-
PDF
- Split View
-
Views
-
Cite
Cite
E. H. Leder, R. G. Danzmann, M. M. Ferguson, The Candidate Gene, Clock, Localizes to a Strong Spawning Time Quantitative Trait Locus Region in Rainbow Trout, Journal of Heredity, Volume 97, Issue 1, January/February 2006, Pages 74–80, https://doi.org/10.1093/jhered/esj004
Close - Share Icon Share
Abstract
We applied a candidate gene mapping approach to an existing quantitative trait loci (QTL) data set for spawning date in rainbow trout (Oncorynchus mykiss) to ascertain whether these genes could potentially account for any observed QTL effects. Several genes were chosen for their known or suspected roles in reproduction, circadian, or circannual timing, including salmon-type gonadotropin-releasing hormone 3A and 3B (GnRH3A and GnRH3B), Clock, Period1, and arylalkylamine N-acetlytransferase−1 and −2 (AANAT-1 and AANAT-2). Genes were sequenced, and polymorphisms were identified in parents of two rainbow trout mapping families, one of which was used previously to detect spawn timing QTL. Interval mapping was used to identify associations between genetic markers and spawning date effects. Using a genetic map that was updated with 574 genetic markers (775 total), we found evidence for 11 significant or suggestive QTL regions. Most QTL were only localized within one of the parents; however, a strong QTL region was identified in both female and male parents on linkage group RT-8 that explained 20% and 50% of trait variance, respectively. The Clock gene mapped to this region. Period1 mapped to a region in the female parent associated with a marginal effect (P = .056) on spawn timing. Other candidate genes were not associated with significant QTL effects.
Seasonal timing of life history events such as maturation and spawning have evolved in many organisms to coincide with optimal environmental conditions and food availability, both for the organism and its offspring. Energetic costs of reproduction are high; therefore, it is advantageous for an organism to devote resources to its future offspring when resources are plentiful. Consequently, annual reproductive cycles tend to coincide with seasonal changes in order for organisms to optimally utilize resources. This seasonal periodicity is believed to be entrained by environmental cues such as light and temperature, but how these cues are interpreted to trigger reproductive events is unknown.
The effects of experimental photoperiod regimens have been examined on a wide variety of fish species (reviewed in Bromage et al. 2001). Although the effects of altered photoperiod on aspects of life history of farmed fishes have been known for some time, the molecular bases for these effects are poorly understood. Recent advances in molecular biology have begun to clarify the role that light plays in the timing of biological functions at a daily and an annual level. This hypothesis is that light acts as an environmental cue or “zeitgeber” that entrains endogenous oscillation of clock genes (Roenneberg and Merrow 2003). Clock genes in turn regulate other genes that require rhythmic daily expression. A number of genes have been implicated in daily and circannual timekeeping. These processes and the genes involved are not completely understood at present, and their complexity varies across species (Dunlap 1999; Falcon 1999; Stanewsky 2003; Whitmore et al. 1998).
Because photoperiod has been shown to have a significant effect on maturation, spawning time, and development in salmonids, circadian rhythm genes are likely candidates for influencing these traits. Clock is common to both invertebrates and vertebrates. It is a transcription factor that forms a dimer with another transcription factor, Bmal1 in vertebrates, to activate transcription of other clock genes including period genes (Dunlap 1999; Stanewsky 2003). The manner in which these genes regulate daily or seasonal processes is not clear, but mutation studies in Drosophila have demonstrated that mating activity (Sakai and Ishida 2001) as well as reproductive fitness (Beaver et al. 2002) are influenced by clock genes.
Melatonin also plays an important role in circadian timekeeping. Melatonin synthesis in the pineal gland oscillates with the light/dark cycle (Falcon 1999). Levels of plasma melatonin peak during the dark and remain high throughout the night. Therefore, the duration of melatonin synthesis corresponds directly to the length of the night. In this manner, seasonality as represented by night length is conveyed to the organism. The daily fluctuations of melatonin result from the activity of arylalkylamine N-acetlytransferase (AANAT), which is the penultimate enzyme in the production of melatonin from serotonin. In trout, it appears that AANAT is regulated by light directly (Begay et al. 1998; Mizusawa et al. 2000), but in other vertebrates the Clock-Bmal1 heterodimer may regulate transcription of AANAT (Chen and Baler 2000; Tosini and Fukuhara 2002). In mammals, this seasonal rhythm of melatonin production seems to have a role in signaling reproduction, although the exact process is still undetermined (Vanecek 1998). There is also a link between photoperiod, melatonin production, and reproduction in fishes, but experimental results have been equivocal (Bromage et al. 2001; Mayer et al. 1997).
Gonadotropin-releasing hormone (GnRH) is one of the key peptides for regulating reproduction through stimulation of synthesis and release of gonadotropins. In fishes, there are two to three forms of GnRH, with the species-specific form believed to be the one involved in reproduction (Somoza et al. 2002). In rockfish, seasonal changes in the abundance of the species-specific form of GnRH were related to reproductive status (Collins et al. 2001). In salmonids, only two forms of the peptide have been discovered, but the species-specific form (sGnRH or GnRH3) is duplicated and messenger RNAs for the duplicated genes are differentially expressed, suggesting a different function (Ferriere et al. 2001; von Schalburg and Sherwood 1999). Therefore, both copies of the species-specific form of GnRH (GnRH3A and GnRH3B) may be important in regulating spawning time in rainbow trout (Oncorynchus mykiss).
To gain a better understanding of the genes involved in the timing of reproduction, a candidate gene approach was applied to an existing quantitative trait loci (QTL) data set for spawning date in rainbow trout (O'Malley et al. 2003; Sakamoto et al. 1999). The search for additional QTL positions was greatly extended in this study through the addition of a large number of amplified fragment length polymorphism (AFLP) and microsatellite markers to the rainbow trout genome. We examine Clock, Period1, AANAT-1 and AANAT-2, and GnRH3A and GnRH3B as potential candidates for spawning date effects in rainbow trout and report an association for the map position of Clock to the strongest QTL region.
Materials and Methods
Reference Family
We extended the genetic map for the rainbow trout reference family lot 44 used by Sakamoto et al. (1999) and O'Malley et al. (2003) to search for spawn timing QTL in this species. Details on the source of this family and experimental design are given in Sakamoto et al. (2000); however, we briefly reiterate herein that the family was generated using a hybrid male from a cross between an autumn × spring spawning line of rainbow trout. This hybrid male was backcrossed to an autumn spawning female. O'Malley et al. (2003) initially used 90 progeny from this family to screen for allelic variation. Five of these progeny were omitted in this analysis (lack of DNA), whereas one additional individual was added. The DNA from this new fish was originally of poor quality, but recent reextractions provided reproducible genotypes. Thus, 86 progeny were used in this analysis.
Genetic Markers
Details on the two mapping panels (lot 25 and lot 44) that were used for the construction of the rainbow trout linkage map are described in Danzmann et al. (2005), including primer sequences for microsatellite markers and genetic polymorphisms. Polymerase chain reaction (PCR) protocols for the microsatellite markers follow those outlined in O'Malley et al. (2003). Details of the cloning, sequencing, and mapping for the GnRH3 genes are presented in Leder et al. (2004). Genomic sequences of the Clock gene were obtained for rainbow trout using primers designed from the partial gene sequence of rainbow trout (AF266745) and a rainbow trout expressed sequence tag (CA384613). Primers for Period1 were designed from the partial rainbow trout sequence (AF228695), whereas those for AANAT were derived from sequences of rainbow trout AANAT-1 (AB007294) and AANAT-2 (AF106006). All candidate gene primers used for mapping polymorphisms in this study are listed in Table 1.
Primer information for candidate genes mapped in this study for mapping families lot 25 and lot 44
. | . | . | Polymorphism . | . | |
|---|---|---|---|---|---|
| Gene . | Primer name . | Primer sequence (5′-3′) . | Lot 25 . | Lot 44 . | |
| Clock | Clk-862-F | ATAGGTTACCTGCCGTTTGA | indel | SSCP | |
| Clk-207-R | ATTTCCCCTTCCCATACTGCA | ||||
| Period1 | Per1-5-F | CATCCCCACCCAGCAGTTCTC | SSCP | SSCP | |
| Per1-337-R | GGTGTACTCTGAGGCGATGTTGTC | ||||
| AANAT-1/i | NAT1a-563-F | GCGAGGACTTCCTGGTTCCC | SSCP | ||
| NAT1a-742-R | TGCAGACTCACTCCTCTGAATCGG | ||||
| AANAT-2/i | NAT2b-232-F | CTAAACAATGCCATAACCAC | RFLP-BstEII | ||
| NAT2b-733-R | GGTAGGGAAAGGTAAAGGTAT | ||||
| NAT2b-1980-F | ATCATACGAAATGGGTGATGGA | SSCP | |||
| NAT2b-1294-R | GGTGCATGTCCCCTCTCTATGT | ||||
| AANAT-2/ii | NAT2-148-F | CCTCCGATGTGTATTTAGTGT | RFLP-RsaI | ||
| NAT2a-589-R | CCCAAAAGCCTCTGTAGTC | ||||
| GnRH3A | GnRH3A-μ-F | GGCAAGTACTATTTACCCTA | microsatellite | ||
| GnRH3A-μ-R | AGTAACGGAATACCCTG | ||||
| GnRH3A-253-F | GGTGATGTTGGCGTTGATAG | microsatellite | |||
| GnRH3A-521-R | GCGATACATTTTTGTGAACGG | ||||
| GnRH3B | GnRH-3B-F | CTCAGCACTGGTCGTATGG | RFLP-AluI | ||
| GnRH-3B-R | ATGCCCCTGCTAATTCCTT | ||||
. | . | . | Polymorphism . | . | |
|---|---|---|---|---|---|
| Gene . | Primer name . | Primer sequence (5′-3′) . | Lot 25 . | Lot 44 . | |
| Clock | Clk-862-F | ATAGGTTACCTGCCGTTTGA | indel | SSCP | |
| Clk-207-R | ATTTCCCCTTCCCATACTGCA | ||||
| Period1 | Per1-5-F | CATCCCCACCCAGCAGTTCTC | SSCP | SSCP | |
| Per1-337-R | GGTGTACTCTGAGGCGATGTTGTC | ||||
| AANAT-1/i | NAT1a-563-F | GCGAGGACTTCCTGGTTCCC | SSCP | ||
| NAT1a-742-R | TGCAGACTCACTCCTCTGAATCGG | ||||
| AANAT-2/i | NAT2b-232-F | CTAAACAATGCCATAACCAC | RFLP-BstEII | ||
| NAT2b-733-R | GGTAGGGAAAGGTAAAGGTAT | ||||
| NAT2b-1980-F | ATCATACGAAATGGGTGATGGA | SSCP | |||
| NAT2b-1294-R | GGTGCATGTCCCCTCTCTATGT | ||||
| AANAT-2/ii | NAT2-148-F | CCTCCGATGTGTATTTAGTGT | RFLP-RsaI | ||
| NAT2a-589-R | CCCAAAAGCCTCTGTAGTC | ||||
| GnRH3A | GnRH3A-μ-F | GGCAAGTACTATTTACCCTA | microsatellite | ||
| GnRH3A-μ-R | AGTAACGGAATACCCTG | ||||
| GnRH3A-253-F | GGTGATGTTGGCGTTGATAG | microsatellite | |||
| GnRH3A-521-R | GCGATACATTTTTGTGAACGG | ||||
| GnRH3B | GnRH-3B-F | CTCAGCACTGGTCGTATGG | RFLP-AluI | ||
| GnRH-3B-R | ATGCCCCTGCTAATTCCTT | ||||
Table includes primer sequences used to obtain an amplicon and the method of scoring the polymorphism.
Primer information for candidate genes mapped in this study for mapping families lot 25 and lot 44
. | . | . | Polymorphism . | . | |
|---|---|---|---|---|---|
| Gene . | Primer name . | Primer sequence (5′-3′) . | Lot 25 . | Lot 44 . | |
| Clock | Clk-862-F | ATAGGTTACCTGCCGTTTGA | indel | SSCP | |
| Clk-207-R | ATTTCCCCTTCCCATACTGCA | ||||
| Period1 | Per1-5-F | CATCCCCACCCAGCAGTTCTC | SSCP | SSCP | |
| Per1-337-R | GGTGTACTCTGAGGCGATGTTGTC | ||||
| AANAT-1/i | NAT1a-563-F | GCGAGGACTTCCTGGTTCCC | SSCP | ||
| NAT1a-742-R | TGCAGACTCACTCCTCTGAATCGG | ||||
| AANAT-2/i | NAT2b-232-F | CTAAACAATGCCATAACCAC | RFLP-BstEII | ||
| NAT2b-733-R | GGTAGGGAAAGGTAAAGGTAT | ||||
| NAT2b-1980-F | ATCATACGAAATGGGTGATGGA | SSCP | |||
| NAT2b-1294-R | GGTGCATGTCCCCTCTCTATGT | ||||
| AANAT-2/ii | NAT2-148-F | CCTCCGATGTGTATTTAGTGT | RFLP-RsaI | ||
| NAT2a-589-R | CCCAAAAGCCTCTGTAGTC | ||||
| GnRH3A | GnRH3A-μ-F | GGCAAGTACTATTTACCCTA | microsatellite | ||
| GnRH3A-μ-R | AGTAACGGAATACCCTG | ||||
| GnRH3A-253-F | GGTGATGTTGGCGTTGATAG | microsatellite | |||
| GnRH3A-521-R | GCGATACATTTTTGTGAACGG | ||||
| GnRH3B | GnRH-3B-F | CTCAGCACTGGTCGTATGG | RFLP-AluI | ||
| GnRH-3B-R | ATGCCCCTGCTAATTCCTT | ||||
. | . | . | Polymorphism . | . | |
|---|---|---|---|---|---|
| Gene . | Primer name . | Primer sequence (5′-3′) . | Lot 25 . | Lot 44 . | |
| Clock | Clk-862-F | ATAGGTTACCTGCCGTTTGA | indel | SSCP | |
| Clk-207-R | ATTTCCCCTTCCCATACTGCA | ||||
| Period1 | Per1-5-F | CATCCCCACCCAGCAGTTCTC | SSCP | SSCP | |
| Per1-337-R | GGTGTACTCTGAGGCGATGTTGTC | ||||
| AANAT-1/i | NAT1a-563-F | GCGAGGACTTCCTGGTTCCC | SSCP | ||
| NAT1a-742-R | TGCAGACTCACTCCTCTGAATCGG | ||||
| AANAT-2/i | NAT2b-232-F | CTAAACAATGCCATAACCAC | RFLP-BstEII | ||
| NAT2b-733-R | GGTAGGGAAAGGTAAAGGTAT | ||||
| NAT2b-1980-F | ATCATACGAAATGGGTGATGGA | SSCP | |||
| NAT2b-1294-R | GGTGCATGTCCCCTCTCTATGT | ||||
| AANAT-2/ii | NAT2-148-F | CCTCCGATGTGTATTTAGTGT | RFLP-RsaI | ||
| NAT2a-589-R | CCCAAAAGCCTCTGTAGTC | ||||
| GnRH3A | GnRH3A-μ-F | GGCAAGTACTATTTACCCTA | microsatellite | ||
| GnRH3A-μ-R | AGTAACGGAATACCCTG | ||||
| GnRH3A-253-F | GGTGATGTTGGCGTTGATAG | microsatellite | |||
| GnRH3A-521-R | GCGATACATTTTTGTGAACGG | ||||
| GnRH3B | GnRH-3B-F | CTCAGCACTGGTCGTATGG | RFLP-AluI | ||
| GnRH-3B-R | ATGCCCCTGCTAATTCCTT | ||||
Table includes primer sequences used to obtain an amplicon and the method of scoring the polymorphism.
Initial amplification reactions for sequencing contained 100 ng total DNA, 0.32 μM each primer, 1.5 mM MgCl2, 200 μM each deoxynucleotide triphosphate (dNTP), 1× PCR buffer, and 2 U of Taq polymerase (Invitrogen, Burlington, Ontario, Canada). The thermal cycler profile consisted of an initial denaturation step of 3 min at 94°C, followed by 35 cycles of 30 s at 94°C, 35 s at 55°C, and 1.25 min at 72°C. Products were purified for cloning using QIAquick Gel Purification Kit (Qiagen, Mississauga, Ontario, Canada). Cleaned PCR products were cloned using pGEM-T vector (Promega, Madison, WI). Plasmid DNA was isolated from the cell culture using QIAprep Miniprep Kit (Qiagen). Cleaned plasmids were sequenced using an ABI 377 automated DNA sequencer (Applied Biosystems, Foster City, CA). Sequences were edited and aligned using Sequencher version 4.0.5 (Gene Codes Corporation, Ann Arbor, MI).
Clock was polymorphic for an insertion/deletion in the lot 25 rainbow trout family. The forward primer was end-labeled, and another reverse primer was designed to amplify a shorter region for genotyping the family. The amplification products were separated by electrophoreses on a 6% acrylamide gel and observed on a Hitachi FMBIOII fluorescence scanner (Hitachi, Tokyo, Japan).
This primer set was also used to amplify the lot 44 rainbow trout family for single-stranded conformational polymorphism (SSCP) analysis. The lot 44 female was polymorphic, and the alleles were separated using the following conditions: 0.5× Mutation Detection Enhancement (MDE) gel (Cambrex Bio Science, Rockland, ME), 0.6× Tris-borate-EDTA (TBE) buffer, 10 W for 5.5 h at 4°C, and observed on a fluorescence scanner.
Clone sequences of rainbow trout were polymorphic for Period1. Additional primers amplifying a smaller portion of the sequence were designed for SSCP. The above-mentioned SSCP conditions were used to separate the alleles.
Initial sequencing of AANAT-1 and AANAT-2 revealed two copies of each gene. Specific primers were designed for each duplicate, and further sequencing of the AANAT-2 duplicates revealed polymorphisms that could be scored by restriction enzyme digests in lot 25 parents (Table 1). Digestions were conducted in 10 μl volumes using 5 U of enzyme, BstEII or RsaI (New England Biolabs, Beverly, MA), 1 μl of manufacturer's reaction buffer, and 3 μl of PCR product. Restriction fragments were observed on a 2% agarose gel stained with ethidium bromide. Another primer set was designed to amplify a product for SSCP to map AANAT-2/i in lot 44 (Table 1) using the above-mentioned SSCP conditions. Polymorphism was detected in only one copy of AANAT-1. Primers were designed to amplify a portion of AANAT-1/i for SSCP (Table 1). Alleles were separated using the following conditions: 0.35× MDE gel, 0.6× TBE buffer, 15 W for 2 h at 4°C, and observed on a fluorescence scanner.
Analysis Methods
The genotypes of parents and offspring were added to the existing linkage data set to determine linkage relationships. The program LINKMFEX (http://www.uoguelph.ca/∼rdanzman/software) was used to determine linkage group affinities (logarithm of odds (LOD) ≥ 4) and marker order. We used the updated nomenclature for rainbow trout linkage groups given in Nichols et al. (2003), and a table presented in that study allows the reader to cross-reference the linkage group designations given in Sakamoto et al. (1999) and O'Malley et al. (2003) to this study. This provides a consolidated framework for discussing rainbow trout linkage group homologies.
MULTIQTL (version 2.4) (Institute of Evolution, Haifa University, Haifa, Israel) was used to analyze the quantitative trait data using an interval mapping procedure (Korol et al. 1995). Significance within a linkage group was determined by permutation testing (Churchill and Doerge 1994). Experiment-wise significance level was determined by using the false-discovery rate (FDR) test at an alpha level of 0.05 (Benjamini and Hochberg 1995). Trait values (scored as number of days to spawning from August 1, in both 1995 and 1996) were combined for both years and analyzed as two separate environments in the MULTIQTL analysis. The analyses for male- and female-specific polymorphic markers were conducted separately. We expected the greatest number of QTL locations to be present in the male parent given the fact that the hybrid male used in this study should possess a high degree of heterozygosity for segregating QTL, and male salmonids may show a higher degree of marker/QTL linkage given the lower recombination rates observed in male salmonids (Sakamoto et al. 2000).
Results
Mapping of Candidate Genes
Clock mapped to RT-8, and Period1 mapped to a segment of RT-15. Both AANAT-1 and AANAT-2 were duplicated, so there were four copies of these genes present: AANAT-1/i, AANAT-1/ii, AANAT-2/i, and AANAT-2/ii. Both AANAT-1/i and AANAT-2/i mapped to RT-9, and one of the duplicates, AANAT-2/ii, mapped to RT-2. GnRH3A mapped to RT-30, and GnRH3B mapped to RT-6 (Leder et al. 2004). Linkage groups RT-2 and RT-9, as well as RT-6 and RT-30, have been identified as putative homeologous (ancestrally duplicated) linkage groups based on the presence of several duplicated genetic markers (Danzmann et al. 2005).
QTL Regions
The genetic map used to detect spawn timing QTL in this study was updated to 775 markers (288 AFLP markers and 487 microsatellite and type I gene markers) from the previous 201 microsatellite markers used by O'Malley et al. (2003). A total of 748 of these markers were assigned to either the male or female genetic maps for this family, and this allowed a more extensive survey of the genomic variation within the family. For example, 102 unique female genetic map positions (across 29 segments within 21 linkage groups) were used by O'Malley et al. (2003) to search for QTL, whereas this study was based on 379 unique genetic map positions for the female map (across 44 segments within 29 linkage groups, with two unassigned segments) (Danzmann et al. 2005). The number of unique genetic map positions in the male parent was considerably lower given the lack of recombination that is generally detected in male salmonids.
O'Malley et al. (2003) described six significant or suggestive QTL regions (P < .05 following permutation testing) for spawn timing in rainbow trout. This analysis extends these findings to report new evidence for spawn timing QTL regions on linkage groups RT-7, RT-11, RT-20, RT-22, and RT-31, in addition to QTL previously reported on RT-2, RT-3, RT-8, RT-18, RT-19, and RT-24 (Table 2). This study was based on a combined analysis of both spawning years and did not support the identification of a spawn timing QTL region on RT-2 because a significant allelic substitution effect at marker OmyRGT42/iiTUF was only detected in 1995. However, the data from both years combined still supported a marginal association with spawn timing in this region (P = .056 following permutation testing). It should also be noted that the O'Malley et al. (2003) initially indicated a marginal association with spawn timing for marker Ots515NWFSC on RT-11, whereas this study suggests a significant association in the male parent. This discrepancy is likely due to the omission of five of the progeny and the addition of a new individual for this analysis.
Male and female parent derived QTL associations for spawning date in their female offspring during 1995 and 1996
. | . | . | . | . | Proportion of Experimental Variance . | . | Additive effecta . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|
. | Linkage group . | Marker . | LOD . | P value . | 1995 . | 1996 . | 1995 . | 1996 . | ||
| Male | RT-3 | Central clusterb | 3.62 | <.001* | 0.173 | 0.201 | −24.4 | −19.8 | ||
| RT-8 | One105ADFG central cluster | 6.15 | <.001* | 0.574 | 0.491 | −47.0 | −32.1 | |||
| RT-11 | Ots515NWFSC | 2.36 | .022* | 0.134 | 0.137 | 21.0 | 15.9 | |||
| RT-18 | OmyCosB/iTUF | 2.89 | .009* | 0.181 | 0.133 | −25.0 | −15.6 | |||
| RT-19 | BHMS349 | 2.80 | .015* | 0.101 | 0.201 | −17.7 | −20.1 | |||
| RT-22 | Central cluster | 2.88 | .012* | 0.154 | 0.158 | 23.0 | 17.5 | |||
| RT-31 | AAC/CTA321 OMM1337 | 2.96 | .035 | 0.209 | 0.152 | 27.6 | 17.3 | |||
| Female | RT-7 | Omy7INRA | 2.84 | .030* | 0.216 | 0.102 | 28.1 | 13.5 | ||
| RT-8 | Central cluster | 3.94 | .001* | 0.199 | 0.203 | 26.4 | 19.9 | |||
| RT-20+2c | ACC/CTG96 | 4.88 | <.001* | 0.270 | 0.205 | 25.5 | 20.0 | |||
| RT-24 | OmyRGT36TUF BHMS377 | 2.90 | .035* | 0.195 | 0.182 | −26.1 | −18.7 | |||
| RT-29 | OtsG43/iUCD | 2.47 | .037 | 0.174 | 0.141 | −24.6 | −16.1 | |||
| RT-31 | OMM1058 | 3.13 | .021* | 0.185 | 0.152 | 25.3 | 16.9 | |||
. | . | . | . | . | Proportion of Experimental Variance . | . | Additive effecta . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|
. | Linkage group . | Marker . | LOD . | P value . | 1995 . | 1996 . | 1995 . | 1996 . | ||
| Male | RT-3 | Central clusterb | 3.62 | <.001* | 0.173 | 0.201 | −24.4 | −19.8 | ||
| RT-8 | One105ADFG central cluster | 6.15 | <.001* | 0.574 | 0.491 | −47.0 | −32.1 | |||
| RT-11 | Ots515NWFSC | 2.36 | .022* | 0.134 | 0.137 | 21.0 | 15.9 | |||
| RT-18 | OmyCosB/iTUF | 2.89 | .009* | 0.181 | 0.133 | −25.0 | −15.6 | |||
| RT-19 | BHMS349 | 2.80 | .015* | 0.101 | 0.201 | −17.7 | −20.1 | |||
| RT-22 | Central cluster | 2.88 | .012* | 0.154 | 0.158 | 23.0 | 17.5 | |||
| RT-31 | AAC/CTA321 OMM1337 | 2.96 | .035 | 0.209 | 0.152 | 27.6 | 17.3 | |||
| Female | RT-7 | Omy7INRA | 2.84 | .030* | 0.216 | 0.102 | 28.1 | 13.5 | ||
| RT-8 | Central cluster | 3.94 | .001* | 0.199 | 0.203 | 26.4 | 19.9 | |||
| RT-20+2c | ACC/CTG96 | 4.88 | <.001* | 0.270 | 0.205 | 25.5 | 20.0 | |||
| RT-24 | OmyRGT36TUF BHMS377 | 2.90 | .035* | 0.195 | 0.182 | −26.1 | −18.7 | |||
| RT-29 | OtsG43/iUCD | 2.47 | .037 | 0.174 | 0.141 | −24.6 | −16.1 | |||
| RT-31 | OMM1058 | 3.13 | .021* | 0.185 | 0.152 | 25.3 | 16.9 | |||
P < .05 for the genome-wide false discovery rate test.
Calendar days.
Indicates that the QTL effect was localized to a large cluster of markers that mapped to the central portion of the linkage group (see Danzmann et al., in press for details).
Small linkage cluster of four markers that possesses a duplicated marker. The duplicated marker maps to RT-20 and RT-14 in other mapping parents. The QTL is tentatively assigned to RT-20+2 although we cannot exclude the possibility that the true linkage group may be RT-14.
Male and female parent derived QTL associations for spawning date in their female offspring during 1995 and 1996
. | . | . | . | . | Proportion of Experimental Variance . | . | Additive effecta . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|
. | Linkage group . | Marker . | LOD . | P value . | 1995 . | 1996 . | 1995 . | 1996 . | ||
| Male | RT-3 | Central clusterb | 3.62 | <.001* | 0.173 | 0.201 | −24.4 | −19.8 | ||
| RT-8 | One105ADFG central cluster | 6.15 | <.001* | 0.574 | 0.491 | −47.0 | −32.1 | |||
| RT-11 | Ots515NWFSC | 2.36 | .022* | 0.134 | 0.137 | 21.0 | 15.9 | |||
| RT-18 | OmyCosB/iTUF | 2.89 | .009* | 0.181 | 0.133 | −25.0 | −15.6 | |||
| RT-19 | BHMS349 | 2.80 | .015* | 0.101 | 0.201 | −17.7 | −20.1 | |||
| RT-22 | Central cluster | 2.88 | .012* | 0.154 | 0.158 | 23.0 | 17.5 | |||
| RT-31 | AAC/CTA321 OMM1337 | 2.96 | .035 | 0.209 | 0.152 | 27.6 | 17.3 | |||
| Female | RT-7 | Omy7INRA | 2.84 | .030* | 0.216 | 0.102 | 28.1 | 13.5 | ||
| RT-8 | Central cluster | 3.94 | .001* | 0.199 | 0.203 | 26.4 | 19.9 | |||
| RT-20+2c | ACC/CTG96 | 4.88 | <.001* | 0.270 | 0.205 | 25.5 | 20.0 | |||
| RT-24 | OmyRGT36TUF BHMS377 | 2.90 | .035* | 0.195 | 0.182 | −26.1 | −18.7 | |||
| RT-29 | OtsG43/iUCD | 2.47 | .037 | 0.174 | 0.141 | −24.6 | −16.1 | |||
| RT-31 | OMM1058 | 3.13 | .021* | 0.185 | 0.152 | 25.3 | 16.9 | |||
. | . | . | . | . | Proportion of Experimental Variance . | . | Additive effecta . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|
. | Linkage group . | Marker . | LOD . | P value . | 1995 . | 1996 . | 1995 . | 1996 . | ||
| Male | RT-3 | Central clusterb | 3.62 | <.001* | 0.173 | 0.201 | −24.4 | −19.8 | ||
| RT-8 | One105ADFG central cluster | 6.15 | <.001* | 0.574 | 0.491 | −47.0 | −32.1 | |||
| RT-11 | Ots515NWFSC | 2.36 | .022* | 0.134 | 0.137 | 21.0 | 15.9 | |||
| RT-18 | OmyCosB/iTUF | 2.89 | .009* | 0.181 | 0.133 | −25.0 | −15.6 | |||
| RT-19 | BHMS349 | 2.80 | .015* | 0.101 | 0.201 | −17.7 | −20.1 | |||
| RT-22 | Central cluster | 2.88 | .012* | 0.154 | 0.158 | 23.0 | 17.5 | |||
| RT-31 | AAC/CTA321 OMM1337 | 2.96 | .035 | 0.209 | 0.152 | 27.6 | 17.3 | |||
| Female | RT-7 | Omy7INRA | 2.84 | .030* | 0.216 | 0.102 | 28.1 | 13.5 | ||
| RT-8 | Central cluster | 3.94 | .001* | 0.199 | 0.203 | 26.4 | 19.9 | |||
| RT-20+2c | ACC/CTG96 | 4.88 | <.001* | 0.270 | 0.205 | 25.5 | 20.0 | |||
| RT-24 | OmyRGT36TUF BHMS377 | 2.90 | .035* | 0.195 | 0.182 | −26.1 | −18.7 | |||
| RT-29 | OtsG43/iUCD | 2.47 | .037 | 0.174 | 0.141 | −24.6 | −16.1 | |||
| RT-31 | OMM1058 | 3.13 | .021* | 0.185 | 0.152 | 25.3 | 16.9 | |||
P < .05 for the genome-wide false discovery rate test.
Calendar days.
Indicates that the QTL effect was localized to a large cluster of markers that mapped to the central portion of the linkage group (see Danzmann et al., in press for details).
Small linkage cluster of four markers that possesses a duplicated marker. The duplicated marker maps to RT-20 and RT-14 in other mapping parents. The QTL is tentatively assigned to RT-20+2 although we cannot exclude the possibility that the true linkage group may be RT-14.
Discussion
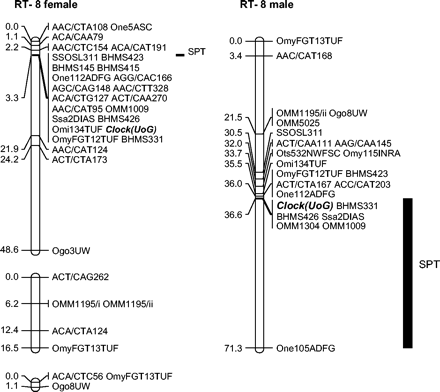
Clock mapped to the QTL region, with largest effect on spawning time in the male and the region with the second largest effect in the female for both years. Segregation at this QTL in the male parent accounted for 50% of the variance in spawning date of the offspring, whereas segregation of the female alleles was associated with approximately 20% of the trait variance. The region bearing the Clock gene was located in a large central cluster of markers within both the male and female parent genetic maps (i.e., a region possessing an apparent low level of recombination) (see Figure 1), and thus we cannot unequivocally localize the effect specifically to the Clock gene itself. Nevertheless, this gene remains the most likely candidate for the phenotypic effects observed, given its known function. The other candidate genes surveyed were not localized within this genetic map to putative QTL regions influencing spawn timing. However, there was marginal evidence that a terminal interval on a segment of the female RT-15 linkage map (spanning the region bearing Period1 and OmyRGT21TUF) was associated with differential spawn timing of the lot 44 female progeny (P = .056, data not shown).
Rainbow trout linkage group RT-8 for male and female mapping parents showing location of the clock gene and associated QTL for spawning date. The first cluster shown in the female map is a combined map for the lot 44 and lot 25 mapping panels, whereas the second cluster depicts an unlinked (LOD 4.0 threshold) cluster in the lot 25 mapping female. The third cluster is an unlinked grouping within the lot 44 mapping female.
Accepting possible marginal QTL effects, O'Malley et al. (2003) identified two homeologous linkage groups that may possess duplicated genes influencing spawn timing (i.e., RT-3/25 and RT-2/9). This study also identifies homeologous linkage groups, RT-7/15 and RT-17/22, as retaining potentially active duplicated QTL that influence this trait (i.e., linkage group RT-17 was reported to have a marginal influence on spawn timing by O'Malley et al. [2003]). The segment of RT-15 bearing a QTL effect is relatively small (19.7 cM), and the QTL effect appears to be localized to a terminal interval on this linkage group segment based on the proximity of Period1 to OmyRGT21TUF, which maps telomerically on RT-15 (Sakamoto et al. 2000). On RT-7, the QTL effect is localized close to the centromere (i.e., syntenic with Omy7INRA). Despite the homeologous affinities of RT-7 and RT-15, we cannot unequivocally confirm that the same QTL region has been detected because shared marker positions adjacent to the QTL are lacking. Within RT-17/22, the QTL effects have been localized to a large central cluster of markers within the male genetic maps for both these linkage groups. Therefore, the exact localization of the QTL region remains difficult to ascertain given the lack of recombination in male salmonids (Sakamoto et al. 2000). Further investigation of these putative duplicated QTL regions requires the reestimation of their chromosomal location based on segregation in female parents or bacterial artificial chromosome contig mapping.
Studies examining the photoperiodic effects on spawning time have been conducted on many fish species since 1937 (reviewed in Bromage et al. 2001), and several other studies have examined the role played by photoperiod on other aspects of reproduction in fish including maturation and somatic growth (Norberg et al. 2001; Rodriguez et al. 2001); follicular growth and egg size (Berrill et al. 2003); and spawning periodicity, fecundity, and egg size (Campos-Mendoza et al. 2004). Despite the amount of research on photoperiod effects and the importance of the timing of reproductive events for aquaculture as well as evolution, there are few studies examining circadian genes and their function in relation to reproductive events in fish. This is the first QTL study implicating circadian rhythm genes (i.e., Clock and possibly Period1) as being associated with a seasonal life history trait in fishes. Our previous expectation was that we would find associations with seasonal spawn timing associated with AANAT genes because they play a significant role in melatonin production, as melatonin appears to be a mediator of photoperiodic regulation of reproduction in salmonids (Bromage et al. 1995, 2001). However, this association between melatonin and reproduction is far from clear (Mayer et al. 1997). Studies using in vivo supplementation of melatonin or pinealectomy to determine the effects of melatonin on reproduction have found equivocal results (Mayer et al. 1997; Bromage et al. 2001). Our results suggest that AANAT as a regulator of melatonin production is not a major gene influencing spawning time in our rainbow trout cross and thus contributes to the lack of evidence supporting the physiological role of melatonin in reproduction (Mayer et al. 1997).
Clock genes have recently been discovered to play a role in reproductive events at several levels in other organisms. In mice, estrous cycles were disrupted by Clock gene mutations (Chappell et al. 2003). Loss of circadian clock function as determined by mutations in specific clock genes (Clock, Period, Timeless, and Cryptochrome) has been shown to decrease the reproductive fitness in Drosophila males, as determined by reduction in sperm output and reduced fertility rates compared with wild-type males (Beaver et al. 2002). In female Drosophila, mating activities were significantly disrupted in Period and Timeless mutants (Sakai and Ishida 2001). Recently, clock genes were implicated in the regulation of pulsatile secretion of GnRH in a mouse hypothalamic cell line suggesting an effect on reproductive rhythms (Chappell et al. 2003). There is also a suggestion that a mutation in one of the clock genes could have a pleiotropic effect on reproductive isolation by altering an organism's perception of daily time, which in turn would affect mating time (Miyatake 2002).
Because seasonality is so critical in the timing of life history events in many species, there must be a molecular connection between environmental inputs and complex biological functions such as maturation and reproduction. Initial research on circadian genes has demonstrated connections between clock genes and diel rhythms (Allada et al. 1998; Stanewsky 2003; Vitaterna et al. 1994), but little is known about how these genes regulate daily functions or how they may regulate annual cycles. Because there exist several clock genes that were not examined in this study and have not yet been identified in salmonids, variation in these genes may account for some of the other QTL effects. Further research should focus on fine-scale mapping of the QTL identified in this study to determine other positional candidate genes for spawning time. Additionally, identifying and examining expression patterns of clock genes and clock-controlled genes in early- and late-spawning strains would help determine how clock genes could potentially regulate reproductive events in rainbow trout.
Corresponding Editor: Martin Tracey
This study was funded by a Strategic Project Grant from the Natural Sciences and Engineering Research Council of Canada and AquaNet, Canada's Network of Centres of Excellence in Aquaculture. The authors acknowledge Xia Yue for laboratory assistance and Karim Gharbi for technical assistance with the SSCP protocols.
References
Allada R, White NE, So WV, Hall JC, and Rosbash M,
Beaver LM, Gvakharia BO, Vollintine TS, Hege DM, Stanewsky R, and Giebultowicz JM,
Begay V, Falcon J, Cahill GM, Klein DC, and Coon SL,
Benjamini Y and Hochberg Y,
Berrill IK, Porter MJR, Smart A, Mitchell D, and Bromage NR,
Bromage N, Porter M, and Randall C,
Bromage NR, Randall CF, Porter MJ, and Davies B,
Campos-Mendoza A, McAndrew BJ, Coward K, and Bromage N,
Chappell PE, White RS, and Mellon PL,
Chen W and Baler R,
Churchill GA and Doerge RW,
Collins PM, O' Neill DF, Barron BR, Moore RK, and Sherwood NM,
Danzmann RG, Cairney M, Ferguson MM, Gharbi K, Guyomard R, Holm L, Hoyheim B, Leder EH, Okamoto N, Ozaki A, Rexroad CE, Sakamoto T, Taggart JB, and Woram RA, in press. A comparative analysis of the rainbow trout genome with two other species of fish (Arctic charr and Atlantic salmon) within the tetraploid derivative Salmonidae family (subfamily: Salmoninae).
Ferriere F, Uzbekova S, Breton B, Jego P, and Bailhache T,
Korol AB, Ronin YI, and Kirzhner VM,
Leder EH, Danzmann RG, and Ferguson MM,
Mayer I, Bornestaf C, and Borg B,
Miyatake T,
Mizusawa K, Iigo M, Masuda T, and Aida K,
Nichols KM, Young WP, Danzmann RG, Robison BD, Rexroad C, Noakes M, Phillips RB, Bentzen P, Spies I, Knudsen K, Allendorf FW, Cunningham BM, Brunelli J, Zhang H, Ristow S, Drew R, Brown KH, Wheeler PA, and Thorgaard GH,
Norberg B, Weltzien FA, Karlsen O, and Holm JC,
O'Malley KG, Sakamoto T, Danzmann RG, and Ferguson MM,
Rodriguez L, Zanuy S, and Carrillo M,
Roenneberg T and Merrow M,
Sakai T and Ishida N,
Sakamoto T, Danzmann RG, Gharbi K, Howard P, Ozaki A, Khoo SK, Woram RA, Okamoto N, Ferguson MM, Holm L, Guyomard R, and Hoyheim B,
Sakamoto T, Danzmann RG, Okamoto N, Ferguson MM, and Ihssen PE,
Somoza GM, Miranda LA, Strobl-Mazzulla P, and Guilgur LG,
Stanewsky R,
Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, and Takahashi JS,
von Schalburg KR and Sherwood N,