-
PDF
- Split View
-
Views
-
Cite
Cite
Duncheng Wang, Shinu A. John, James L. Clements, Dean H. Percy, Kevin P. Barton, Lee Ann Garrett-Sinha, Ets-1 deficiency leads to altered B cell differentiation, hyperresponsiveness to TLR9 and autoimmune disease, International Immunology, Volume 17, Issue 9, September 2005, Pages 1179–1191, https://doi.org/10.1093/intimm/dxh295
Close - Share Icon Share
Abstract
It has been shown that mice with a targeted mutation in the Ets-1 gene exhibit increased B cell terminal differentiation to IgM-secreting plasma cells. Here, we show that mice, formerly described to lack Ets-1 protein, actually express low levels of an internally deleted Ets-1 protein. Mice harboring this Ets-1 hypomorphic allele possess very few marginal zone B cells and have increased expression of activation markers on follicular B cells. Adoptive transfer experiments indicate that this activated phenotype can be reversed upon transfer of Ets-1-deficient B cells to a wild-type host, suggesting a role for B cell-extrinsic factors in regulating the activated state. Supporting this observation, the reverse transfer experiment of wild-type B cells into an Ets-1-deficient host resulted in increased expression of activation markers on the transferred B cells. However, there are also cell-intrinsic changes in Ets-1-deficient B cells as demonstrated by their increased differentiation to plasma cells in vitro in response to stimulation with cytosine-phosphate-guanine DNA sequence-containing oligodeoxynucleotide [CpG DNA, a Toll-like receptor (TLR) 9 ligand]. Consistent with the activated phenotype and increased terminal differentiation of Ets-1-deficient B cells, Ets-1 mutant mice develop autoimmune disease. Hence, our studies establish Ets-1 as an important regulator of peripheral B cell differentiation and B cell responses to TLR9 activation.
Introduction
Terminal differentiation of B cells to Ig-secreting plasma cells is a critical step in the immune response. However, excessive B cell activation and differentiation can contribute to autoimmune disease. Thus, the formation of antibody-secreting plasma cells is tightly regulated, in part by the action of particular transcription factors. The transcription factors BCL-6, Pax-5 and Mitf negatively regulate the differentiation of plasma cells from precursors (1–4). The transcription factor Ets-1 also appears to play a role in restricting plasmacytic differentiation since Ets-1-deficient mice have an excess of IgM-secreting plasma cells (5–8).
Ets transcription factors bind DNA via a winged helix-turn-helix motif known as the Ets domain. In addition, several Ets proteins including Ets-1 contain a conserved region known as the ‘Pointed’ domain that is involved in protein–protein interactions. In Ets-1, the Pointed domain serves as a docking site for extracellular regulated kinases (Erk) kinases, which subsequently phosphorylate the protein at threonine 38 located upstream of the Pointed domain (9). The phosphorylation of this conserved residue is correlated with enhanced transcriptional activity of Ets-1 (10) by promoting the recruitment of co-activators such as p300/CREB binding protein (CBP) (11).
In adult mice, high levels of Ets-1 expression are restricted to lymphoid organs (12, 13), suggesting that Ets-1 plays an important role in the development or functional differentiation of lymphoid cells. Indeed, the generation of Ets-1-deficient mice by two separate research groups (resulting in two Ets-1-targeted alleles) confirmed an important role for Ets-1 in the differentiation of B cells, T cells, NK cells and NK T cells (5–8, 14, 15). In one Ets-1 gene-targeted allele, the third and fourth exons of the gene, encoding the Pointed domain, were deleted (6), whereas in the other allele, the last two exons (exons 8 and 9), encoding the Ets DNA-binding domain, were deleted (5).
Similar defects in lymphoid cell development were observed in mice with both the Ets-1-targeted alleles. The total number of B cells was within normal limits in Ets-1-deficient mice, but some B cells had a B220loIgM+ phenotype (5–8). Increased numbers of IgM-secreting plasma cells were detected in the peripheral lymphoid organs and serum IgM levels were increased by 5- to 10-fold in Ets-1-deficient mice as compared with control mice (5, 7, 8). Taken together, these data indicate that Ets-1 is not essential for the development of B cells, but it does play an important role in limiting their terminal differentiation. In this report, we further explore the requirement for Ets-1 in regulating B cell differentiation and functional responses.
Methods
Mice
Mice utilized in this study have been previously described (7, 14) and possess a mixed genetic background (C57BL/6 and 129Sv). Mice were housed in a conventional facility, but maintained in microisolator cages, fed autoclaved food and water and handled only in biosafety cabinets.
Reverse transcriptase–PCR analysis of Ets-1
To clone the mutant version of Ets-1 expressed by Ets-1 gene-targeted mice, we designed primers flanking the open reading frame of the murine Ets-1 cDNA (forward primer: CGCCGGCCCCGTCGACTCCGGGCACCATG, reverse primer: GCAGGTTCCCGCGGCCGCTTCCTTCTG). These primers contain SalI and NotI restriction sites that allowed us to clone the resulting products in-frame with the hemagglutinin (HA) epitope tag of the vector pCMV-HA (BD Biosciences, San Diego, CA, USA). RNA isolated from purified B cells was reverse transcribed and amplified using Taq polymerase with the primers described above. PCR products were then subcloned into pCMV-HA and sequenced to confirm their identity.
Immunoblotting
B cells were purified from the spleens of mice using CD43 microbeads and a VarioMACS magnetic column (Miltenyi Biotec, Auburn, CA, USA) to isolate a negatively selected population. B cell lysates were separated on SDS PAGE gels and transferred to polyvinylidene fluoride (PVDF) membranes. Antibodies used in western blotting were rabbit polyclonal anti-mouse Ets-1 (C-20) from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and mouse anti-β-tubulin (clone KMX-1) from Chemicon (Temecula, CA, USA).
Flow cytometry
Single-cell suspensions were prepared from spleen and lymph nodes of mice. Fluorescently labeled antibodies were obtained from BD Biosciences or Biolegend (San Diego, CA, USA) and included CD23–PE (B3B4), CD21–FITC (7G6), CD40–PE (1C10), CD45R/B220–FITC or –PE (RA3-6B2), CD80–PE (16-10A1), CD86–PE (PO3 or GL-1), CD138–PE (281-2), IgM–FITC or –allophycocyanin (11/41), IgD–FITC (11-26c.2a) and MHC class II (I-A and I-E) (M5/114.15.2). Carboxyfluorescein [carboxyfluorescein diacetate succinimidyl ester (CFSE)] was obtained from Molecular Probes (Eugene, OR, USA). Samples were analyzed on a Becton–Dickinson FACSCalibur and the resulting data were evaluated using FlowJo software. A total of 10 000–20 000 events were obtained in each analysis except for the adoptive transfer experiments in which 100 000 events were obtained. Mean fluorescent intensity (MFI) of staining of each marker was analyzed on B cells or B cell subsets.
Immunofluorescence
Spleens and kidneys of mice were embedded in Tissue-Tek O.C.T. compound (Sakura-Finetec, Torrance, CA, USA), frozen in liquid nitrogen and sectioned at 6 μm. After blocking, tissue sections were labeled with specific antibodies for 1 h at room temperature. Kidney sections were incubated with monoclonal anti-mouse IgM–FITC (11/41, BD Biosciences), polyclonal anti-mouse IgG–FITC (Jackson ImmunoResearch, West Grove, PA, USA) or polyclonal anti-mouse complement C3–FITC (MP Biomedical, Irvine, CA, USA). Spleen sections were incubated with a combination of biotinylated mouse anti-mouse CD22 (Cy34, BD Biosciences) and rat anti-mouse metallophilic macrophage mAb (Moma-1; Serotec, Raleigh, NC, USA). After washing, the spleen sections were incubated with secondary reagents: streptavidin-conjugated Alexa-Fluor 488- and Alexa-Fluor 568-labeled goat anti-rat secondary antibody (Molecular Probes). Stained slides were mounted using VectaShield (Vector Labs, Burlingame, CA, USA) and photographed with a Nikon FXA fluorescence microscope.
Adoptive transfer
B cells were purified from the spleens of donor mice (as indicated in the figure legends) using magnetic depletion of CD43+ non-B cells. For some experiments, expression of activation markers (CD23, CD80, CD86 and MHC II) was assessed on bulk B cells prior to adoptive transfer. Purified B cells were labeled with 1 μM CFSE for 3 min, washed and then injected retro-orbitally into anesthetized C57BL/6 × 129Sv hybrid wild-type or Ets-1p/p host mice. Three to five days post-transfer, spleens of host mice were harvested and the phenotype of transferred B cells was analyzed by flow cytometry. Data were analyzed by gating on the CFSE+ population in the spleen and assessing the expression of surface markers. MFI of each marker on the CFSE+ population was determined.
ELISA
Serum was harvested from mice via eye bleed or cardiac puncture and stored at −20°C until analyzed. ELISA capturing agents were coated onto NUNC-Immuno Maxi-Sorp 96-well plates at 10 μg ml−1. The capturing agents used were trinitrophenol (TNP) (Sigma Chemical, St Louis, MO, USA), double-stranded sheared salmon sperm DNA (Eppendorf Scientific, Westbury, NY, USA), a natural mixture of histones H1, H2A, H2B, H3 and H4 from calf thymus (Roche Applied Sciences, Indianapolis, IN, USA), bovine heart cardiolipin (Sigma Chemical), bovine brain MBP (Sigma Chemical) and a mixture of mouse IgG antibodies (SouthernBiotech, Birmingham, AL, USA). Mouse serum was diluted 1: 1000 in PBS plus 4% BSA and incubated on the coated ELISA plates. After washing, the plates were incubated with biotin-conjugated anti-mouse kappa and lambda light chain antibodies (for detection of antibodies against DNA, histones, cardiolipin and MBP) or with biotin-conjugated anti-mouse IgM (for detection of antibodies against mouse IgG or TNP). Plates were subsequently incubated with avidin–HRP complex (eBiosciences, San Diego, CA, USA) and developed using 3,3′,5,5′-tetramethylbenzidine substrate solution (eBiosciences). Absorbances were read at 450 nm on a Bio-Rad ELISA microplate reader.
B cell stimulation and ELISPOT
B cells were purified from the spleens of mice as described above. Purified B cells were cultured at 37°C with 5% CO2 in RPMI-1640 medium supplemented with 10% FCS, penicillin/streptomycin, glutamine and 50 μM β-mercaptoethanol. Cells were either unstimulated or stimulated for 72 h with 10 μg ml−1 goat F(ab′)2 anti-mouse IgM antibody μ chain specific (Jackson ImmunoResearch), 10 μg ml−1 bacterial LPS (Sigma Chemical) or 5 μg ml−1 phosphorothioate oligodeoxynucleotides (ODN) [the previously described (16) stimulatory ODN 1826 (5′ TCCATGACGTTCCTGACGTT 3′) and control ODN 2138 (5′ TCCATGAGCTTCCTGAGCTT 3′), a kind gift of Arthur Krieg and Coley Pharmaceuticals]. In some experiments, 0.1 μM chloroquine diphosphate (Sigma Chemicals) was added to cells stimulated with ODN 1826. For ELISPOT analysis, Millipore MultiScreen 96-well plates with Immobilon-P membrane were coated with 10 μg ml−1 of polyclonal goat anti-mouse Ig (SouthernBiotech). B cells, stimulated as described above, were plated at 250, 1000, 5000 and 20 000 cells per well and incubated overnight at 37°C. IgM-secreting plasma cells were detected using a biotin-conjugated rat anti-mouse IgM (R6-60.2; BD Biosciences) detection antibody followed by avidin–HRP complex (VectaStain ABC Peroxidase Kit; Vector Labs) and 3-amino-9-ethyl carbazole (Sigma Chemical). ELISPOT plates were counted with an automated reader (Zellnet Consulting, Fort Lee, NJ, USA).
Statistics
Data are represented as mean ± SEM. Statistical significance of differences in Tables and Figures was assessed using a paired two-tailed Student's t-test.
Methods for Online Supplementary Data
Differentiation of LPS- and cytosine-phosphate-guanine DNA sequence (CpG)-stimulated B cells was assessed by immunofluorescent staining for cytoplasmic IgM. Cells stimulated for 72 h were allowed to settle onto polylysine coated cover slips. Attached cells were then fixed in 100% methanol and stained with FITC-coupled anti-mouse IgM antibody. Stained slides were mounted using VectaShield and photographed with a Nikon FXA fluorescence microscope.
HeLa cervical carcinoma cells were grown in DMEM supplemented with 10% FCS, glutamine and penicillin/streptomycin. The day prior to transfection, 150 000 cells were plated in each well of a 6-well plate. Cells were transfected the next day with Fugene-6 reagent (Roche Applied Sciences) according to the directions supplied by the manufacturer. A total of 200 ng of a plasmid driving expression of HA-tagged mouse Ets-1 (pCMV-HA-Ets1) was co-transfected with 500 ng of Ets-dependent reporter (E74×3)-TK-Luc, containing three copies of an Ets-binding site cloned upstream of the TK minimal promoter and a firefly luciferase reporter gene (a kind gift of Satrajit Sinha, SUNY at Buffalo, Buffalo, NY, USA). Cells were co-transfected with varying amounts (0, 10, 50, 200, 800 or 2000 ng) of a plasmid-driving expression of HA-tagged mutant Ets-1 cloned from Ets-1p/p mice (pCMV-HA-Ets1ΔP). Cells were also co-transfected with 200 ng of an internal control plasmid pEF-RLuc, containing the constitutively expressed EF1α promoter driving expression of Renilla luciferase (a kind gift of Xin Lin, Anderson Cancer Center, University of Texas, Houston, TX, USA). Forty-eight hours post-transfection, cells were harvested and lysed. Firefly and Renilla luciferase values were measured using the Dual-luciferase kit (Promega) and expressed as ratios of firefly luciferase to Renilla luciferase.
Results
Ets-1-deficient mice express a mutant version of Ets-1 lacking the Pointed domain
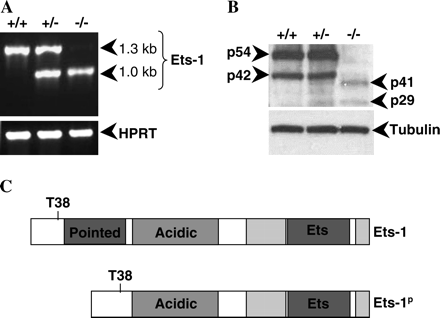
Generation of the Ets-1 gene-targeted mutant mice used in this study has previously been described (6, 7). The gene-targeting event that gave rise to the Ets-1 knockout allele results in deletion of exon 3 and a portion of exon 4, which encode the Pointed domain of Ets-1. Analysis of the genomic organization of Ets-1 revealed that exons 2 and 5 were in the same reading frame, suggesting that splicing around the inserted neomycin resistance gene could potentially lead to production of a transcript encoding an in-frame fusion of Ets-1 lacking the Pointed domain. To test this possibility, we performed reverse transcriptase (RT)–PCR analysis using RNA derived from purified B cells and primers flanking the open reading frame of the Ets-1 gene. As shown in Fig. 1(A), wild-type B cells produce an RT–PCR product of the expected size (1.3 kb) whereas Ets-1−/− B cells produce a smaller transcript of ∼1.0 kb and Ets-1+/− B cells produce both transcripts. We cloned and sequenced the 1.0-kb PCR fragment derived from Ets-1 gene-targeted B cells and showed that it indeed encoded an mRNA species lacking exons 3 and 4 and in which exon 2 was fused in-frame to exon 5. This transcript presumably arises due to alternate splicing around the neomycin gene.
Ets-1 gene-targeted mice express a hypomorphic allele of Ets-1. (A) RT–PCR amplification of cDNA derived from purified B cells using primers flanking the Ets-1 open reading frame or primers specific for the hypoxanthine phosphoribosyl transferase (HPRT) internal control. (B) Western blot analysis of protein isoforms in wild-type, heterozygous and homozygous Ets-1 mutant thymocytes using an antibody specific for the C-terminus of Ets-1. The membrane was stripped and reprobed with an anti-β-tubulin antibody to confirm equal loading of the lysates. (C) Predicted protein product of the mutant Ets-1 locus. The mutant protein contains the Erk phosphorylation site (T38), the acidic activation domain (acidic), the autoinhibitory domains (light gray boxes flanking the Ets domain) and the Ets DNA-binding domain, but lacks the Pointed domain (Pointed).
To determine whether the mutant mRNA species gives rise to a protein product, we performed western blot analysis using lysates prepared from Ets-1+/+, Ets-1+/− and Ets-1−/− thymocytes. In lysates from wild-type mice, two major isoforms of Ets-1 (p54 Ets-1 and p42 Ets-1) are detected, which arise via alternate splicing of exon 7 (17, 18). As shown in Fig. 1(B), both p54 and p42 Ets-1 can be detected in extracts from Ets-1+/+ and Ets-1+/− thymocytes. As expected, Ets-1−/− thymocyte extracts lack both p54 and p42 Ets-1, but contains two novel immunoreactive species of ∼41 and 29 kDa. The 41- and 29-kDa species could also be detected weakly in Ets-1+/− thymocyte lysates. Densitometry analysis indicated that these forms of Ets-1 are ∼1–5% as abundant as the p54 and p42 Ets-1 isoforms in wild-type thymocyte lysates. Based on these results, we have designated this Ets-1 mutant allele as Ets-1p to indicate that the allele produces a version of Ets-1 lacking the Pointed domain and now refer to the Ets-1-deficient animals as Ets-1p/p mice. Figure 1(C) shows the predicted domain structure of the mutant protein, which retains the Erk kinase phosphorylation site, acidic transactivation domain, autoinhibitory domains and Ets DNA-binding domain.
Mice expressing the hypomorphic allele of Ets-1 exhibit striking lymphoid abnormalities as described previously (6, 7, 14) and in the data presented below. Since expression of the deleted form of the Ets-1 protein is very low as compared with expression of the wild-type protein, we propose that the Ets-1p/p phenotype is likely to be the result of near total loss of Ets-1 activity. However, we cannot exclude the possibility that expression of a mutant form of Ets-1 lacking the Pointed domain may also have effects on B cell differentiation.
B cell development is altered in Ets-1p/p mice
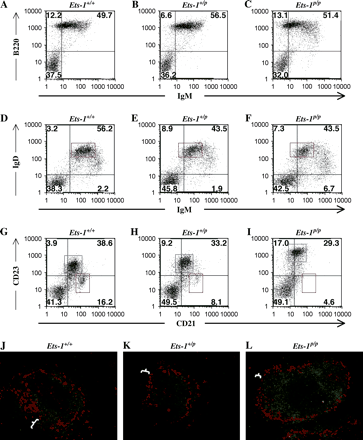
Loss of Ets-1 activity has previously been shown to result in increased numbers of B220loIgM+ B cells in the lymphoid organs (5–8), which we have confirmed (Fig. 2A–C). In order to distinguish immature, transitional and mature B cell populations in the peripheral lymphoid tissues, we stained spleen cells with antibodies specific for IgM and IgD. Two differences were noted in the IgM/IgD staining profiles between Ets-1p/p mice and wild-type mice. First, the mature IgDhiIgMlo B cells express lower than normal surface levels of IgM, but normal levels of surface IgD (Fig. 2D–F, see also Table 1). Second, Ets-1-deficient spleens contain more B cells with an immature phenotype (IgDloIgMhi).
B cell development is altered in Ets-1p/p mice. (A–C) Spleen cells were stained with fluorescent antibodies to B220 and IgM and analyzed by flow cytometry. (D–F) Spleen cells were stained with fluorescent antibodies to IgM and IgD and analyzed by flow cytometry. Mature B cells are indicated by the red box. (G–I) Spleen cells were stained with fluorescent antibodies to CD21 and CD23 and analyzed by flow cytometry. Marginal zone B cells are indicated by the red box and follicular B cells are indicated by the blue box. (J–L) Immunofluorescent staining of B cells (anti-CD22, green) and metallophilic macrophages (anti-Moma-1, red) shows that Ets-1p/p mice have fewer marginal zone B cells (denoted by the white markers), which are normally found surrounding the metallophilic macrophages.
Ets-1p/p B cells have an activated phenotype
Marker . | Spleen . | . | . | Lymph node . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | Number analyzed . | Average ± SE . | P-value . | Number analyzed . | Average ± SE . | P-value . | ||||
| IgM (mature B) | 4 | 4 | ||||||||
| Ets-1+/+ | 158 ± 22 | 174 ± 39 | ||||||||
| Ets-1+/p | 110 ± 21 | 0.01* | 124 ± 36 | 0.03* | ||||||
| Ets-1p/p | 84 ± 7 | 0.03* | 99 ± 24 | 0.02* | ||||||
| CD23 (follicular) | 9 | 3 | ||||||||
| Ets-1+/+ | 224 ± 35 | 232 ± 77 | ||||||||
| Ets-1+/p | 353 ± 68 | 0.04* | 295 ± 165 | 0.56 | ||||||
| Ets-1p/p | 1106 ± 116 | 0.00004* | 651 ± 160 | 0.05* | ||||||
| CD40 | 6 | 5 | ||||||||
| Ets-1+/+ | 64 ± 26 | 50 ± 16 | ||||||||
| Ets-1+/p | 59 ± 18 | 0.62 | 53 ± 17 | 0.20 | ||||||
| Ets-1p/p | 71 ± 18 | 0.52 | 60 ± 20 | 0.14 | ||||||
| CD80 | 5 | 4 | ||||||||
| Ets-1+/+ | 17 ± 4 | 11 ± 4 | ||||||||
| Ets-1+/p | 19 ± 5 | 0.28 | 15 ± 5 | 0.09 | ||||||
| Ets-1p/p | 29 ± 6 | 0.01* | 18 ± 5 | 0.01* | ||||||
| CD86 | 9 | 8 | ||||||||
| Ets-1+/+ | 19 ± 5 | 18 ± 4 | ||||||||
| Ets-1+/p | 22 ± 7 | 0.10 | 23 ± 5 | 0.06 | ||||||
| Ets-1p/p | 35 ± 9 | 0.002* | 41 ± 9 | 0.008* | ||||||
| MHC II | 9 | 7 | ||||||||
| Ets-1+/+ | 487 ± 93 | 555 ± 87 | ||||||||
| Ets-1+/p | 494 ± 91 | 0.83 | 466 ± 104 | 0.22 | ||||||
| Ets-1p/p | 676 ± 112 | 0.0016* | 668 ± 155 | 0.29 | ||||||
Marker . | Spleen . | . | . | Lymph node . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | Number analyzed . | Average ± SE . | P-value . | Number analyzed . | Average ± SE . | P-value . | ||||
| IgM (mature B) | 4 | 4 | ||||||||
| Ets-1+/+ | 158 ± 22 | 174 ± 39 | ||||||||
| Ets-1+/p | 110 ± 21 | 0.01* | 124 ± 36 | 0.03* | ||||||
| Ets-1p/p | 84 ± 7 | 0.03* | 99 ± 24 | 0.02* | ||||||
| CD23 (follicular) | 9 | 3 | ||||||||
| Ets-1+/+ | 224 ± 35 | 232 ± 77 | ||||||||
| Ets-1+/p | 353 ± 68 | 0.04* | 295 ± 165 | 0.56 | ||||||
| Ets-1p/p | 1106 ± 116 | 0.00004* | 651 ± 160 | 0.05* | ||||||
| CD40 | 6 | 5 | ||||||||
| Ets-1+/+ | 64 ± 26 | 50 ± 16 | ||||||||
| Ets-1+/p | 59 ± 18 | 0.62 | 53 ± 17 | 0.20 | ||||||
| Ets-1p/p | 71 ± 18 | 0.52 | 60 ± 20 | 0.14 | ||||||
| CD80 | 5 | 4 | ||||||||
| Ets-1+/+ | 17 ± 4 | 11 ± 4 | ||||||||
| Ets-1+/p | 19 ± 5 | 0.28 | 15 ± 5 | 0.09 | ||||||
| Ets-1p/p | 29 ± 6 | 0.01* | 18 ± 5 | 0.01* | ||||||
| CD86 | 9 | 8 | ||||||||
| Ets-1+/+ | 19 ± 5 | 18 ± 4 | ||||||||
| Ets-1+/p | 22 ± 7 | 0.10 | 23 ± 5 | 0.06 | ||||||
| Ets-1p/p | 35 ± 9 | 0.002* | 41 ± 9 | 0.008* | ||||||
| MHC II | 9 | 7 | ||||||||
| Ets-1+/+ | 487 ± 93 | 555 ± 87 | ||||||||
| Ets-1+/p | 494 ± 91 | 0.83 | 466 ± 104 | 0.22 | ||||||
| Ets-1p/p | 676 ± 112 | 0.0016* | 668 ± 155 | 0.29 | ||||||
MFI and SEM are shown for the expression of each marker on the bulk B cell population or on the B cell subsets indicated (IgMloIgDhi mature B cells or CD21loCD23hi follicular B cells). Statistical significance of the differences in MFI (comparing Ets-1+/+ with Ets-1+/p or Ets-1p/p) was analyzed. Asterisks indicate P-values that are statistically significant (P ≤ 0.05).
Ets-1p/p B cells have an activated phenotype
Marker . | Spleen . | . | . | Lymph node . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | Number analyzed . | Average ± SE . | P-value . | Number analyzed . | Average ± SE . | P-value . | ||||
| IgM (mature B) | 4 | 4 | ||||||||
| Ets-1+/+ | 158 ± 22 | 174 ± 39 | ||||||||
| Ets-1+/p | 110 ± 21 | 0.01* | 124 ± 36 | 0.03* | ||||||
| Ets-1p/p | 84 ± 7 | 0.03* | 99 ± 24 | 0.02* | ||||||
| CD23 (follicular) | 9 | 3 | ||||||||
| Ets-1+/+ | 224 ± 35 | 232 ± 77 | ||||||||
| Ets-1+/p | 353 ± 68 | 0.04* | 295 ± 165 | 0.56 | ||||||
| Ets-1p/p | 1106 ± 116 | 0.00004* | 651 ± 160 | 0.05* | ||||||
| CD40 | 6 | 5 | ||||||||
| Ets-1+/+ | 64 ± 26 | 50 ± 16 | ||||||||
| Ets-1+/p | 59 ± 18 | 0.62 | 53 ± 17 | 0.20 | ||||||
| Ets-1p/p | 71 ± 18 | 0.52 | 60 ± 20 | 0.14 | ||||||
| CD80 | 5 | 4 | ||||||||
| Ets-1+/+ | 17 ± 4 | 11 ± 4 | ||||||||
| Ets-1+/p | 19 ± 5 | 0.28 | 15 ± 5 | 0.09 | ||||||
| Ets-1p/p | 29 ± 6 | 0.01* | 18 ± 5 | 0.01* | ||||||
| CD86 | 9 | 8 | ||||||||
| Ets-1+/+ | 19 ± 5 | 18 ± 4 | ||||||||
| Ets-1+/p | 22 ± 7 | 0.10 | 23 ± 5 | 0.06 | ||||||
| Ets-1p/p | 35 ± 9 | 0.002* | 41 ± 9 | 0.008* | ||||||
| MHC II | 9 | 7 | ||||||||
| Ets-1+/+ | 487 ± 93 | 555 ± 87 | ||||||||
| Ets-1+/p | 494 ± 91 | 0.83 | 466 ± 104 | 0.22 | ||||||
| Ets-1p/p | 676 ± 112 | 0.0016* | 668 ± 155 | 0.29 | ||||||
Marker . | Spleen . | . | . | Lymph node . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | Number analyzed . | Average ± SE . | P-value . | Number analyzed . | Average ± SE . | P-value . | ||||
| IgM (mature B) | 4 | 4 | ||||||||
| Ets-1+/+ | 158 ± 22 | 174 ± 39 | ||||||||
| Ets-1+/p | 110 ± 21 | 0.01* | 124 ± 36 | 0.03* | ||||||
| Ets-1p/p | 84 ± 7 | 0.03* | 99 ± 24 | 0.02* | ||||||
| CD23 (follicular) | 9 | 3 | ||||||||
| Ets-1+/+ | 224 ± 35 | 232 ± 77 | ||||||||
| Ets-1+/p | 353 ± 68 | 0.04* | 295 ± 165 | 0.56 | ||||||
| Ets-1p/p | 1106 ± 116 | 0.00004* | 651 ± 160 | 0.05* | ||||||
| CD40 | 6 | 5 | ||||||||
| Ets-1+/+ | 64 ± 26 | 50 ± 16 | ||||||||
| Ets-1+/p | 59 ± 18 | 0.62 | 53 ± 17 | 0.20 | ||||||
| Ets-1p/p | 71 ± 18 | 0.52 | 60 ± 20 | 0.14 | ||||||
| CD80 | 5 | 4 | ||||||||
| Ets-1+/+ | 17 ± 4 | 11 ± 4 | ||||||||
| Ets-1+/p | 19 ± 5 | 0.28 | 15 ± 5 | 0.09 | ||||||
| Ets-1p/p | 29 ± 6 | 0.01* | 18 ± 5 | 0.01* | ||||||
| CD86 | 9 | 8 | ||||||||
| Ets-1+/+ | 19 ± 5 | 18 ± 4 | ||||||||
| Ets-1+/p | 22 ± 7 | 0.10 | 23 ± 5 | 0.06 | ||||||
| Ets-1p/p | 35 ± 9 | 0.002* | 41 ± 9 | 0.008* | ||||||
| MHC II | 9 | 7 | ||||||||
| Ets-1+/+ | 487 ± 93 | 555 ± 87 | ||||||||
| Ets-1+/p | 494 ± 91 | 0.83 | 466 ± 104 | 0.22 | ||||||
| Ets-1p/p | 676 ± 112 | 0.0016* | 668 ± 155 | 0.29 | ||||||
MFI and SEM are shown for the expression of each marker on the bulk B cell population or on the B cell subsets indicated (IgMloIgDhi mature B cells or CD21loCD23hi follicular B cells). Statistical significance of the differences in MFI (comparing Ets-1+/+ with Ets-1+/p or Ets-1p/p) was analyzed. Asterisks indicate P-values that are statistically significant (P ≤ 0.05).
Mature B cell populations in the spleen can be divided into two subsets, follicular B cells and marginal zone B cells, which can be differentiated both by their localization within the splenic follicles and by their relative expression of the cell-surface markers CD21 and CD23. In wild-type mice, ∼90% of B cells are of the follicular type (CD21loCD23hi), whereas ∼10% are of the marginal zone type (CD21hiCD23lo). In Ets-1p/p spleens, very few marginal zone B cells could be detected (Fig. 2G–I). Moreover, follicular B cells in Ets-1p/p mice exhibited strikingly increased staining for CD23 and slightly reduced staining for CD21 (Fig. 2I versus G, see also Table 1). To confirm the decreases in the marginal zone population, we performed immunofluorescent staining with spleen sections derived from Ets-1+/+, Ets-1+/p and Ets-1p/p mice. Marginal zone B cells are localized to the outer margin of the B cell follicles at the border of the red and white pulp of the spleen. They are separated from the follicular B cells by a specialized subset of macrophages known as metallophilic macrophages. As shown in Fig. 2(J–L), Ets-1p/p spleens possess B cell follicles surrounded by metallophilic macrophages, but have very few marginal zone B cells at the outer rim of the follicle. In contrast to the significant decrease in marginal zone B cell numbers in Ets-1p/p mice, numbers of B-1a B cells in the peritoneal cavity are normal (Supplementary Figure 1, available at International Immunology Online).
Ets-1p/p B cells express increased levels of activation markers
CD23 is known to be up-regulated on B cells by CD40 ligation or IL-4 stimulation (19, 20). Thus, increased expression of CD23 on B cells from Ets-1p/p mice may reflect an activated state. To explore this possibility in more detail, we stained spleen and lymph node suspensions with antibodies specific for surface markers that are up-regulated in activated B cells (MHC II, CD40, CD80 and CD86). Table 1 summarizes the average MFI of staining for each of these markers on splenic and lymph node B cells from Ets-1+/+, Ets-1+/p and Ets-1p/p mice. As can be appreciated from this Table, Ets-1p/p B cells express modestly increased surface levels of CD80 and CD86 in both the lymph node and the spleen. MHC II expression was also significantly increased in splenic Ets-1p/p B cells as compared with Ets-1+/+ B cells. Although MHC II levels were slightly higher in lymph node B cells from Ets-1p/p mice, this difference did not reach statistical significance (Table 1). In contrast to the increases observed with CD23, CD80, CD86 and MHC II, we did not detect a statistically significant change in surface staining for CD40. Together, these results indicate that Ets-1p/p B cells have a weakly activated phenotype.
Because Ets-1 expression is not restricted to B cells, it is possible that the activated phenotype of Ets-1p/p B cells may not be B cell intrinsic. To determine whether mutant B cells remain activated and differentiate at high rates to plasma cells in a wild-type environment, we purified Ets-1+/+, Ets-1+/p and Ets-1p/p splenic B cells, stained them with CFSE and then adoptively transferred them into non-irradiated wild-type hosts. Three to five days post-transfer, expression of IgM, CD23, CD80, CD86, CD138 and MHC II was assessed on CFSE+ splenocytes. The majority of CFSE+ cells expressed IgM and MHC II, confirming that they were B cells. In a wild-type environment, the expression of activation markers (CD23, CD80, CD86 and MHC II) on Ets-1p/p B cells was not significantly elevated relative to their expression on adoptively transferred control B cells (Table 2). Particularly striking is the observation that CD23 staining intensity was greatly decreased in adoptively transferred Ets-1p/p B cells as compared with freshly isolated Ets-1p/p B cells (compare CD23 staining in Tables 1 and 2). Few or none of the CFSE+ cells stained with the plasma cell-specific marker CD138 (data not shown), suggesting that these cells had not differentiated to plasma cells. Hence, the activated phenotype and increased terminal differentiation of Ets-1p/p B cells are reversed when the cells are transferred to wild-type hosts, indicating that factors in the wild-type environment function to limit the activity of Ets-1-deficient B cells.
The activated phenotype of Ets-1p/p B cells can be reversed by transfer to wild-type hosts
Marker . | Number analyzed . | Average ± SE . | P-value . |
|---|---|---|---|
| IgM | 7 | ||
| Ets-1+/+ | 300 ± 135 | ||
| Ets-1+/p | 140 ± 44 | 0.16 | |
| Ets-1p/p | 155 ± 69 | 0.44 | |
| CD23 | 9 | ||
| Ets-1+/+ | 96 ± 28 | ||
| Ets-1+/p | 126 ± 47 | 0.34 | |
| Ets-1p/p | 132 ± 56 | 0.43 | |
| CD80 | 4 | ||
| Ets-1+/+ | 61 ± 53 | ||
| Ets-1+/p | 25 ± 14 | 0.41 | |
| Ets-1p/p | 29 ± 14 | 0.48 | |
| CD86 | 9 | ||
| Ets-1+/+ | 21 ± 9 | ||
| Ets-1+/p | 23 ± 9 | 0.41 | |
| Ets-1p/p | 26 ± 14 | 0.40 | |
| MHC II | 9 | ||
| Ets-1+/+ | 574 ± 154 | ||
| Ets-1+/p | 670 ± 172 | 0.20 | |
| Ets-1p/p | 619 ± 246 | 0.79 |
Marker . | Number analyzed . | Average ± SE . | P-value . |
|---|---|---|---|
| IgM | 7 | ||
| Ets-1+/+ | 300 ± 135 | ||
| Ets-1+/p | 140 ± 44 | 0.16 | |
| Ets-1p/p | 155 ± 69 | 0.44 | |
| CD23 | 9 | ||
| Ets-1+/+ | 96 ± 28 | ||
| Ets-1+/p | 126 ± 47 | 0.34 | |
| Ets-1p/p | 132 ± 56 | 0.43 | |
| CD80 | 4 | ||
| Ets-1+/+ | 61 ± 53 | ||
| Ets-1+/p | 25 ± 14 | 0.41 | |
| Ets-1p/p | 29 ± 14 | 0.48 | |
| CD86 | 9 | ||
| Ets-1+/+ | 21 ± 9 | ||
| Ets-1+/p | 23 ± 9 | 0.41 | |
| Ets-1p/p | 26 ± 14 | 0.40 | |
| MHC II | 9 | ||
| Ets-1+/+ | 574 ± 154 | ||
| Ets-1+/p | 670 ± 172 | 0.20 | |
| Ets-1p/p | 619 ± 246 | 0.79 |
CFSE-labeled Ets-1+/+, Ets-1+/p and Ets-1p/p B cells were transferred to unirradiated wild-type C57BL/6 × 129Sv hosts and analyzed by flow cytometry after 3–5 days. MFI and SEM are shown for the expression of IgM, MHC II, CD86, CD80 and CD23 on the CFSE+ population.
The activated phenotype of Ets-1p/p B cells can be reversed by transfer to wild-type hosts
Marker . | Number analyzed . | Average ± SE . | P-value . |
|---|---|---|---|
| IgM | 7 | ||
| Ets-1+/+ | 300 ± 135 | ||
| Ets-1+/p | 140 ± 44 | 0.16 | |
| Ets-1p/p | 155 ± 69 | 0.44 | |
| CD23 | 9 | ||
| Ets-1+/+ | 96 ± 28 | ||
| Ets-1+/p | 126 ± 47 | 0.34 | |
| Ets-1p/p | 132 ± 56 | 0.43 | |
| CD80 | 4 | ||
| Ets-1+/+ | 61 ± 53 | ||
| Ets-1+/p | 25 ± 14 | 0.41 | |
| Ets-1p/p | 29 ± 14 | 0.48 | |
| CD86 | 9 | ||
| Ets-1+/+ | 21 ± 9 | ||
| Ets-1+/p | 23 ± 9 | 0.41 | |
| Ets-1p/p | 26 ± 14 | 0.40 | |
| MHC II | 9 | ||
| Ets-1+/+ | 574 ± 154 | ||
| Ets-1+/p | 670 ± 172 | 0.20 | |
| Ets-1p/p | 619 ± 246 | 0.79 |
Marker . | Number analyzed . | Average ± SE . | P-value . |
|---|---|---|---|
| IgM | 7 | ||
| Ets-1+/+ | 300 ± 135 | ||
| Ets-1+/p | 140 ± 44 | 0.16 | |
| Ets-1p/p | 155 ± 69 | 0.44 | |
| CD23 | 9 | ||
| Ets-1+/+ | 96 ± 28 | ||
| Ets-1+/p | 126 ± 47 | 0.34 | |
| Ets-1p/p | 132 ± 56 | 0.43 | |
| CD80 | 4 | ||
| Ets-1+/+ | 61 ± 53 | ||
| Ets-1+/p | 25 ± 14 | 0.41 | |
| Ets-1p/p | 29 ± 14 | 0.48 | |
| CD86 | 9 | ||
| Ets-1+/+ | 21 ± 9 | ||
| Ets-1+/p | 23 ± 9 | 0.41 | |
| Ets-1p/p | 26 ± 14 | 0.40 | |
| MHC II | 9 | ||
| Ets-1+/+ | 574 ± 154 | ||
| Ets-1+/p | 670 ± 172 | 0.20 | |
| Ets-1p/p | 619 ± 246 | 0.79 |
CFSE-labeled Ets-1+/+, Ets-1+/p and Ets-1p/p B cells were transferred to unirradiated wild-type C57BL/6 × 129Sv hosts and analyzed by flow cytometry after 3–5 days. MFI and SEM are shown for the expression of IgM, MHC II, CD86, CD80 and CD23 on the CFSE+ population.
To determine whether wild-type B cells could respond to the factors in the Ets-1p/p environment that induce B cell activation, we reversed the transfer experiments and introduced purified CFSE-labeled wild-type B cells into Ets-1p/p hosts. After 3 days post-transfer, we analyzed the expression of activation markers on CFSE+ wild-type B cells. As can be seen in Table 3, wild-type B cells transferred into Ets-1p/p hosts significantly up-regulated expression of CD23 and CD80. Expression of MHC II and CD86 was also elevated although the difference did not reach statistical significance. Together, these data indicate that there are alterations to the Ets-1p/p environment that contribute to the activation of B cells.
Wild-type quiescent B cells up-regulate activation markers on transfer to Ets-1p/p hosts
Marker . | Number analyzed . | Average ± SE (before transfer) . | Average ± SE (after transfer) . | P-value . |
|---|---|---|---|---|
| MHC II | 4 | 640 ± 109 | 1022 ± 101 | 0.06 |
| CD23 | 4 | 179 ± 45 | 949 ± 145 | 0.007* |
| CD80 | 4 | 2.5 ± 0.1 | 6.9 ± 0.8 | 0.02* |
| CD86 | 4 | 6.1 ± 0.1 | 13.8 ± 3.9 | 0.25 |
Marker . | Number analyzed . | Average ± SE (before transfer) . | Average ± SE (after transfer) . | P-value . |
|---|---|---|---|---|
| MHC II | 4 | 640 ± 109 | 1022 ± 101 | 0.06 |
| CD23 | 4 | 179 ± 45 | 949 ± 145 | 0.007* |
| CD80 | 4 | 2.5 ± 0.1 | 6.9 ± 0.8 | 0.02* |
| CD86 | 4 | 6.1 ± 0.1 | 13.8 ± 3.9 | 0.25 |
CFSE-labeled C57BL/6 wild-type B cells were transferred to unirradiated Ets-1p/p hosts and analyzed by flow cytometry after 3 days. MFI and SEM are shown for the expression of MHC II, CD23, CD80 and CD86 on total B cells prior to transfer and on the CFSE+ population after transfer. Asterisks indicate P-values that are statistically significant (P ≤ 0.05).
Wild-type quiescent B cells up-regulate activation markers on transfer to Ets-1p/p hosts
Marker . | Number analyzed . | Average ± SE (before transfer) . | Average ± SE (after transfer) . | P-value . |
|---|---|---|---|---|
| MHC II | 4 | 640 ± 109 | 1022 ± 101 | 0.06 |
| CD23 | 4 | 179 ± 45 | 949 ± 145 | 0.007* |
| CD80 | 4 | 2.5 ± 0.1 | 6.9 ± 0.8 | 0.02* |
| CD86 | 4 | 6.1 ± 0.1 | 13.8 ± 3.9 | 0.25 |
Marker . | Number analyzed . | Average ± SE (before transfer) . | Average ± SE (after transfer) . | P-value . |
|---|---|---|---|---|
| MHC II | 4 | 640 ± 109 | 1022 ± 101 | 0.06 |
| CD23 | 4 | 179 ± 45 | 949 ± 145 | 0.007* |
| CD80 | 4 | 2.5 ± 0.1 | 6.9 ± 0.8 | 0.02* |
| CD86 | 4 | 6.1 ± 0.1 | 13.8 ± 3.9 | 0.25 |
CFSE-labeled C57BL/6 wild-type B cells were transferred to unirradiated Ets-1p/p hosts and analyzed by flow cytometry after 3 days. MFI and SEM are shown for the expression of MHC II, CD23, CD80 and CD86 on total B cells prior to transfer and on the CFSE+ population after transfer. Asterisks indicate P-values that are statistically significant (P ≤ 0.05).
Ets-1p/p B cells differentiate at higher rates in response to CpG
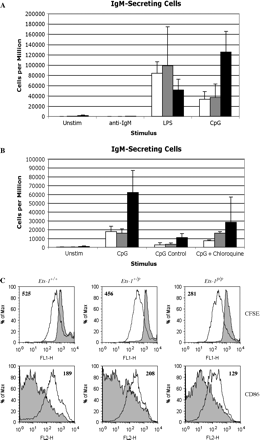
Ets-1p/p B cells have a partially activated phenotype in vivo and differentiate at high rates to IgM-secreting plasma cells. However, the adoptive transfer experiments suggest that this phenotype may be, at least in part, B cell extrinsic. To explore whether Ets-1p/p B cells also have intrinsic changes in their responses to stimuli, we purified splenic B cells from Ets-1+/+, Ets-1+/p and Ets-1p/p mice and stimulated them for 72 h with anti-IgM F(ab′)2 BCR cross-linking antibodies, LPS or CpG ODN or left them unstimulated and then measured their differentiation to IgM-secreting cells using ELISPOT. These assays demonstrated that, in the absence of overt stimulation, ∼0.2% of Ets-1p/p B cells underwent differentiation to IgM-secreting cells, whereas such cells were not found in unstimulated cultures of wild-type B cells (Fig. 3A). These IgM-secreting cells do not represent contamination of the purified B cell population with plasma cells because their development could be strongly suppressed by stimulation with anti-IgM BCR cross-linking antibody. Thus, Ets-1p/p B cells have an intrinsic propensity to differentiate to IgM-secreting cells, which can be suppressed by BCR ligation.
Ets-1p/p B cells differentiate at increased rates to IgM-secreting plasma cells in response to TLR9 stimulation. (A) Purified splenic B cells from wild-type mice (white bars), Ets-1+/p mice (light gray bars) or Ets-1p/p mice (black bars) were stimulated for 72 h with anti-IgM F(ab′)2 BCR cross-linking antibody (anti-IgM), bacterial LPS (LPS), CpG containing ODN (CpG) or left unstimulated (Unstim). IgM-secreting plasma cells were enumerated using ELISPOT analysis. (B) ELISPOT assays were performed as in part A (except that the cells were incubated on ELISPOT plates for a shorter time of 6 h versus 18 h in A). Purified splenic B cells stimulated with CpG containing ODN (CpG), GpC containing control ODN (CpG Control), CpG containing ODN plus 0.1 μM chloroquine (CpG + chloroquine) or left unstimulated (Unstim). A very low concentration of chloroquine was employed in these assays because higher doses of the drug caused toxicity. (C) CFSE-labeled purified splenic B cells were stimulated for 72 h with CpG containing ODN (unfilled histograms) or left unstimulated (filled histograms). CFSE and CD86 staining were assessed by flow cytometry.
In addition, Ets-1p/p B cells were hyperresponsive to the differentiation cues provided by Toll-like receptor (TLR) 9, as a stimulatory CpG ODN (ODN 1826) induced the development of 3- to 5-fold more IgM-secreting plasma cells in Ets-1p/p B cell cultures as compared with Ets-1+/+ or Ets-1+/p B cell cultures (P = 0.015) (Fig. 3A and B). Even a control ODN (ODN 2138) containing GpC motifs, rather than CpG motifs, was capable of inducing some differentiation of Ets-1p/p B cells (Fig. 3B). This result is in agreement with previous observations that the control ODN utilized in our studies (ODN 2138) induces weak TLR9-dependent signaling leading to Th2-biased in vivo responses (16).
To further explore the responses of mutant B cells to TLR9 activation, we incubated purified B cells with CpG ODN in the presence of a low concentration (0.1 μM) of the endosomal maturation inhibitor chloroquine, which blocks p38 kinase and AP-1 activation induced by CpG ODN (21, 22). We used a low concentration of chloroquine in our studies because higher levels led to significant toxicity over the 72-h incubation period. These experiments confirmed that Ets-1p/p B cells are hyperresponsive to CpG stimulation and that this response can be partially inhibited by low levels of chloroquine (Fig. 3B). Note that we detected lower numbers of IgM-secreting cells in these experiments as compared with those detected in the experiments shown in Fig. 3(A). This difference can be explained by the fact that the B cells were incubated for a shorter time on the ELISPOT plates (6 h for the experiments shown in Fig. 3B versus 18 h in the experiments shown in Fig. 3A). However, the overall trend of hyperresponsiveness of Ets-1p/p B cells to CpG ODN stimulation is evident in both sets of experiments. To verify that each population of B cells responded to CpG ODN, we measured B cell proliferation (indicated by the dilution of CFSE staining) and activation (indicated by the up-regulation of CD86) after 72 h of incubation with ODN 1826. As shown in Fig. 3(C), B cells of each genotype underwent similar levels of proliferation and up-regulated CD86 to similar extents. Differentiation of B cells in response to LPS and CpG ODN was further confirmed using immunofluorescent staining of cytoplasmic IgM (Supplementary Figure 2, available at International Immunology Online).
In contrast to the hyperresponsiveness of Ets-1p/p B cells to CpG ODN, there was no enhanced differentiation response to BCR cross-linking antibody or to bacterial LPS (a TLR4 agonist) (Fig. 3A). Indeed, Ets-1p/p B cells exhibited somewhat lower responses to LPS than did Ets-1+/+ B cells, although this difference did not reach statistical significance (P = 0.094). Taken together, these data indicate that purified Ets-1p/p B cells have a specific propensity to differentiate spontaneously and in response to TLR9 stimuli, but not BCR or TLR4 stimuli.
Ets-1p/p mice develop autoimmune disease
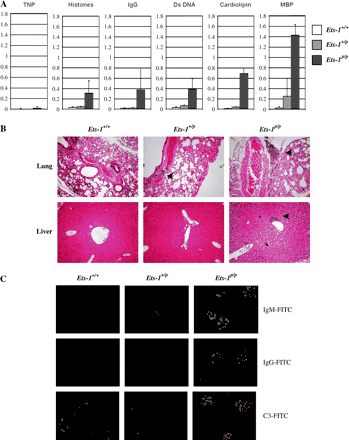
Polyclonally activated B cells and increased numbers of plasma cells are detected in many autoimmune mouse strains. Because Ets-1p/p mice exhibit signs of polyclonal B cell activation and have increased numbers of plasma cells, we examined these mice for evidence of autoimmune disease. Ets-1+/+, Ets-1+/p and Ets-1p/p serum was tested by ELISA with a foreign antigen (TNP) or with potential self-antigens as the capturing agents. As shown in Fig. 4(A), Ets-1p/p mice produce low, but detectable, levels of antibody specific for a foreign antigen. This presumably reflects low levels of non-antigen-specific differentiation of Ets-1p/p B cells to Ig-secreting cells (perhaps driven by responses to CpG). In contrast, there are greatly increased levels of autoreactive antibodies specific for each potential self-antigen tested (double-stranded DNA, histones, cardiolipin, MBP and IgG) in the serum of Ets-1p/p mice. Analysis of the isotypes of the auto-antibodies indicated that both IgM and IgG auto-antibodies are produced (data not shown). These data suggest that auto-antibody production is antigen driven and likely involves interaction of B cells with autoreactive T cells.
Ets-1p/p mice develop autoimmune disease. (A) ELISA analysis of the levels of anti-TNP, anti-histone, anti-IgG, anti-double-stranded DNA (Ds DNA), anti-cardiolipin and anti-MBP antibodies in the serum of Ets-1p/p and control mice. Shown are the absorbance readings and standard deviations from the analysis of serum of three–four mice of each genotype. (B) Hematoxylin and eosin-stained tissue sections from the lung and liver of wild-type, Ets-1+/p and Ets-1p/p mice. Inflammatory infiltrates are indicated by the black arrows. (C) Immune complex deposition in the kidney of Ets-1p/p mice as detected with FITC-labeled anti-mouse IgM, anti-mouse IgG and anti-mouse complement C3 antibodies. Original magnification: ×200 for panels B and C.
To determine whether the hyperactive B cells and auto-antibodies found in Ets-1p/p mice lead to organ pathology, we examined sections of various organs for evidence of inflammation or other pathological changes. The spleen and lymph nodes of Ets-1p/p mice were frequently enlarged and contained foci of plasma cells. In addition, there was often extramedullary hematopoiesis in the spleen. The lungs of nearly all Ets-1p/p mice (five of six) examined contained mild inflammatory infiltrates into the perivascular, peribronchial and sub-pleural regions (Fig. 4B). Such infiltrates could also be detected in the majority of Ets-1+/p mice (four of five), but not Ets-1+/+ mice (zero of four). Lung infiltration tended to be less prominent in heterozygous mutant mice than homozygous mutant mice. Mild inflammatory infiltrates were also detected in the livers of most Ets-1p/p mice (4 of 6), but only one of five Ets-1+/p mice and zero of four Ets-1+/+ mice (Fig. 4B). These infiltrates were mainly detected in the region surrounding the gall bladder and the common bile duct. Although we cannot rule out infection with pathogenic organisms, we hypothesize that the infiltrates in the lungs and livers of Ets-1p/p mice represent an autoimmune attack by self-reactive lymphocytes.
Kidneys from Ets-1p/p mice generally exhibited mild to moderate thickening of the glomerular basement membrane occasionally accompanied by infiltrating lymphocytes. Kidney sections from 6- to 12-week-old Ets-1p/p mice exhibited extensive glomerular staining with anti-mouse IgM, anti-mouse IgG and anti-mouse complement C3 antibodies, demonstrating the deposition of immune complexes in this tissue (Fig. 4C). Ets-1+/p kidneys also exhibited weak staining with anti-IgM, anti-IgG and anti-complement C3 antibodies, whereas kidneys from wild-type mice did not exhibit specific staining (Fig. 4C). Despite the extensive immune complexes, only rare and mild inflammatory infiltrates were detected in the kidneys of mutant mice. Moreover, measurements of urine protein levels in mice up to the age of 29 weeks failed to show increased urinary protein concentrations in mutant mice. Together, our results indicate that Ets-1p/p mice develop a systemic autoimmune disease affecting multiple organs.
Discussion
The mice used in our study, previously described as being completely deficient in Ets-1 protein, actually expressed low levels of a hypomorphic allele of Ets-1 lacking the Pointed domain. The Pointed domain of Ets-1 interacts with Erk kinases, which subsequently phosphorylate Ets-1 on a threonine residue (T38) located upstream of the Pointed domain (9, 10, 23). Phosphorylation of this residue results in recruitment of the co-activator proteins CBP and p300 to Ets-1, leading to an enhanced transcriptional activation function (11). The Ets-1p mutant protein could theoretically function as a dominant negative transcription factor by binding to DNA but failing to activate transcription efficiently. Indeed, in transient transfection assays, the Ets-1p protein was capable of dominantly inhibiting activation of a reporter gene by wild-type Ets-1 (Supplementary Figure 3, available at International Immunology Online). However, such inhibition required very high concentrations of the mutant protein. Since we have shown by western blotting that in Ets-1p/p mice the Pointed domain-deficient mutant protein is expressed at very low levels, it is unlikely that it serves as a dominant negative in vivo. Moreover, the alterations in lymphocyte development found in Ets-1p/p mice are very similar to those found in a separate line of Ets-1 gene-targeted mice in which exons 8 and 9 (encoding the Ets DNA-binding domain) were deleted (5, 8, 15). Hence, we propose that the phenotype of these mice results from the near total absence of Ets-1 activity rather than from expression of a mutant version of Ets-1.
Loss of Ets-1 leads to alterations in the development of B lymphocytes that share some similarities with those found in other strains of gene-targeted mutant mice. In particular, mice deficient in the tyrosine kinase Lyn have large numbers of IgM-secreting plasma cells in their peripheral lymphoid organs and produce auto-antibodies (24, 25). Ets-1p/p B cells express normal levels of Lyn (by western analysis, unpublished results), suggesting that the defect in these mice is not attributable to reduced expression of Lyn. Ets-1p/p mice also exhibit a number of phenotypic similarities to mice lacking the transcription factor Aiolos. In Aiolos−/− mice, follicular B cells exhibit an activated cell-surface phenotype and secrete auto-antibodies, leading to the deposition of immune complexes in the kidney (26, 27). Aiolos−/− mice, like Ets-1p/p mice, lack marginal zone B cells, although they differ from Ets-1p/p mice in that they also lack B-1 B cells (26). Ets-1p/p mice also differ from Aiolos−/− mice in that they do not appear to form spontaneous germinal centers and lack elevated serum titers of IgG or IgE.
Ets-1p/p mice have normal numbers of B-1a B cells in the peritoneal cavity and B-2 B cells in the lymphoid organs, but lack marginal zone B cells in the spleen. This is in contrast to a report by Eyquem et al. (8) that mice harboring an independently generated Ets-1 mutation lack both B-1a B cells and marginal zone B cells. The explanation for this difference in phenotype is currently unclear. However, B-1a B cell development may require only residual levels of Ets-1 activity (present in Ets-1p/p mice), but may be incompatible with a complete absence of Ets-1 activity (reported for mice harboring the other Ets-1-targeted allele). Alternatively, the difference may be explained by genetic background effects (Ets-1p/p mice are on a mixed 129Sv × C57BL/6 background, whereas the mice of Eyquem et al. are on a C57BL/6 background).
Ets-1p/p mice and the Ets-1-deficient mice described by Eyquem et al. lack marginal zone B cells, indicating a critical role for Ets-1 in regulating the development or survival of this population. Ets-1 may be required for the expression of one or more genes needed for efficient development of the marginal zone B cell population. Alternatively, marginal zone B cells in Ets-1p/p mice may be depleted via rapid terminal differentiation to IgM-secreting plasma cells. We favor this latter explanation because previous studies have demonstrated that marginal zone B cells are highly responsive to T cell-independent stimuli that drive plasmacytic differentiation (28, 29). We hypothesize that Ets-1p/p marginal zone B cells are even more prone to the differentiation signals than are Ets-1+/+ marginal zone B cells and therefore undergo rapid terminal differentiation, leading to their depletion in vivo.
Ets-1p/p mature B cells express reduced levels of surface IgM, suggesting the possibility that Ets-1 may be required for expression of the Ig heavy chain gene. Indeed, Ets-1 has been reported to directly bind to the Ig heavy chain intronic enhancer and to contribute to the activity of this enhancer element (30). However, Ets-1p/p B cells express normal surface levels of IgD and Ets-1p/p plasma cells have high levels of intracellular IgM, indicating that Ets-1 is not absolutely required for the expression of the Ig heavy chain. Surface IgM is down-regulated on anergic B cells expressing a transgenic BCR specific for hen egg lysozyme (HEL) exposed to self-antigen (31). Many Ets-1p/p B cells may be autoreactive as suggested by the presence of high titers of autoreactive antibodies. Hence, it is possible that low expression of IgM on mature Ets-1p/p B cells reflects their chronic activation by self-antigens. However, we have recently generated Ets-1p/p mice harboring a transgenic BCR specific for HEL (but lacking the expression of HEL as a self-antigen). In these transgenic mice, IgM levels on follicular B cells are still reduced as compared with those on wild-type HEL BCR transgenic B cells (Shinu A. John and Lee Ann Garrett-Sinha, unpublished results). Hence, antigenic stimulation does not appear to be involved in modulating the levels of surface IgM on Ets-1p/p B cells. Instead, Ets-1 may regulate genes involved in IgM trafficking.
The expression of CD23 on Ets-1p/p follicular B cells is strikingly elevated as compared with wild-type follicular B cells, with Ets-1+/p B cells exhibiting an intermediate level of staining. MHC II, CD80 and CD86 showed smaller, but reproducible, increases in Ets-1p/p B cells as compared with wild-type or Ets-1+/p B cells. The expression of all these activation markers can be induced on B cells by exposure to activation signals including T cell-derived signals such as IL-4 or CD40L (32–38). Thus, increased levels of CD23, CD80, CD86 and MHC II on Ets-1p/p B cells could potentially reflect increased T cell activity. Supporting this model is the data from our adoptive transfer experiments in which Ets-1p/p B cells down-regulated activation markers when transferred into wild-type hosts and Ets-1+/+ B cells up-regulated activation markers when transferred into Ets-1p/p hosts. Indeed, flow cytometry analysis has shown that an increased proportion of T cells in Ets-1p/p mice expresses surface markers consistent with a memory or effector phenotype (Shinu A. John and Lee Ann Garrett-Sinha, unpublished results). The presence of activated T cells in these animals would be consistent with the production of autoreactive IgG antibodies.
Our data demonstrate that Ets-1-deficient B cells also have cell-intrinsic changes as they exhibited increased rates of differentiation to IgM-secreting plasma cells when stimulated in vitro via TLR9. This observation suggests that Ets-1 functions to limit TLR9 signaling pathways and thereby limits B cell terminal differentiation. To our knowledge, this represents the first description of a transcription factor that acts to inhibit TLR9 signaling pathways and it will be of considerable interest to identify Ets-1 target genes that regulate the TLR9 signaling pathway in future experiments. Several proteins have already been described that limit signaling through TLR pathways including interleukin-1 receptor associated kinase (IRAK-2), IRAK-M, Triad3A and single Ig IL-1R-related molecule (SIGIRR) (39). Further studies will be required to determine if one or more of the genes encoding these proteins is misexpressed in Ets-1p/p B cells.
The regulation of the TLR9 response by Ets-1 is an interesting observation because TLR9-mediated signals have been implicated in the development or progression of a number of autoimmune diseases using animal models (40–45). Recent evidence suggests that TLR9-mediated activation of immune cells may also be relevant to human autoimmune diseases (46). Mechanisms by which CpG-containing DNA complexes can stimulate autoreactive B cells have been studied using anti-IgG (rheumatoid factor) B cells and anti-DNA B cells (47, 48). In these autoreactive cells, binding of the BCR to DNA molecules or to immune complexes containing DNA leads to internalization of the DNA, stimulation of TLR9-dependent signaling pathways and activation of B cell proliferation (47, 48). Thus, CpG motifs represent a potential danger to the organism by promoting the activation of autoreactive B cells. The transcription factor Ets-1, by restraining B cell differentiation in response to CpG motifs, may play an important role in limiting TLR9-mediated promotion of autoimmune diseases.
In contrast to the excessive differentiation induced by TLR9 activation, purified Ets-1-deficient B cells did not exhibit enhanced differentiation in response to LPS. Indeed, the numbers of IgM-secreting plasma cells detected in LPS-stimulated Ets-1p/p cultures were reduced as compared with those found in control cultures. Although the decreased response to LPS may reflect inherent alterations in this pathway, it is also possible that the decreased responses to LPS activation reflect the reduced numbers of marginal zone B cells in Ets-1p/p mice since marginal zone B cells are reported to be much more responsive to LPS-induced differentiation than are follicular B cells (28, 29).
The increased B cell activity in Ets-1p/p mice is correlated with the development of autoimmune disease. Mice with autoimmune syndromes often exhibit the deposition of immune complexes in their kidneys (24, 49–54). Such complexes can function to recruit other inflammatory cells and to activate the complement, leading to severe kidney damage and proteinuria. Although Ets-1p/p mice have significant immune complexes in their kidneys, only mild glomerulonephritis was present and increased urinary protein levels were not detected in mice (up to ∼6 months of age). The fairly mild inflammation found in the kidney is somewhat counter-intuitive, given the high levels of auto-antibodies in the serum and the extensive immune complex deposition. However, it should be noted that Ets-1p/p mice have alterations in the T cell, NK cell and NK T cell compartments (6, 7, 14), which likely influence inflammatory cell recruitment and activation.
Our studies have shown that Ets-1-deficient B cells have an intrinsic propensity to undergo differentiation to plasma cells as demonstrated by their increased in vitro differentiation in response to CpG ODN. These results support the idea that Ets-1 is a critical negative regulator of B cell terminal differentiation induced by TLR9. As such, Ets-1 may be essential for limiting B cell responses to T cell-independent signaling pathways in vivo and for limiting the activation of autoreactive B cells exposed to TLR9 ligands.
Both authors contributed equally to these studies.
Transmitting editor: P. Ohashi
We thank Brian Grabiner and Rose-Anne Romano for their assistance with flow cytometry. These studies were supported by a Brian D. Novis Research Grant from the International Myeloma Foundation.
References
Reljic, R., Wagner, S. D., Peakman, L. J. and Fearon, D. T.
Lin, K. I., Angelin-Duclos, C., Kuo, T. C. and Calame, K.
Tunyaplin, C., Shaffer, A. L., Angelin-Duclos, C. D., Yu, X., Staudt, L. M. and Calame, K. L.
Lin, L., Gerth, A. J. and Peng, S. L.
Bories, J. C., Willerford, D. M., Grevin, D. et al.
Muthusamy, N., Barton, K. and Leiden, J. M.
Barton, K., Muthusamy, N., Fischer, C. et al.
Eyquem, S., Chemin, K., Fasseu, M. et al.
Seidel, J. J. and Graves, B. J.
Yang, B. S., Hauser, C. A., Henkel, G. et al.
Foulds, C. E., Nelson, M. L., Blaszczak, A. G. and Graves, B. J.
Maroulakou, I. G., Papas, T. S. and Green, J. E.
Kola, I., Brookes, S., Green, A. R. et al.
Walunas, T. L., Wang, B., Wang, C. R. and Leiden, J. M.
Eyquem, S., Chemin, K., Fasseu, M. and Bories, J. C.
Vollmer, J., Weeratna, R. D., Jurk, M. et al.
Pognonec, P., Boulukos, K. E., Bosselut, R., Boyer, C., Schmitt-Verhulst, A. M. and Ghysdael, J.
Koizumi, S., Fisher, R. J., Fujiwara, S. et al.
Dierks, S. E., Campbell, K. A., Studer, E. J. and Conrad, D. H.
Maliszewski, C. R., Grabstein, K., Fanslow, W. C., Armitage, R., Spriggs, M. K. and Sato, T. A.
Hacker, H., Mischak, H., Miethke, T. et al.
Yi, A. K. and Krieg, A. M.
Wasylyk, C., Bradford, A. P., Gutierrez-Hartmann, A. and Wasylyk, B.
Hibbs, M. L., Tarlinton, D. M., Armes, J. et al.
Nishizumi, H., Taniuchi, I., Yamanashi, Y. et al.
Wang, J. H., Avitahl, N., Cariappa, A. et al.
Sun, J., Matthias, G., Mihatsch, M. J., Georgopoulos, K. and Matthias, P.
Oliver, A. M., Martin, F., Gartland, G. L., Carter, R. H. and Kearney, J. F.
Oliver, A. M., Martin, F. and Kearney, J. F.
Nelsen, B., Tian, G., Erman, B. et al.
Goodnow, C. C., Crosbie, J., Adelstein, S. et al.
Conrad, D. H., Waldschmidt, T. J., Lee, W. T. et al.
Hasbold, J., Johnson-Leger, C., Atkins, C. J., Clark, E. A. and Klaus, G. G.
Roy, M., Aruffo, A., Ledbetter, J., Linsley, P., Kehry, M. and Noelle, R.
Hathcock, K. S., Laszlo, G., Pucillo, C., Linsley, P. and Hodes, R. J.
Stack, R. M., Lenschow, D. J., Gray, G. S., Bluestone, J. A. and Fitch, F. W.
Noelle, R. J., Kuziel, W. A., Maliszewski, C. R., McAdams, E., Vitetta, E. S. and Tucker, P. W.
Noelle, R., Krammer, P. H., Ohara, J., Uhr, J. W. and Vitetta, E. S.
Li, X. and Qin, J.
Waldner, H., Collins, M. and Kuchroo, V. K.
Anders, H. J., Vielhauer, V., Eis, V. et al.
Darabi, K., Karulin, A. Y., Boehm, B. O. et al.
Hasegawa, K. and Hayashi, T.
Segal, B. M., Chang, J. T. and Shevach, E. M.
Tsunoda, I., Tolley, N. D., Theil, D. J., Whitton, J. L., Kobayashi, H. and Fujinami, R. S.
Means, T. K., Latz, E., Hayashi, F., Murali, M. R., Golenbock, D. T. and Luster, A. D.
Leadbetter, E. A., Rifkin, I. R., Hohlbaum, A. M., Beaudette, B. C., Shlomchik, M. J. and Marshak-Rothstein, A.
Viglianti, G. A., Lau, C. M., Hanley, T. M., Miko, B. A., Shlomchik, M. J. and Marshak-Rothstein, A.
Shultz, L. D. and Green, M. C.
Andrews, B. S., Eisenberg, R. A., Theofilopoulos, A. N. et al.
Strasser, A., Whittingham, S., Vaux, D. L. et al.
Bouillet, P., Metcalf, D., Huang, D. C. et al.
Mackay, F., Woodcock, S. A., Lawton, P. et al.