-
PDF
- Split View
-
Views
-
Cite
Cite
Salvador Resino, José Mª Bellón, José Tomás Ramos, Milagros Gonzalez-Rivera, Mª Isabel de José, Mª Isabel González, Dolores Gurbindo, Mª José Mellado, Esther Cabrero, Mª Ángeles Muñoz-Fernaández, Positive virological outcome after lopinavir/ritonavir salvage therapy in protease inhibitor-experienced HIV-1-infected children: a prospective cohort study, Journal of Antimicrobial Chemotherapy, Volume 54, Issue 5, November 2004, Pages 921–931, https://doi.org/10.1093/jac/dkh431
Close - Share Icon Share
Abstract
Background: Lopinavir/ritonavir has demonstrated antiviral activity in the HIV-infected patient.
Objective: To analyse virological response to lopinavir/ritonavir therapy in previously protease inhibitor (PI)-experienced HIV-1-infected children.
Materials and methods: Sixty-seven HIV-1-children on lopinavir/ritonavir were studied in a multicentre prospective cohort observational study. The outcome variables were undetectable viral load (uVL; VL ≤400 copies/mL) and virological failure after uVL with a rebound of VL >400 copies/mL. VL and genotype of HIV-1-isolates were measured using standard assays.
Results: 83.5% of children had a 1 log10 VL decrease including 65.6% who reached uVL. Children with >2 changes of antiretroviral therapy (ART) or >5 drugs needed a median time of 3–4 months higher than children with ≤2 changes of ART or ≤5 drugs previous to lopinavir/ritonavir, to reach those values, and the relative proportions (RP) were 2.2 (P =0.038) and 1.9 (P=0.050), respectively. Children with CD4+>15% (P=0.122), VL ≤30 000 (P < 0.001) copies/mL, and age >12 years (P=0.096) achieved an earlier control of VL during the follow-up. The children with virological failure or rebound of VL had higher baseline VL and lower CD4+ T-lymphocytes/mm3 and had taken a greater number of drugs previous to lopinavir/ritonavir. HIV-children with a new nucleoside reverse transcriptase inhibitor (NRTI), or protease inhibitor (PI) or PI plus non-nucleoside reverse transcriptase inhibitors (NNRTI) in the current regimen had a better virological response than children without these new drugs. Also, children with <6 protease mutations had an RP of 2.31 of achieving uVL.
Conclusions: Highly active antiretroviral therapy (HAART) including lopinavir/ritonavir induces beneficial effects in terms of virological outcome responses, and it is an effective option for salvage therapy in PI-experienced HIV-1-infected children.
Introduction
One of the most important characteristics of HIV-1 infection in children is the presence of an immature immune system unable to control the HIV-1 replication efficiently allowing a higher plasma viral load (VL) than adults.1 Many adults on highly active antiretroviral therapy (HAART) achieve suppression of VL below the limits of assay detection [undetectable viral load (uVL): ≤400 copies/mL] along with an increase in CD4+ T-lymphocytes,2–4 with good outcome.5–7 However, HIV-1-infected children do not always reach and maintain uVL.5,8–11 Moreover, virological failure takes place quickly if a maximum suppression of the viral replication is not reached, allowing the appearance of HIV-1-quasispecies resistant to antiretroviral drugs,12 and thus, draining the limited arsenal of antiretroviral drugs available to fight against HIV-1 infections.13
Lopinavir (formerly ABT-378) is a protease inhibitor (PI) that, co-formulated with low-dose ritonavir (lopinavir/ritonavir), has shown potent antivirological activity in both antiretroviral-naive14 and single PI-experimented HIV-1-infected subjects.15,16 However, toxicity may also be increased and the benefit of such combinations must therefore be balanced against potential adverse events.
Genotypic and phenotypic analyses show that HIV-1 isolates, harbouring resistance mutations and displaying loss of susceptibility to currently available PIs, are prevalent among treated patients experiencing virological failure.17 The patterns of mutations selected by lopinavir/ritonavir in naive subjects have not been determined so far. However, genetic data correlated with reduced in vitro susceptibility to lopinavir in viral isolates from subjects failing on therapy with other PIs have been characterized.18 Those analyses revealed mutations in 11 amino acids of the HIV protease (L10F/I/R/V, K20M/R, L24I, M46I/L, F53L, I54L/T/V, L63P, A71I/L/T/V, V82A/F/T, I84V and L90M) that are likely to contribute to the reduced susceptibility to lopinavir. The number of these 11 mutations (referred to as the lopinavir mutation score) provides a potential method to evaluate virological response to lopinavir/ritonavir as a baseline genotype function.
We designed the present prospective cohort study to assess the virological, immunological and clinical response to lopinavir/ritonavir in combination with nucleoside reverse transcriptase inhibitor (NRTI) and protease inhibitor (PI) and/or non-nucleoside reverse transcriptase inhibitors (NNRTI) in a selected population of HIV-1-infected children with previous virological failure and on treatment with the three types of available antiretrovirals. In addition, we have addressed the safety and tolerability of lopinavir/ritonavir in these PI-experienced HIV-1-infected children.
Materials and methods
Selection of patients and study design
This study was a multicentre, observational, open, and prospective cohort study designed to evaluate the virological outcome after the association of lopinavir/ritonavir in triple, quadruple or quintuple combination antiretroviral therapy. The study population consisted of a cohort of 84 HIV-1-infected children followed in 12 Spanish hospitals between June 2000 and March 2003, in whom lopinavir/ritonavir had been given as salvage therapy after previous antiretroviral treatment and virological failure (VL >5000 copies/mL). The inclusion criteria were: (i) virological failure to antiretroviral therapy (ART) with a PI; (ii) VL >5000 copies/mL at entry of the study; (iii) having had at least 3 months on follow-up. Out of the 84 children, 67 children were included in this study to complete the inclusion criteria. Out of the 67 HIV-1-children, 44 children achieved uVL and were included in a sub-study of VL rebound. Four children with uVL were excluded because their follow-up periods were shorter than 3 months with uVL.
Laboratory markers of HIV-1 infection
T-lymphocyte subsets in peripheral blood were quantified by flow cytometry (FACScan; Becton-Dickinson Immunocytometry Systems, San Jose, CA, USA),19 and VL was measured in 200 μL of plasma using a quantitative assay (Amplicor monitor; Roche Diagnostic Systems, Brandenburg, NJ, USA).
Analysis of HIV-1 isolate genotype
Plasma samples for baseline viral genotype were collected between day −60 (60 days before initiation of lopinavir/ritonavir treatment) and day 1. Genotypic HIV-1 drug resistance was determined from plasma-associated HIV-1 RNA using the TruGene HIV-1 Resistance Kit (Visible Genetics, Toronto, Canada). The entire HIV-1 protease gene was analysed using Gene Objects software (Visible Genetics). Drug resistances were defined according to the IAS–USA consensus statement.20
Baseline genotypic resistances to PI were studied in 51 out of 67 samples of HIV-1-infected children because there was no biological sample for 16 children.
Study evaluation
A thorough medical history was taken, and physical examination was carried out during the screening period. A complete set of laboratory analyses (haematology, blood and urine biochemistry) was carried out at each study visit. The response to therapy was evaluated every 3 months by serial measurements of %CD4+ and %CD8+, VL, and by collecting clinical data, according to published guidelines.21
The dose of lopinavir/ritonavir every 12 h was dependent on the child weight: (i) 7–15 kg, the dose was 12 mg lopinavir/kg + 3 mg ritonavir/kg; (ii) >15–40 kg, the dose was 10 mg lopinavir/kg + 2.5 mg ritonavir/kg; (iii) >40 kg, the dose was 400 mg lopinavir + 100 mg ritonavir. Switches in clinical classification and ART were made according to clinical, immunological and virological evolution and following the aforementioned Centers for Disease Control (CDC) and European guidelines in paediatric treatment21–23 upon obtaining written informed consent from parents or legal guardians. Reduction of drug doses and temporal or permanent discontinuation followed strict protocol guidelines.
Each subject was closely monitored every 3 months throughout the course of study for the development of any clinical and/or laboratory evidence of an adverse event such as hypercholesterolaemia or triglyceridaemia, hepatotoxicity associated with antiretroviral therapy, hyperlactaemia, hypersensitivity reaction to ART, etc.
Exposure and outcome variables
During the whole follow-up period, all 67 HIV-1-children received lopinavir/ritonavir in triple, quadruple or quintuple combination therapy (Table 1). We analysed all data from the moment that children started treatment with lopinavir/ritonavir until they discontinued their regimens21–23 or, if they remained in the study, until their last clinic visit and last VL data available. We stratified the children according to baseline %CD4+, baseline VL, and baseline age. Also, we stratified the children according to number of ART protocol switches; number of antiretroviral drugs used, new drugs used in the current regimen (excluding lopinavir), and AIDS status before lopinavir/ritonavir.
We calculated the number of children that presented a decrease of 1 log10 in VL, or uVL (VL ≤400 copies/mL). We carried out an additional sub-study in 40 children to study the rebound of VL (VL > 400, 5000 and 10 000 copies/mL) after achieving uVL.
Statistics
Kaplan–Meier methods were used to determine the time taken to achieve a virological response to lopinavir/ritonavir. This was considered as the time between the baseline time point and the appearance of event. We used Kaplan–Meier curves to evaluate the virological response (uVL) according to baseline characteristics. We examined VL decreases of 1 log10 copies/mL following the start of lopinavir/ritonavir. Cox regression analyses were carried out in order to assess the relative proportion (RP) of uVL according to baseline characteristics.
Results
Characteristics of the HIV-1-infected children
Sixty-seven children on lopinavir/ritonavir were studied throughout a follow-up period of 16.8 months (range: 6–27.8) in 12 Spanish hospitals. Table 1 show ART regimens administered to the 67 children pre-lopinavir/ritonavir as well as those after beginning the treatment with lopinavir/ritonavir. During the whole clinical follow-up period, none of the children progressed to AIDS or death. Clinical, immunological and virological characteristics at baseline (entry of the study) are shown in Table 2.
Evolution of plasma viral load and CD4+ T-lymphocytes
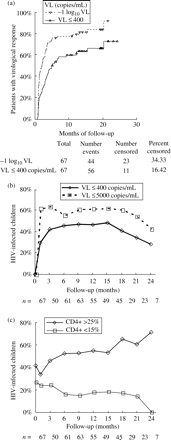
Figure 1(a) shows the proportion of HIV-1-infected children who achieved a decrease of 1 log10 in VL (–1 log10 copies/mL) or uVL (≤400 copies/mL) after starting a new HAART regimen that included lopinavir/ritonavir. During the follow-up period, 83.5% of children had at least a drop of 1 log10 in their VL, and 65.6% children achieved uVL. In those children, the median time to achieve uVL was 4.9±1.4 months (95% CI: 2.2; 7.7). Since virological failure at baseline was defined as VL > 5000 copies/mL (3.7 log10 copies/mL), we also analysed the percentage of children who achieved ≤5000 copies/mL or uVL during the follow-up period (Figure 1b). Next, we analysed the percentage of children who achieved >25% CD4+ T-lymphocytes (Figure 1c). Therefore, after treatment with lopinavir/ritonavir in combination therapy, the percentage of HIV-1-infected children with CD4+ >25% increased until the end of the study.
Complete virological response during follow-up according to previous ART to lopinavir/ritonavir and new drugs in HAART with lopinavir
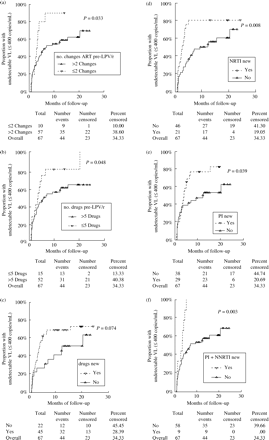
HIV-1-infected children with a history of >2 changes of ART or >5 different drugs used previous to lopinavir/ritonavir presented a worse response to lopinavir/ritonavir (Figure 2a and b and Table 3). Thus, those children had a median of time to achieve uVL 3–4 months higher than children who had only ≤2 changes of ART or ≤5 drugs previous to lopinavir/ritonavir (Figure 2a and b) and RP statistically significant (Table 3). Forty-five HIV-infected children had new drugs in the current HAART protocol (without regarding lopinavir), and they presented a better virological response than children without any new drug (Figure 2c and Table 3), although the differences were not statistically significant (P=0.074). However, HIV-infected children with a new NRTI, or PI or PI plus NNRTI in the current regimen had a better virological response than children without these new drugs (Figure 2d– and Table 3). In contrast, the number of drugs received in the current regimen did not show an association with the virological response. In the Cox regression multivariate analysis, VL ≤30 000 copies/mL (RP: 3.37; 95% CI: 1.57; 7.23), >12 years old (RP: 2.26; 95% CI: 1.11; 4.61), and current HAART with new NRTI (RP: 1.94; 95% CI: 0.97; 3.88) had a positive association with uVL during the follow-up.
Complete virological response during follow-up according to baseline characteristics
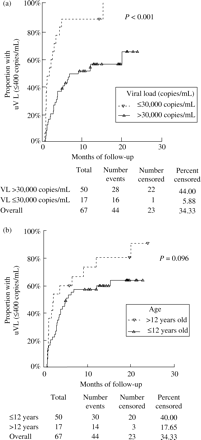
Children with CD4+ T-lymphocytes >15% at baseline achieved uVL in a shorter time than children with CD4+ ≤15%, although the differences were not statistically significant (P=0.122). The percentage of children who reached it was 61.5% and 50%, respectively. In addition, children who had being diagnosed with AIDS before the new treatment with lopinavir/ritonavir did not have a statistically significant different virological response compared with those who did not have AIDS (61.7% and 69.7% reached uVL, respectively). In contrast, lower values of VL (VL ≤30 000 copies/mL) or older age at baseline (>12 years) were directly associated with achieving uVL earlier (Table 3). Moreover, children with VL ≤30 000 copies/mL or with age >12 years achieved an earlier control of their VL during the follow-up period, compared with those children with VL >30 000 copies/mL or younger (Figure 3a and b). Thus, 94.2% of children with VL ≤ 30 000 copies/mL and 82.5% of children with age >12 years achieved uVL during the follow-up.
Virological failure after lopinavir/ritonavir treatment
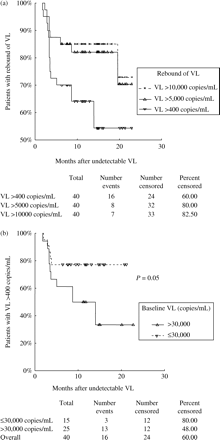
As we mentioned before, 44 out of 67 children on lopinavir/ritonavir achieved uVL. Four children with uVL were excluded because their follow-up periods were shorter than 3 months on follow-up with uVL. The immunological, virological, and antiretroviral therapy characteristics of 40 HIV-children are shown in Table 4. The children who presented a rebound of VL were those who at baseline showed higher values of VL, higher numbers of antiretroviral drugs before lopinavir/ritonavir and lower CD4+ T-lymphocyte counts (Table 4). Figure 4(a) shows the rebound of VL during the follow-up period. We have shown that a lower percentage of children with VL ≤30 000 copies/mL at baseline had less rebound of VL during the follow-up period than those with VL >30 000 copies/mL (Figure 4b). Thus, children with VL >30 000 copies/mL at baseline had an RP of rebound of VL (VL >400 copies/mL) of 3.2 (95% CI: 0.9; 11.3; P=0.068).
Protease mutations at baseline
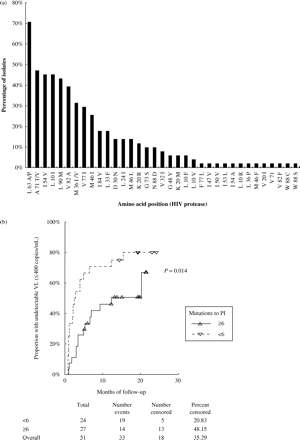
Baseline genotypic resistances to PI were studied in 51 out of 67 samples of HIV-1-infected children (Figure 5a). At baseline, the median number of protease mutations was 6 (1–11). In seven out of 51 (13.7%) children, we did not find any mutations and in four (7.8%) we only found one protease mutation. Forty (78.4%) HIV-1-infected children had HIV-1 isolates with more than one mutation associated with PI, reflecting the ART history. Twenty-six (65%) out of these 40 children had >7 protease mutations at baseline.
The most frequent protease mutations were L63A/P (71%), A71T/V (47%), I54V (45%), L90M (43%) and V82 (39%) (Figure 5a). We did not find any particular mutations associated with virological failure. However, children with HIV-1 isolates with <6 protease mutations at baseline achieved uVL more quickly than children with HIV-1 isolates with >6 protease mutations (Figure 5b). Thus, HIV-1-infected children with viral isolates with <6 protease mutations had an RP of 2.31 (95% CI: 1.15; 4.64) to achieve uVL. These results indicate that the number of protease mutations can predict more accurately the response to lopinavir/ritonavir than the presence of any single mutation or specific patterns of multiple mutations.
Adverse events
Adverse events during the follow-up study were reported in 30 out of 67 (44.7%) children, mainly during the first month of treatment. The most frequent events were gastrointestinal: diarrhoea in 19 (28%) children, vomiting in 12 (18%), and nausea in two (3%) children. In addition, abdominal pain (grade 1–2) occurred in eight (12%) children and exanthema in one child. Paired fasting cholesterol and triglyceride plasma levels were determined during the follow-up. Cholesterol levels were >250 mg/dL in 23.7% of children, non-fasting triglyceride levels >400 mg/dL in 23% of children, and glucose levels >110 mg/100 mL in 15% of children. There were not significant increases in the remaining laboratory parameters over time, including haematology markers, aminotransferases, amylase, alkaline phosphatase or creatinine levels. Four out of 67 (6%) HIV-1-children changed their ART due to toxicity and/or intolerance. Another two out of 67 children temporarily interrupted their ART due to diarrhoea and vomiting during 1–2 weeks and were introduced again in the study later.
Discussion
This is one of the first studies to characterize the clinical, virological and immunological response to a combined lopinavir/ritonavir therapy in heavily pre-treated HIV-1-infected children. These children had virological failure before starting on combination therapy with lopinavir/ritonavir. ART failure is a common, significant problem and as many as 50% of HIV-1-infected patients have detectable VL despite being on combination ART.24 Those patients who do not reach viral suppression during first-line PI-based therapies are less likely to have a virological response to a second-line PI therapy.8,25 In our study, 44 (65.6%) children reached uVL (≤400 copies/mL) during the follow-up. These results are in agreement with the median times for achieving a decrease in VL in heavily pre-treated HIV-1-infected adults26,27 and children.8,10 Interestingly, the virological response of HIV-infected children on first-line HAART was faster than the virological response of HIV-infected children on second-line HAART.8 Moreover, an increase in CD4+ and clinical improvement took place,28,29 even in children with virological failure.30,31
In addition to their demonstrated antiviral efficacy, the ability of PIs to induce immune recovery is widely recognized.8,32,33 Although there are not yet any published data on the effectiveness of lopinavir/ritonavir in restoring specific immune responses, in our study we observed a significant increase in CD4+ T-lymphocytes in all lopinavir/ritonavir-treated children. This occurred despite the lack of a complete virological response or even in children who had already experienced a significant CD4 T-lymphocyte response with their previous antiretroviral regimens.
Our study shows that prior ART treatments of the experienced children are associated with virological failure, as has previously been reported for adults34,35 and children.8,36 This is noteworthy, given the clinical concern regarding the effectiveness of the combination therapy with lopinavir/ritonavir in children. Sustained suppression of viral replication can be achieved in HIV-1-infected children on HAART.37,38 However, it is difficult to obtain a sustained decrease in VL below the detection limits of the assays.6,39 The influences of the treatment regimen, baseline VL, and prior ART on HAART effectiveness have already been reported.26,34,40 In our study, children who had not taken any new NNRTI and PI in their previous regimen to lopinavir/ritonavir were more likely to suppress VL, as has been reported.41 However, when a new NNRTI and PI were present in their current lopinavir/ritonavir regimen, their chances to suppress VL increased, just as when adding a new NRTI or PI (see Results). Previous reports have shown unfavourable interaction between lopinavir/ritonavir and amprenavir.42,43 In our study, only four children received amprenavir as a third PI, but we did not observe a worse virological response. This may be due to the reduced sample size. The influence of having AIDS before the new treatment with lopinavir/ritonavir was not statistically significantly associated either positively or negatively with virological response (achieving uVL).
Factors associated with therapeutic failure may have a different significance. Our data indicate that the children with a rebound in VL were those who had worse clinical pre-lopinavir/ritonavir evolution, with a more deteriorated immune system, leading to a poorer control of viral replication and more treatment switches, with a greater number of drugs before combination therapy with lopinavir/ritonavir. Thus, we have shown that at baseline, a VL > 30 000 copies/mL leads to VL rebound after achieving uVL, as has been previously reported in other PI studies.8,26,34,40,44–46 This failure to reach or maintain the suppression of VL may be attributed to a variety of factors, including, but not limited to, suboptimal pharmacokinetics, marginal potency, side effects, and inadequate adherence to complicated dosing requirements of regimens. Persistent viral replication as a consequence of inadequate drug levels or intermittent adherence may result in loss of antiviral activity due to the development of drug resistances.
On the other hand, HIV-infected children more than 12 years old had a positive virological response. This can be due to the natural selection occurring in the study population. The children better adapted to HIV-infection and surviving for a longer time, were accumulated with newly-recruited HIV-infected children on HAART, accounting for the overall increase in their survival and mean age observed.
In this study, we also analysed the effect of the viral genotype at baseline on the virological response to combination therapy with lopinavir/ritonavir in multiple PI-experienced children. We did not find specific mutations associated with virological outcome. We observed that the presence of ≥6 protease mutations at baseline was associated with a reduced virological response to lopinavir/ritonavir. This would explain why these children achieved uVL more slowly than children with HIV-1 isolates with <6 protease mutations. Previous studies reported that an increasing number of protease baseline mutations generally worsened the virological response15,47,48 although this did not always happen.41 In the multivariate analyses we did not find an association between specific baseline protease mutations and virological response. Despite several studies,15,18,49,50 the exact pattern of lopinavir/ritonavir resistance is not yet known and the beneficial role for lopinavir/ritonavir in salvage antiretroviral therapy remains under debate. Thus, investigation of specific mutation patterns associated with diminished response to lopinavir/ritonavir in HIV-1-infected children is needed.
Regarding tolerance, combination therapy with lopinavir/ritonavir in PI-experienced children was well tolerated. The most common drug-related adverse events were of gastrointestinal nature, and the most common laboratory abnormality was lipid elevation. These are also the most common adverse events seen in adult patients.16,51–53 However, the increases in plasma cholesterol or triglycerides in the studied children were lower than those present in adults.54 Furthermore, it is very important to take into account that all the children were ART-experienced before starting combination therapy with lopinavir/ritonavir. This suggests that those various ART drugs may have also increased cholesterol, as shown by the proportion of children with elevated lipid at entry into the study. Additional follow-ups of large cohorts of children for longer periods of time will be required to establish the clinical significance of the elevated lipid levels observed during lopinavir/ritonavir treatment, especially with respect to the impact on body fat distribution abnormalities and long-term cardiovascular risk.
This is a prospective study; it may have some potential limitations since antiretroviral treatment was not randomized, and the selection of drug regimen, toxicity management and assessment of virological failure were all carried out according to the primary care physicians. In addition, patients were taking a number of different antiretroviral regimens, which differed in their composition. Moreover, adherence was difficult to evaluate. Thus, the antiretroviral treatments introduce heterogeneity in our study. Also, HIV-infected patients with virological suppression on HAART show intermittent episodes of low-level of VL in correlation with slower decay rates of latently infected cells and increased levels of viral evolution.55 In our present study, we assessed VL every 3 months. This means that VL may have increased >400 copies/mL between measurements. Abrupt increases in VL levels are observed whenever the therapeutic regimen is reduced.56 In some patients, an increase in VL below the threshold of detection (0–400 copies/mL) may represent an overall less effective viral suppression. This intermittent viraemia increases the emergence of subpopulations of drug-resistant viruses.57
In conclusion, our results indicate a beneficial clinical effect and a good immunological and virological response to lopinavir/ritonavir in treatment-experienced HIV-1-infected children with previous virological failure and with HIV-1 isolates with protease mutations, and support their prescription in salvage therapies. Lopinavir/ritonavir is an effective option for the treatment of HIV-1-infected children used in combination with other antiretroviral agents. Therefore it may be used as a component of initial therapy or salvage therapy; future studies will better define its place in therapy.
Kaplan–Meier estimates for viral load (VL) evolution with respect to baseline levels during the follow-up with lopinavir/ritonavir (a), and the proportion of HIV-1-children with uVL (b) and >25% CD4 cells during follow-up (c). n, number of HIV-infected children.
Kaplan–Meier estimates for undetectable viral load (VL) during the follow-up period according to ART previous to lopinavir/ritonavir (a and b) and new drugs the children received (c–f).
Kaplan–Meier estimates for undetectable viral load (VL) during the follow-up period according to baseline characteristics.
Kaplan–Meier for rebound of viral load (VL) after achieving an undetectable VL (a), and according to baseline VL (b).
Genotypic HIV-1-mutations to protease inhibitor. Prevalence of baseline mutations associated with protease inhibitor resistance (a). Kaplan–Meier estimates for undetectable viral load (VL) according to baseline HIV-1-mutations (b).
Characteristics of antiretroviral treatment of the HIV-1-infected children previous to baseline and at baseline
| Characteristics . | Values . | |
|---|---|---|
| Previous to baseline | ||
| time (months) with ARTa | 79.3±4.5 (13.3; 156) | |
| ART-protocols switchesa | 5.7±0.4 (1; 15) | |
| drug counta | 7.3±0.26 (3; 12) | |
| HAART-protocol with PI | 67 | |
| time (months) with PIa | 26.8±1.5 (3.2; 55.1) | |
| HAART-protocol with NNRTI | 14 | |
| time (months) with NNRTIa | 10.8±1.6 (0.5; 23.0) | |
| HAART-protocol with NNRTI + PI | 40 | |
| time (months) with NNRTI + PIa | 18.9±1.8 (0.7; 50.5) | |
| During the follow-up study | ||
| time (months) with LPV/ra | 16.7±0.6 (5; 27.8) | |
| HAART protocol (new drugs in children exclude LPV) | 45 | |
| NRTI | 21 | |
| NNRTI | 15 | |
| PI | 29 | |
| HAART protocol (NRTIs + …) | ||
| LPV/r | 35 | |
| LPV/r + 1 PI | 8 | |
| LPV/r + 2 PI | 1 | |
| LPV/r + 1 NNRTI | 21 | |
| LPV/r + 1 PI + 1 NNRTI | 2 | |
| protease inhibitor with LPV/r | ||
| saquinavir | 4 | |
| nelfinavir | 4 | |
| amprenavir | 4 | |
| non-nucleoside analogue with LPV/r | ||
| nevirapine | 5 | |
| efavirenz | 18 | |
| Characteristics . | Values . | |
|---|---|---|
| Previous to baseline | ||
| time (months) with ARTa | 79.3±4.5 (13.3; 156) | |
| ART-protocols switchesa | 5.7±0.4 (1; 15) | |
| drug counta | 7.3±0.26 (3; 12) | |
| HAART-protocol with PI | 67 | |
| time (months) with PIa | 26.8±1.5 (3.2; 55.1) | |
| HAART-protocol with NNRTI | 14 | |
| time (months) with NNRTIa | 10.8±1.6 (0.5; 23.0) | |
| HAART-protocol with NNRTI + PI | 40 | |
| time (months) with NNRTI + PIa | 18.9±1.8 (0.7; 50.5) | |
| During the follow-up study | ||
| time (months) with LPV/ra | 16.7±0.6 (5; 27.8) | |
| HAART protocol (new drugs in children exclude LPV) | 45 | |
| NRTI | 21 | |
| NNRTI | 15 | |
| PI | 29 | |
| HAART protocol (NRTIs + …) | ||
| LPV/r | 35 | |
| LPV/r + 1 PI | 8 | |
| LPV/r + 2 PI | 1 | |
| LPV/r + 1 NNRTI | 21 | |
| LPV/r + 1 PI + 1 NNRTI | 2 | |
| protease inhibitor with LPV/r | ||
| saquinavir | 4 | |
| nelfinavir | 4 | |
| amprenavir | 4 | |
| non-nucleoside analogue with LPV/r | ||
| nevirapine | 5 | |
| efavirenz | 18 | |
ART, antiretroviral therapy; HAART, highly active antiretroviral therapy; PI, protease inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; LPV/r, lopinavir/ritonavir.
Values are expressed as mean±S.E.M. (min; max).
Characteristics of antiretroviral treatment of the HIV-1-infected children previous to baseline and at baseline
| Characteristics . | Values . | |
|---|---|---|
| Previous to baseline | ||
| time (months) with ARTa | 79.3±4.5 (13.3; 156) | |
| ART-protocols switchesa | 5.7±0.4 (1; 15) | |
| drug counta | 7.3±0.26 (3; 12) | |
| HAART-protocol with PI | 67 | |
| time (months) with PIa | 26.8±1.5 (3.2; 55.1) | |
| HAART-protocol with NNRTI | 14 | |
| time (months) with NNRTIa | 10.8±1.6 (0.5; 23.0) | |
| HAART-protocol with NNRTI + PI | 40 | |
| time (months) with NNRTI + PIa | 18.9±1.8 (0.7; 50.5) | |
| During the follow-up study | ||
| time (months) with LPV/ra | 16.7±0.6 (5; 27.8) | |
| HAART protocol (new drugs in children exclude LPV) | 45 | |
| NRTI | 21 | |
| NNRTI | 15 | |
| PI | 29 | |
| HAART protocol (NRTIs + …) | ||
| LPV/r | 35 | |
| LPV/r + 1 PI | 8 | |
| LPV/r + 2 PI | 1 | |
| LPV/r + 1 NNRTI | 21 | |
| LPV/r + 1 PI + 1 NNRTI | 2 | |
| protease inhibitor with LPV/r | ||
| saquinavir | 4 | |
| nelfinavir | 4 | |
| amprenavir | 4 | |
| non-nucleoside analogue with LPV/r | ||
| nevirapine | 5 | |
| efavirenz | 18 | |
| Characteristics . | Values . | |
|---|---|---|
| Previous to baseline | ||
| time (months) with ARTa | 79.3±4.5 (13.3; 156) | |
| ART-protocols switchesa | 5.7±0.4 (1; 15) | |
| drug counta | 7.3±0.26 (3; 12) | |
| HAART-protocol with PI | 67 | |
| time (months) with PIa | 26.8±1.5 (3.2; 55.1) | |
| HAART-protocol with NNRTI | 14 | |
| time (months) with NNRTIa | 10.8±1.6 (0.5; 23.0) | |
| HAART-protocol with NNRTI + PI | 40 | |
| time (months) with NNRTI + PIa | 18.9±1.8 (0.7; 50.5) | |
| During the follow-up study | ||
| time (months) with LPV/ra | 16.7±0.6 (5; 27.8) | |
| HAART protocol (new drugs in children exclude LPV) | 45 | |
| NRTI | 21 | |
| NNRTI | 15 | |
| PI | 29 | |
| HAART protocol (NRTIs + …) | ||
| LPV/r | 35 | |
| LPV/r + 1 PI | 8 | |
| LPV/r + 2 PI | 1 | |
| LPV/r + 1 NNRTI | 21 | |
| LPV/r + 1 PI + 1 NNRTI | 2 | |
| protease inhibitor with LPV/r | ||
| saquinavir | 4 | |
| nelfinavir | 4 | |
| amprenavir | 4 | |
| non-nucleoside analogue with LPV/r | ||
| nevirapine | 5 | |
| efavirenz | 18 | |
ART, antiretroviral therapy; HAART, highly active antiretroviral therapy; PI, protease inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; LPV/r, lopinavir/ritonavir.
Values are expressed as mean±S.E.M. (min; max).
Clinical, immunological and virological parameters of the HIV-1-infected children at baseline
| Characteristics . | Values . | |
|---|---|---|
| No. HIV-1 children | 67 | |
| Age (years)a | 9.4±0.5 (1.4; 17.1) | |
| Clinical category (CDC)b | ||
| A | 10 (15%) | |
| B | 23 (34%) | |
| C | 34 (51%) | |
| Immunological category (CDC)b | ||
| >25% CD4+ T-lymphocytes | 18 (26.9%) | |
| 15–25% CD4+ T-lymphocytes | 21 (31.9%) | |
| <15% CD4+ T-lymphocytes | 28 (41.8%) | |
| Immunological parametersa | ||
| % CD4+ T-lymphocytes | 21.5±10.1 (0.5; 49) | |
| CD4+ T-lymphocytes/mm3 | 698.8±72.1 (6; 3244) | |
| % CD8+ T-lymphocytes | 46.5±13.2 (17; 81) | |
| CD8+ T-lymphocytes/mm3 | 1476.1±131.5 (216; 4500) | |
| Virological characteristics | ||
| log10 VL (copies/mL)a | 4.89±0.07 (3.8; 6) | |
| VL ≤10 000 copies/mLb | 3 (4.5%) | |
| VL 10 000–30 000 copies/mLb | 14 (20.9%) | |
| VL 30 000–100 000 copies/mLb | 23 (34.3%) | |
| VL >100 000 copies/mLb | 27 (40.3%) | |
| Characteristics . | Values . | |
|---|---|---|
| No. HIV-1 children | 67 | |
| Age (years)a | 9.4±0.5 (1.4; 17.1) | |
| Clinical category (CDC)b | ||
| A | 10 (15%) | |
| B | 23 (34%) | |
| C | 34 (51%) | |
| Immunological category (CDC)b | ||
| >25% CD4+ T-lymphocytes | 18 (26.9%) | |
| 15–25% CD4+ T-lymphocytes | 21 (31.9%) | |
| <15% CD4+ T-lymphocytes | 28 (41.8%) | |
| Immunological parametersa | ||
| % CD4+ T-lymphocytes | 21.5±10.1 (0.5; 49) | |
| CD4+ T-lymphocytes/mm3 | 698.8±72.1 (6; 3244) | |
| % CD8+ T-lymphocytes | 46.5±13.2 (17; 81) | |
| CD8+ T-lymphocytes/mm3 | 1476.1±131.5 (216; 4500) | |
| Virological characteristics | ||
| log10 VL (copies/mL)a | 4.89±0.07 (3.8; 6) | |
| VL ≤10 000 copies/mLb | 3 (4.5%) | |
| VL 10 000–30 000 copies/mLb | 14 (20.9%) | |
| VL 30 000–100 000 copies/mLb | 23 (34.3%) | |
| VL >100 000 copies/mLb | 27 (40.3%) | |
VL, viral load; CDC, Centers for Disease Control.
Values are expressed as: amean±S.E.M. (min; max); and
absolute (percentage).
Clinical, immunological and virological parameters of the HIV-1-infected children at baseline
| Characteristics . | Values . | |
|---|---|---|
| No. HIV-1 children | 67 | |
| Age (years)a | 9.4±0.5 (1.4; 17.1) | |
| Clinical category (CDC)b | ||
| A | 10 (15%) | |
| B | 23 (34%) | |
| C | 34 (51%) | |
| Immunological category (CDC)b | ||
| >25% CD4+ T-lymphocytes | 18 (26.9%) | |
| 15–25% CD4+ T-lymphocytes | 21 (31.9%) | |
| <15% CD4+ T-lymphocytes | 28 (41.8%) | |
| Immunological parametersa | ||
| % CD4+ T-lymphocytes | 21.5±10.1 (0.5; 49) | |
| CD4+ T-lymphocytes/mm3 | 698.8±72.1 (6; 3244) | |
| % CD8+ T-lymphocytes | 46.5±13.2 (17; 81) | |
| CD8+ T-lymphocytes/mm3 | 1476.1±131.5 (216; 4500) | |
| Virological characteristics | ||
| log10 VL (copies/mL)a | 4.89±0.07 (3.8; 6) | |
| VL ≤10 000 copies/mLb | 3 (4.5%) | |
| VL 10 000–30 000 copies/mLb | 14 (20.9%) | |
| VL 30 000–100 000 copies/mLb | 23 (34.3%) | |
| VL >100 000 copies/mLb | 27 (40.3%) | |
| Characteristics . | Values . | |
|---|---|---|
| No. HIV-1 children | 67 | |
| Age (years)a | 9.4±0.5 (1.4; 17.1) | |
| Clinical category (CDC)b | ||
| A | 10 (15%) | |
| B | 23 (34%) | |
| C | 34 (51%) | |
| Immunological category (CDC)b | ||
| >25% CD4+ T-lymphocytes | 18 (26.9%) | |
| 15–25% CD4+ T-lymphocytes | 21 (31.9%) | |
| <15% CD4+ T-lymphocytes | 28 (41.8%) | |
| Immunological parametersa | ||
| % CD4+ T-lymphocytes | 21.5±10.1 (0.5; 49) | |
| CD4+ T-lymphocytes/mm3 | 698.8±72.1 (6; 3244) | |
| % CD8+ T-lymphocytes | 46.5±13.2 (17; 81) | |
| CD8+ T-lymphocytes/mm3 | 1476.1±131.5 (216; 4500) | |
| Virological characteristics | ||
| log10 VL (copies/mL)a | 4.89±0.07 (3.8; 6) | |
| VL ≤10 000 copies/mLb | 3 (4.5%) | |
| VL 10 000–30 000 copies/mLb | 14 (20.9%) | |
| VL 30 000–100 000 copies/mLb | 23 (34.3%) | |
| VL >100 000 copies/mLb | 27 (40.3%) | |
VL, viral load; CDC, Centers for Disease Control.
Values are expressed as: amean±S.E.M. (min; max); and
absolute (percentage).
Results of Cox regression analysis to achieve undetectable viral load (VL ≤ 400 copies/mL) during follow-up
| . | RP . | 95% CI . | P value . | |||
|---|---|---|---|---|---|---|
| ART previous to LPV/r | ||||||
| ART-drug switches ≤2 | 2.2 | 1.04; 4.66 | 0.038 | |||
| drugs count pre-LPV ≤5 | 1.9 | 0.99; 3.66 | 0.050 | |||
| NNRTI | 0.8 | 0.41; 1.81 | 0.714 | |||
| Drugs new in current HAART | ||||||
| LPV + drug newa | 1.8 | 0.93; 3.6 | 0.079 | |||
| LPV + NRTI newa | 2.2 | 1.20; 4.13 | 0.011 | |||
| LPV + NNRTI newa | 1.4 | 0.71; 2.82 | 0.318 | |||
| LPV + PI newa | 1.8 | 1.02; 3.44 | 0.043 | |||
| LPV/r + PI + NNRTI newa | 2.9 | 1.38; 6.43 | 0.005 | |||
| Baseline characteristics | ||||||
| no-AIDS | 1.3 | 0.72; 2.37 | 0.382 | |||
| >12 years old | 1.7 | 0.90; 3.22 | 0.100 | |||
| >15% CD4 cells | 1.7 | 0.83; 3.63 | 0.140 | |||
| ≤30 000 copies/mL VL | 3.6 | 1.90; 6.77 | <0.001 | |||
| . | RP . | 95% CI . | P value . | |||
|---|---|---|---|---|---|---|
| ART previous to LPV/r | ||||||
| ART-drug switches ≤2 | 2.2 | 1.04; 4.66 | 0.038 | |||
| drugs count pre-LPV ≤5 | 1.9 | 0.99; 3.66 | 0.050 | |||
| NNRTI | 0.8 | 0.41; 1.81 | 0.714 | |||
| Drugs new in current HAART | ||||||
| LPV + drug newa | 1.8 | 0.93; 3.6 | 0.079 | |||
| LPV + NRTI newa | 2.2 | 1.20; 4.13 | 0.011 | |||
| LPV + NNRTI newa | 1.4 | 0.71; 2.82 | 0.318 | |||
| LPV + PI newa | 1.8 | 1.02; 3.44 | 0.043 | |||
| LPV/r + PI + NNRTI newa | 2.9 | 1.38; 6.43 | 0.005 | |||
| Baseline characteristics | ||||||
| no-AIDS | 1.3 | 0.72; 2.37 | 0.382 | |||
| >12 years old | 1.7 | 0.90; 3.22 | 0.100 | |||
| >15% CD4 cells | 1.7 | 0.83; 3.63 | 0.140 | |||
| ≤30 000 copies/mL VL | 3.6 | 1.90; 6.77 | <0.001 | |||
LPV/r, lopinavir/ritonavir; RP, relative proportion; 95% CI, confidence interval of 95%; P, level of significance; ART, antiretroviral therapy; VL, viral load.
Beginning of HAART protocol with LPV and other new drugs.
Results of Cox regression analysis to achieve undetectable viral load (VL ≤ 400 copies/mL) during follow-up
| . | RP . | 95% CI . | P value . | |||
|---|---|---|---|---|---|---|
| ART previous to LPV/r | ||||||
| ART-drug switches ≤2 | 2.2 | 1.04; 4.66 | 0.038 | |||
| drugs count pre-LPV ≤5 | 1.9 | 0.99; 3.66 | 0.050 | |||
| NNRTI | 0.8 | 0.41; 1.81 | 0.714 | |||
| Drugs new in current HAART | ||||||
| LPV + drug newa | 1.8 | 0.93; 3.6 | 0.079 | |||
| LPV + NRTI newa | 2.2 | 1.20; 4.13 | 0.011 | |||
| LPV + NNRTI newa | 1.4 | 0.71; 2.82 | 0.318 | |||
| LPV + PI newa | 1.8 | 1.02; 3.44 | 0.043 | |||
| LPV/r + PI + NNRTI newa | 2.9 | 1.38; 6.43 | 0.005 | |||
| Baseline characteristics | ||||||
| no-AIDS | 1.3 | 0.72; 2.37 | 0.382 | |||
| >12 years old | 1.7 | 0.90; 3.22 | 0.100 | |||
| >15% CD4 cells | 1.7 | 0.83; 3.63 | 0.140 | |||
| ≤30 000 copies/mL VL | 3.6 | 1.90; 6.77 | <0.001 | |||
| . | RP . | 95% CI . | P value . | |||
|---|---|---|---|---|---|---|
| ART previous to LPV/r | ||||||
| ART-drug switches ≤2 | 2.2 | 1.04; 4.66 | 0.038 | |||
| drugs count pre-LPV ≤5 | 1.9 | 0.99; 3.66 | 0.050 | |||
| NNRTI | 0.8 | 0.41; 1.81 | 0.714 | |||
| Drugs new in current HAART | ||||||
| LPV + drug newa | 1.8 | 0.93; 3.6 | 0.079 | |||
| LPV + NRTI newa | 2.2 | 1.20; 4.13 | 0.011 | |||
| LPV + NNRTI newa | 1.4 | 0.71; 2.82 | 0.318 | |||
| LPV + PI newa | 1.8 | 1.02; 3.44 | 0.043 | |||
| LPV/r + PI + NNRTI newa | 2.9 | 1.38; 6.43 | 0.005 | |||
| Baseline characteristics | ||||||
| no-AIDS | 1.3 | 0.72; 2.37 | 0.382 | |||
| >12 years old | 1.7 | 0.90; 3.22 | 0.100 | |||
| >15% CD4 cells | 1.7 | 0.83; 3.63 | 0.140 | |||
| ≤30 000 copies/mL VL | 3.6 | 1.90; 6.77 | <0.001 | |||
LPV/r, lopinavir/ritonavir; RP, relative proportion; 95% CI, confidence interval of 95%; P, level of significance; ART, antiretroviral therapy; VL, viral load.
Beginning of HAART protocol with LPV and other new drugs.
Baseline characteristics of 40 HIV-1-infected children who achieved VL ≤400 copies/mL according to rebound of viral load during follow-up
| Characteristic . | VL ≤400 copies/mL (no rebound) . | VL >400 copies/mL (rebound) . | P value . | |||
|---|---|---|---|---|---|---|
| No. HIV-1 children | 24 | 16 | ||||
| Baseline | ||||||
| age (years) | 9.3±1 (1.6; 17.5) | 10.8±0.9 (3.1; 16.9) | 0.267 | |||
| lymphocyte subsets | ||||||
| % CD4+ T-lymphocytes | 26.4±1.7 (12; 42) | 22.3±2.9 (6; 44) | 0.145 | |||
| CD4+ T-lymphocytes/mm3 | 1027±152 (251; 2874) | 684±112 (66; 1535) | 0.078 | |||
| % CD8+ T-lymphocytes | 41.1±2.3 (23; 71) | 47.5±3.4 (21; 67) | 0.144 | |||
| CD8+ T-lymphocytes/mm3 | 1533±180 (526; 3761) | 1418±232 (231; 3712) | 0.562 | |||
| VL | ||||||
| VL (copies/mL) | 91 930±26 512 (6300; 500 000) | 217 979.6±71 750.9 (12 539; 1 000 000) | 0.054 | |||
| VL ≤30 000 copies/mL | 12 (50%) | 3 (18.8%) | 0.047 | |||
| VL >30 000 copies/mL | 12 (50%) | 13 (81.2%) | 0.047 | |||
| Previous LPV/r | ||||||
| time with ART | 70.5±7.8 (13; 156.5) | 79.2±8.2 (25.1; 148.9) | 0.462 | |||
| ART-protocols switches | 5.08±0.68 (1; 15) | 6.13±0.68 (2; 11) | 0.183 | |||
| drug count | 6.33±0.45 (3; 10) | 7.94±0.49 (5; 11) | 0.034 | |||
| NNRTI | 13 (54.2%) | 11 (68.8%) | 0.278 | |||
| Characteristic . | VL ≤400 copies/mL (no rebound) . | VL >400 copies/mL (rebound) . | P value . | |||
|---|---|---|---|---|---|---|
| No. HIV-1 children | 24 | 16 | ||||
| Baseline | ||||||
| age (years) | 9.3±1 (1.6; 17.5) | 10.8±0.9 (3.1; 16.9) | 0.267 | |||
| lymphocyte subsets | ||||||
| % CD4+ T-lymphocytes | 26.4±1.7 (12; 42) | 22.3±2.9 (6; 44) | 0.145 | |||
| CD4+ T-lymphocytes/mm3 | 1027±152 (251; 2874) | 684±112 (66; 1535) | 0.078 | |||
| % CD8+ T-lymphocytes | 41.1±2.3 (23; 71) | 47.5±3.4 (21; 67) | 0.144 | |||
| CD8+ T-lymphocytes/mm3 | 1533±180 (526; 3761) | 1418±232 (231; 3712) | 0.562 | |||
| VL | ||||||
| VL (copies/mL) | 91 930±26 512 (6300; 500 000) | 217 979.6±71 750.9 (12 539; 1 000 000) | 0.054 | |||
| VL ≤30 000 copies/mL | 12 (50%) | 3 (18.8%) | 0.047 | |||
| VL >30 000 copies/mL | 12 (50%) | 13 (81.2%) | 0.047 | |||
| Previous LPV/r | ||||||
| time with ART | 70.5±7.8 (13; 156.5) | 79.2±8.2 (25.1; 148.9) | 0.462 | |||
| ART-protocols switches | 5.08±0.68 (1; 15) | 6.13±0.68 (2; 11) | 0.183 | |||
| drug count | 6.33±0.45 (3; 10) | 7.94±0.49 (5; 11) | 0.034 | |||
| NNRTI | 13 (54.2%) | 11 (68.8%) | 0.278 | |||
Values are expressed as mean ± S.E.M. (min; max) and absolute (percentage). LPV/r, lopinavir/ritonavir; VL, viral load; ART, antiretroviral therapy.
Baseline characteristics of 40 HIV-1-infected children who achieved VL ≤400 copies/mL according to rebound of viral load during follow-up
| Characteristic . | VL ≤400 copies/mL (no rebound) . | VL >400 copies/mL (rebound) . | P value . | |||
|---|---|---|---|---|---|---|
| No. HIV-1 children | 24 | 16 | ||||
| Baseline | ||||||
| age (years) | 9.3±1 (1.6; 17.5) | 10.8±0.9 (3.1; 16.9) | 0.267 | |||
| lymphocyte subsets | ||||||
| % CD4+ T-lymphocytes | 26.4±1.7 (12; 42) | 22.3±2.9 (6; 44) | 0.145 | |||
| CD4+ T-lymphocytes/mm3 | 1027±152 (251; 2874) | 684±112 (66; 1535) | 0.078 | |||
| % CD8+ T-lymphocytes | 41.1±2.3 (23; 71) | 47.5±3.4 (21; 67) | 0.144 | |||
| CD8+ T-lymphocytes/mm3 | 1533±180 (526; 3761) | 1418±232 (231; 3712) | 0.562 | |||
| VL | ||||||
| VL (copies/mL) | 91 930±26 512 (6300; 500 000) | 217 979.6±71 750.9 (12 539; 1 000 000) | 0.054 | |||
| VL ≤30 000 copies/mL | 12 (50%) | 3 (18.8%) | 0.047 | |||
| VL >30 000 copies/mL | 12 (50%) | 13 (81.2%) | 0.047 | |||
| Previous LPV/r | ||||||
| time with ART | 70.5±7.8 (13; 156.5) | 79.2±8.2 (25.1; 148.9) | 0.462 | |||
| ART-protocols switches | 5.08±0.68 (1; 15) | 6.13±0.68 (2; 11) | 0.183 | |||
| drug count | 6.33±0.45 (3; 10) | 7.94±0.49 (5; 11) | 0.034 | |||
| NNRTI | 13 (54.2%) | 11 (68.8%) | 0.278 | |||
| Characteristic . | VL ≤400 copies/mL (no rebound) . | VL >400 copies/mL (rebound) . | P value . | |||
|---|---|---|---|---|---|---|
| No. HIV-1 children | 24 | 16 | ||||
| Baseline | ||||||
| age (years) | 9.3±1 (1.6; 17.5) | 10.8±0.9 (3.1; 16.9) | 0.267 | |||
| lymphocyte subsets | ||||||
| % CD4+ T-lymphocytes | 26.4±1.7 (12; 42) | 22.3±2.9 (6; 44) | 0.145 | |||
| CD4+ T-lymphocytes/mm3 | 1027±152 (251; 2874) | 684±112 (66; 1535) | 0.078 | |||
| % CD8+ T-lymphocytes | 41.1±2.3 (23; 71) | 47.5±3.4 (21; 67) | 0.144 | |||
| CD8+ T-lymphocytes/mm3 | 1533±180 (526; 3761) | 1418±232 (231; 3712) | 0.562 | |||
| VL | ||||||
| VL (copies/mL) | 91 930±26 512 (6300; 500 000) | 217 979.6±71 750.9 (12 539; 1 000 000) | 0.054 | |||
| VL ≤30 000 copies/mL | 12 (50%) | 3 (18.8%) | 0.047 | |||
| VL >30 000 copies/mL | 12 (50%) | 13 (81.2%) | 0.047 | |||
| Previous LPV/r | ||||||
| time with ART | 70.5±7.8 (13; 156.5) | 79.2±8.2 (25.1; 148.9) | 0.462 | |||
| ART-protocols switches | 5.08±0.68 (1; 15) | 6.13±0.68 (2; 11) | 0.183 | |||
| drug count | 6.33±0.45 (3; 10) | 7.94±0.49 (5; 11) | 0.034 | |||
| NNRTI | 13 (54.2%) | 11 (68.8%) | 0.278 | |||
Values are expressed as mean ± S.E.M. (min; max) and absolute (percentage). LPV/r, lopinavir/ritonavir; VL, viral load; ART, antiretroviral therapy.
We thank Dolores García-Alonso for her excellent technical assistance. We would like to thank Vanessa Campo-Ruiz, MD PhD for her assistance in translating and editing this manuscript. Financial Support: Fundación para la Investigación y la Prevención del SIDA en España, FIPSE (grant 36365/02, 12456/03), Comunidad de Madrid (grant 08.5/0034/2001), the Red Temática Cooperativa de Investigación en SIDA (grant RIS G03/173) of FIS, the Red Temática Cooperativa de Investigación en Genética Clínica y Molecular (grant RIG C03/07) of FIS, Abbott Laboratories, and Plan Nacional de Salud (grant SAF 2003–09209). Salvador Resino is supported by a grant from FIS (grant CM0300038).
Participating hospitals and sites: Madrid: Hospital 12 Octubre: J. T. Ramos, P. Carreño, J. Ruiz, J. Clemente. Hospital Gregorio Marañón: J. M. Bellón, M. D. Gurbindo, M. L. Navarro, S. Resino, M. A. Muñoz-Fernández. Hospital la Paz: M. I. Isabel de José. Hospital Carlos III: P. Martín-Fontelos, M. J. Mellado, J. Villota. Seville: Hospital Virgen del Rocio: J. A. León Leal. Barcelona: Hospital S.Juan de Dios: C. Fortuny, L. M. Tello. Hospital Valle de Hebrón: J. M. Bertrán, L. García. Hospital del Mar: A. Mur. Alicante: Hospital S. Juan: R. González-Montero. Bilbao: Hospital de Cruces: I. Pocheville, C. Gutierrez. Palma de Mallorca: Hospital Son Dureta: L. Ciria, J. Dueñas. Valencia: Hospital La Fe: A. Orti, M. C. Otero, F. Asensi. Saragossa: Hospital Clínico: M. Gracia. Abbott Laboratories, Spain: E. Cabrero, L. Usán.
References
Shearer, W. T., Quinn, T. C., LaRussa, P. et al. (
Borkowsky, W., Stanley, K., Douglas, S. D. et al. (
Wintergerst, U., Hoffmann, F., Solder, B. et al. (
Mueller, B. U., Nelson, R. P., Jr, Sleasman, J. et al. (
Wiznia, A., Stanley, K., Krogstad, P. et al. (
Rutstein, R. M., Feingold, A., Meislich, D. et al. (
Resino, S., Bellón, J., Resino, R. et al. (
Resino, S., Bellón, J., Gurbindo, D. et al. (
Nachman, S. A., Stanley, K., Yogev, R. et al. (
Starr, S. E., Fletcher, C. V., Spector, S. A. et al. (
Starr, S. E., Fletcher, C. V., Spector, S. A. et al. (
Hirsch, M. S., Conway, B., D'Aquila, R. T. et al. (
Aleman, S., Soderbarg, K., Visco-Comandini, U. et al. (
Murphy, R. L., Brun, S., Hicks, C. et al. (
Kempf, D. J., Isaacson, J. D., King, M. S. et al. (
Saez-Llorens, X., Violari, A., Deetz, C. O. et al. (
Condra, J. H., Schleif, W. A., Blahy, O. M. et al. (
Kempf, D. J., Isaacson, J. D., King, M. S. et al. (
Munoz-Fernandez, M. A., Obregon, E., Navarro, J. et al. (
Hirsch, M. S., Brun-Vezinet, F., D'Aquila, R. T. et al. (
Centers for Disease Control and Prevention (
Centers for Disease Control and Prevention (
Sharland, M., di Zub, G. C., Ramos, J. T. et al. (
Lucas, G. M., Chaisson, R. E. & Moore, R. D. (
Mocroft, A., Phillips, A. N., Miller, V. et al. (
Mocroft, A., Devereux, H., Kinloch-de-Loes, S. et al. (
Romano, L., Peduzzi, C., Venturi, G. et al. (
Melvin, A. J., Mohan, K. M., Arcuino, L. A. et al. (
van Rossum, A. M., Niesters, H. G., Geelen, S. P. et al. (
Deeks, S. G., Barbour, J. D., Grant, R. M. et al. (
Resino, S., Bellón, J. M., Gurbindo, D. et al. (
Resino, S., Correa, R., Bellon, J. et al. (
Resino, S., Sanchez-Ramon, S., Bellon, J. M. et al. (
Mocroft, A., Gill, M. J., Davidson, W. et al. (
Fatkenheuer, G., Theisen, A., Rockstroh, J. et al. (
Nadal, D., Steiner, F., Cheseaux, J. J. et al. (
Resino, S., Bellón, J., Gurbindo, D. et al. (
Melvin, A. J., Rodrigo, A. G., Mohan, K. M. et al. (
Purswani, M., Johann-Liang, R., Cervia, J. et al. (
Staszewski, S., Miller, V., Sabin, C. et al. (
Gilleece, Y. C., Qazi, N. A., Morlese, J. F. et al. (
Fatkenheuer, G., Romer, K., Kamps, R. et al. (
Mauss, S., Schmutz, G. & Kuschak, D. (
Bratt, G., Karlsson, A., Leandersson, A. C. et al. (
Le Moing, V., Chene, G., Carrieri, M. P. et al. (
Mellors, J. W., Munoz, A., Giorgi, J. V. et al. (
Bongiovanni, M., Bini, T., Adorni, F. et al. (
Monno, L., Saracino, A., Scudeller, L. et al. (
Prado, J. G., Wrin, T., Beauchaine, J. et al. (
Tsuchiya, K., Matsuoka, S., Hachiya, A. et al. (
Cooper, C. L., Parbhakar, M. A. & Angel, J. B. (
Clumeck, N., Goebel, F., Rozenbaum, W. et al. (
Gutierrez, F., Padilla, S., Navarro, A. et al. (
Havlir, D. V., Bassett, R., Levitan, D. et al. (
Havlir, D. V., Marschner, I. C., Hirsch, M. S. et al. (
Author notes
1Laboratory of Immuno-Molecular Biology, Hospital Gregorio Marañón, Madrid; 2Department of Paediatrics, Hospital ‘12 de Octubre’, Madrid; 3Department of Paediatrics, Hospital la Paz, Madrid; 4Department of Paediatrics, Hospital Gregorio Marañón, Madrid; 5Department of Paediatrics, Hospital Carlos III, Madrid; 6Abbott Laboratories, Madrid, Spain