fixed effect meta-analysis |
| Treatment failure: moderate outcome-specific quality | |||||||||
| 153 total | RCTs and observational | No true control arm; most studies are hospital based; many observational (−0.5) | Heterogeneity from meta-analysis (−0.5) | South/Southeast Asia, Eastern Mediterranean, Latin America, North/South/East/West Africa, Eastern Europe and Apache reservations in USA | About 26 used low osmolarity ORS About 26 used rice- or cereal-based ORS | 1283/18 084 episodes | NA | 0.2%* (0.1–0.2%) | *Pooled failure rate Random effects meta-analysis |
| 10421−124 | RCTs | No true control arm; most studies hospital based | Heterogeneity from meta-analysis (−0.5) | South/Southeast Asia, Eastern Mediterranean, Latin America and North/West/East Africa | About 22 used low osmolarity ORS (total osmolarity ≤ 250 mmol/L); approximately 26 used rice- or cereal-based ORS | 734/9449 episodes | NA | 0.2%* (0.1–0.2%) | *Pooled failure rate Random effects meta-analysis |
| 49125–172 (DEC Ashley et al., Unpublished, 1980.) | Observational | No adjustment for confounding | Heterogeneity from meta-analysis (−0.5) | Latin America, South/Southeast Asia, Eastern Mediterranean, North/South/East/ West Africa, Eastern Europe and Apache reservations in USA | About four studies used low-osmolarity ORS | 549/8635 episodes | NA | 0.3%* (0.2–0.4%) | *Pooled failure rate Random effects meta-analysis |
aSee Walker et al.15 for a description of the quality assessment and grading methods.
Quality assessment of trials of ORS
| No. of studies . | Quality assessmenta . | Summary of findings . | Comments . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Directness . | No. of events . | Effect . | ||||
| Design . | Limitations . | Consistency . | Generalizability to population of interest . | Generalizability to intervention of interest . | Intervention . | Control . | Relative reduction (95% CI) . | ||
| Diarrhoea mortality rate: low outcome-specific quality | |||||||||
| Three18–20 | Quasi experimental (−1) | No adjustment for confounding variables (−0.5); ORS used in control arms in most cases, but at a lower rate than in intervention arms | Consistent benefit from all studies | India, Bangladesh and Burma (−0.5) | No studies used reduced osmolarity ORS | 27/11696 child-years | 41/5295 child years | 69% (51–80%) | Fixed effect meta-analysis |
| Treatment failure: moderate outcome-specific quality | |||||||||
| 153 total | RCTs and observational | No true control arm; most studies are hospital based; many observational (−0.5) | Heterogeneity from meta-analysis (−0.5) | South/Southeast Asia, Eastern Mediterranean, Latin America, North/South/East/West Africa, Eastern Europe and Apache reservations in USA | About 26 used low osmolarity ORS About 26 used rice- or cereal-based ORS | 1283/18 084 episodes | NA | 0.2%* (0.1–0.2%) | *Pooled failure rate Random effects meta-analysis |
| 10421−124 | RCTs | No true control arm; most studies hospital based | Heterogeneity from meta-analysis (−0.5) | South/Southeast Asia, Eastern Mediterranean, Latin America and North/West/East Africa | About 22 used low osmolarity ORS (total osmolarity ≤ 250 mmol/L); approximately 26 used rice- or cereal-based ORS | 734/9449 episodes | NA | 0.2%* (0.1–0.2%) | *Pooled failure rate Random effects meta-analysis |
| 49125–172 (DEC Ashley et al., Unpublished, 1980.) | Observational | No adjustment for confounding | Heterogeneity from meta-analysis (−0.5) | Latin America, South/Southeast Asia, Eastern Mediterranean, North/South/East/ West Africa, Eastern Europe and Apache reservations in USA | About four studies used low-osmolarity ORS | 549/8635 episodes | NA | 0.3%* (0.2–0.4%) | *Pooled failure rate Random effects meta-analysis |
| No. of studies . | Quality assessmenta . | Summary of findings . | Comments . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Directness . | No. of events . | Effect . | ||||
| Design . | Limitations . | Consistency . | Generalizability to population of interest . | Generalizability to intervention of interest . | Intervention . | Control . | Relative reduction (95% CI) . | ||
| Diarrhoea mortality rate: low outcome-specific quality | |||||||||
| Three18–20 | Quasi experimental (−1) | No adjustment for confounding variables (−0.5); ORS used in control arms in most cases, but at a lower rate than in intervention arms | Consistent benefit from all studies | India, Bangladesh and Burma (−0.5) | No studies used reduced osmolarity ORS | 27/11696 child-years | 41/5295 child years | 69% (51–80%) | Fixed effect meta-analysis |
| Treatment failure: moderate outcome-specific quality | |||||||||
| 153 total | RCTs and observational | No true control arm; most studies are hospital based; many observational (−0.5) | Heterogeneity from meta-analysis (−0.5) | South/Southeast Asia, Eastern Mediterranean, Latin America, North/South/East/West Africa, Eastern Europe and Apache reservations in USA | About 26 used low osmolarity ORS About 26 used rice- or cereal-based ORS | 1283/18 084 episodes | NA | 0.2%* (0.1–0.2%) | *Pooled failure rate Random effects meta-analysis |
| 10421−124 | RCTs | No true control arm; most studies hospital based | Heterogeneity from meta-analysis (−0.5) | South/Southeast Asia, Eastern Mediterranean, Latin America and North/West/East Africa | About 22 used low osmolarity ORS (total osmolarity ≤ 250 mmol/L); approximately 26 used rice- or cereal-based ORS | 734/9449 episodes | NA | 0.2%* (0.1–0.2%) | *Pooled failure rate Random effects meta-analysis |
| 49125–172 (DEC Ashley et al., Unpublished, 1980.) | Observational | No adjustment for confounding | Heterogeneity from meta-analysis (−0.5) | Latin America, South/Southeast Asia, Eastern Mediterranean, North/South/East/ West Africa, Eastern Europe and Apache reservations in USA | About four studies used low-osmolarity ORS | 549/8635 episodes | NA | 0.3%* (0.2–0.4%) | *Pooled failure rate Random effects meta-analysis |
aSee Walker et al.15 for a description of the quality assessment and grading methods.
For the outcomes of diarrhoea mortality and treatment failure, there was evidence to support the effectiveness of ORS. A fixed effect meta-analysis showed a 69% (95% CI: 51–80%) pooled relative reduction in diarrhoea mortality in communities in which ORS was promoted compared with comparison areas, with no indication of heterogeneity (Table 1). A random effects meta-analysis similarly showed a very low-pooled treatment failure rate (0.2%; 95% CI: 0.1–0.2%) for ORS. Studies reporting treatment failure were almost universally conducted in hospital or clinic settings.
For the outcome of hospitalization/referral, 6 of 20 studies included a relevant comparison arm: five quasi-experimental studies and one pre/post study. Of the five quasi-experimental studies, two did not clearly report the coverage achieved, one provided regular home visits by nurses and a health education component in the intervention but not comparison arms and none discussed care-seeking practices in intervention and control areas. The outcomes of these studies were mixed, with two studies reporting increases in hospitalization in the intervention relative to the control area, whereas the other studies reported 47–57% relative decrease in hospitalization and 29–89% relative decreases in referrals to health centres in the intervention areas.
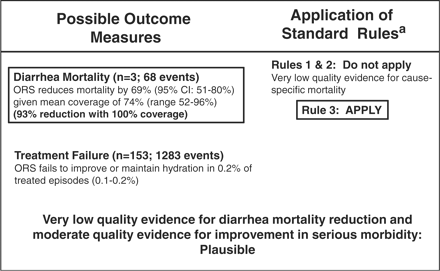
We applied the CHERG Rules for Evidence Review15 to the evidence presented in Table 1. We used the pooled effect size for diarrhoea mortality, as it was more conservative than the effect size for severe morbidity (treatment failure). The mean and median coverage levels in the intervention arms of the diarrhoea mortality studies were 74%; assuming a linear relationship between coverage and mortality reduction, at 100% coverage a 93% relative reduction in diarrhoea mortality would be expected (Figure 2).
Application of standardized rules for choice of final outcome to estimate effect of ORS on the reduction of diarrhoea mortality. aSee Walker et al.15 for a description of the CHERG Rules for Evidence Review
RHFs
We identified and abstracted five studies reporting diarrhoea mortality, one reporting all-cause mortality, five reporting hospitalization or referral and 14 reporting treatment failure for RHFs (Supplementary table 2). For the outcomes of diarrhoea and all-cause mortality, no studies met the required study quality criteria for inclusion in the meta-analyses.
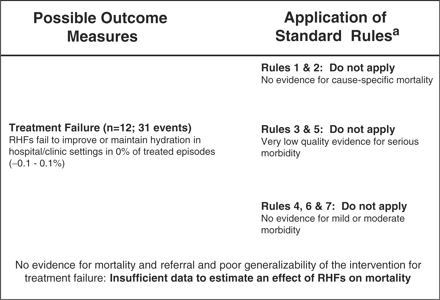
We included 12 studies in the meta-analysis of treatment failure and found a pooled failure rate of 0% (95% CI: −0.1 to 0.1%) (Table 2). However, each of these studies included dehydrated patients and was conducted in a hospital or clinic setting. Studies assessed sugar solution and sugar- or cereal–salt solutions; none assessed other RHFs such as plain water or rice water. Due to the low quality of evidence (i.e. no community-based studies) for serious morbidity and the lack of well-designed studies assessing the effect of RHFs on mortality, we did not estimate an effect of RHFs on diarrhoea mortality (Figure 3).
Quality assessment and summary outcomes of trials of RHFs
| . | Quality assessmenta . | Summary of findings . | Comments . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | Directness . | No of events . | Effect . | |||
| No of studies . | Design . | Limitations . | Consistency . | Generalizability to population of interest . | Generalizability to intervention of interest . | Intervention . | Control . | Relative reduction (95% CI) . | |
| Treatment failure: low/very low outcome-specific quality | |||||||||
| 1227,29,39, 40,53,67, 73,87,98, 118,123,173 | RCTs | Studies clinic- or hospital based (−0.5); no true control arm | Heterogeneity from meta- analysis (−0.5) | South/Southeast Asia, North/West/East Africa and Latin America. RHFs not tested in the home (−1) | Nine used cereal/ sugar–salt solution; one used sugar solution; two did not define RHF (−0.5) | 31/784 episodes | NA | 0* (−0.1, 0.1%) | *Pooled failure rate Random effects meta-analysis |
| . | Quality assessmenta . | Summary of findings . | Comments . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | Directness . | No of events . | Effect . | |||
| No of studies . | Design . | Limitations . | Consistency . | Generalizability to population of interest . | Generalizability to intervention of interest . | Intervention . | Control . | Relative reduction (95% CI) . | |
| Treatment failure: low/very low outcome-specific quality | |||||||||
| 1227,29,39, 40,53,67, 73,87,98, 118,123,173 | RCTs | Studies clinic- or hospital based (−0.5); no true control arm | Heterogeneity from meta- analysis (−0.5) | South/Southeast Asia, North/West/East Africa and Latin America. RHFs not tested in the home (−1) | Nine used cereal/ sugar–salt solution; one used sugar solution; two did not define RHF (−0.5) | 31/784 episodes | NA | 0* (−0.1, 0.1%) | *Pooled failure rate Random effects meta-analysis |
aSee Walker et al.15 for a description of the quality assessment and grading methods.
Quality assessment and summary outcomes of trials of RHFs
| . | Quality assessmenta . | Summary of findings . | Comments . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | Directness . | No of events . | Effect . | |||
| No of studies . | Design . | Limitations . | Consistency . | Generalizability to population of interest . | Generalizability to intervention of interest . | Intervention . | Control . | Relative reduction (95% CI) . | |
| Treatment failure: low/very low outcome-specific quality | |||||||||
| 1227,29,39, 40,53,67, 73,87,98, 118,123,173 | RCTs | Studies clinic- or hospital based (−0.5); no true control arm | Heterogeneity from meta- analysis (−0.5) | South/Southeast Asia, North/West/East Africa and Latin America. RHFs not tested in the home (−1) | Nine used cereal/ sugar–salt solution; one used sugar solution; two did not define RHF (−0.5) | 31/784 episodes | NA | 0* (−0.1, 0.1%) | *Pooled failure rate Random effects meta-analysis |
| . | Quality assessmenta . | Summary of findings . | Comments . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | Directness . | No of events . | Effect . | |||
| No of studies . | Design . | Limitations . | Consistency . | Generalizability to population of interest . | Generalizability to intervention of interest . | Intervention . | Control . | Relative reduction (95% CI) . | |
| Treatment failure: low/very low outcome-specific quality | |||||||||
| 1227,29,39, 40,53,67, 73,87,98, 118,123,173 | RCTs | Studies clinic- or hospital based (−0.5); no true control arm | Heterogeneity from meta- analysis (−0.5) | South/Southeast Asia, North/West/East Africa and Latin America. RHFs not tested in the home (−1) | Nine used cereal/ sugar–salt solution; one used sugar solution; two did not define RHF (−0.5) | 31/784 episodes | NA | 0* (−0.1, 0.1%) | *Pooled failure rate Random effects meta-analysis |
aSee Walker et al.15 for a description of the quality assessment and grading methods.
Application of standardized rules for choice of final outcome to estimate effect of RHFs on the reduction of diarrhoea mortality (aSee Walker et al.15 for a description of the CHERG Rules for Evidence Review.)
ORT
We found 14 studies reporting diarrhoea mortality, one reporting all-cause mortality and five reporting dehydration or treatment failure for ORT that met our inclusion/exclusion criteria (Supplementary table 3). The studies abstracted are suggestive of an effect of joint promotion of ORS and/or RHF on diarrhoea mortality. Of the studies reporting mortality data with comparison groups (including historical comparison groups), all reported a decline in diarrhoea or all-cause mortality, although the magnitude of the declines, time periods over which they occurred and the associated coverage levels varied greatly. These studies primarily used pre/post designs and in most cases did not adjust for confounding; thus, it is not possible to determine whether the declines were causally associated with the use of ORS and RHFs, or with other interventions and changes occurring in the community during the same time period.
Discussion
We found a large body of evidence evaluating the efficacy and effectiveness of ORS, and a more limited number of studies assessing RHFs. Based on this evidence, we estimated that ORS may reduce diarrhoea mortality by up to 93%, but were unable to estimate the effectiveness of RHFs against diarrhoea mortality because no studies were conducted outside hospital setting, which is inconsistent with the definition of ‘home fluids’ (Figures 2 and 3). Whereas the overall quality of evidence supporting the effectiveness of ORS against diarrhoea mortality was low as a result of non-randomized study designs, our conclusions are strengthened by the consistency of the effect size and direction among the studies included and those excluded from the meta-analysis. Moreover, the biological basis for ORS, co-transport of glucose and sodium across the epithelial layer in the small intestine is well established and supports a protective effect of ORS against fluid losses and electrolyte imbalances.16,17
We correlated ORS effectiveness with coverage, using the absolute coverage levels reported for the intervention arms. However, in most community-based studies, ORS was also available and used at a low level in the comparison arms. The effective coverage level (difference in coverage between the intervention and comparison arms) was thus lower than the absolute coverage level used in our calculations. For this reason, our approach is conservative and likely overestimates the coverage needed to achieve a particular mortality reduction.
RHFs were not designed as an intervention to directly decrease diarrhoea mortality, but were instead intended to be used for home-based fluid management to prevent dehydration, with possible indirect effects on mortality. However, the only well-controlled studies of RHFs were conducted in hospital settings and included only sugar–salt solution and cereal–salt solution. Whereas we included these studies in our meta-analysis of RHF treatment failure, the results cannot be generalized to the administration of RHFs by a caregiver in the home and cannot be assumed to be representative of all current RHFs. Community-based studies of RHFs, which are not only inherently less controlled but also more relevant than hospital-based studies, have been conducted and were abstracted into Supplementary table 2, but either did not include a relevant comparison arm or failed to adequately document the coverage achieved, making it difficult to interpret their results. Moreover, we were unable to find studies meeting our inclusion criteria that assessed other RHFs such as water and rice water. Thus, our findings may not be representative of the full range of RHFs.
ORS is a simple, proven intervention that can be used at the community and facility level to prevent and treat diarrhoeal dehydration and decrease diarrhoea mortality. Whereas ORS is highly effective, coverage levels remain low in most countries. It is essential that ORS coverage be increased to achieve reductions in diarrhoea mortality; to do so, operations and implementation research is needed to better understand how to deliver ORS effectively and promote its use at home and facility level as part of appropriate case management of diarrhoea.
In contrast to ORS, RHFs were designed and recommended as a home-based intervention to prevent diarrhoeal dehydration, but this message has become confused as diarrhoea control programmes have evolved. Moreover, for RHFs to be used appropriately at home, caregivers must be able to assess whether a child is dehydrated and correctly determine whether to provide RHFs or ORS. Thus, whereas there is evidence suggesting that RHFs may be effective in preventing dehydration, its correct implementation and the associated behaviour change communication messages are complex. From a programmatic perspective, promoting the use of ORS for all diarrhoea episodes might, therefore, be both simpler and more effective than promoting ORS and RHFs as a package and teaching caregivers when and how to use each.
Supplementary data
Supplementary data are available here.
Funding
US Fund for UNICEF from the Bill & Melinda Gates Foundation (grant 43386 to ‘Promote evidence-based decision making in designing maternal, neonatal and child health interventions in low- and middle-income countries’). MKM is supported by a training grant from the U.S. National Institutes of Health (grant T32HD046405 for ‘International Maternal and Child Health’).
Acknowledgement
We thank our colleagues at WHO and UNICEF for their review of the manuscript and valuable feedback.
Conflict of interest: none declared.