-
PDF
- Split View
-
Views
-
Cite
Cite
Andrea M. Polanowski, Sarah M. Robinson-Laverick, David Paton, Simon N. Jarman, Variation in the Tyrosinase Gene Associated with a White Humpback Whale (Megaptera novaeangliae), Journal of Heredity, Volume 103, Issue 1, January-February 2012, Pages 130–133, https://doi.org/10.1093/jhered/esr108
Close - Share Icon Share
Abstract
Tyrosinase-negative oculocutaneous albinism (OCA1A) is characterized by lifelong white hair and skin, a phenotype that has been described in most mammalian species worldwide. Tyrosinase is the key enzyme in melanin biosynthesis, and mutations in the tyrosinase gene result in OCA1A. We examined sequence variation at exon 1 of the tyrosinase gene in 66 humpback whale samples collected from the east coast of Australia, including an anomalously white humpback whale known as “Migaloo.” We identified 3 novel variants, including a cytosine deletion that results in a premature stop codon in exon 1. The deletion truncates the tyrosinase protein including the putative catalytic domains that are essential for tyrosinase enzymatic activity. Migaloo was homozygous for this deletion, suggesting that the albino phenotype is a consequence of inactive tyrosinase caused by the frameshift in the tyrosinase gene.
Animals external colors are important adaptations. The color and patterning of wild animals provide camouflage for reduced visibility to predators or enhanced ability to hunt prey. The color of skin is also important for protecting skin from damage by short wavelength radiation, especially UV-B (Martinez-Levasseur et al. 2010). In mammals, the most important compound involved in producing dark skin and in protecting skin from UV-B damage is melanin (Oetting and King 1993). Animals that cannot make functional melanin are significantly more visible than the wild type, and they have a greatly increased susceptibility to damage by UV-B. Despite the strong selection pressures against albino animals, many mammalian species have a surprisingly frequent occurrence of albino individuals.
Tyrosinase is the key enzyme that regulates melanin biosynthesis in mammals. It is a transmembrane copper-containing enzyme that is responsible for the first 2 steps in the melanin biosynthesis pathway, converting tyrosine to L-dihydroxy-phenylalanine (DOPA) and subsequently to DOPAquinone (Lerner and Fitzpatrick 1950). The enzyme contains 529 amino acids, a signal peptide, 2 putative copper-binding sites, 17 cysteine residues grouped in 2 cysteine-rich domains, and a transmembrane region at the C-terminal end (Camand et al. 2001). It has 5 exons, with exon 1 containing half of the coding region spanning from codon 1 to 272 (Oetting and King 1993).
Oculocutaneous albinism (OCA) is an autosomal recessive disorder characterized by reduced or absent biosynthesis of melanin in the skin, eyes, and hair. In the most severe form of OCA, tyrosinase-negative OCA (OCA1A), tyrosinase gene (TYR, MIM 606933) mutations are responsible for an inactive enzyme resulting in a lifelong absence of melanin production (Gronskov et al. 2007). Mutations in the tyrosinase gene have been extensively studied in humans, with 219 different mutations reported on the albinism database (http://albinismdb.med.umn.edu/21/04/2011). Frameshift and nonsense mutations are randomly dispersed in the coding region and produce truncated proteins that are associated with a complete lack of enzymatic activity. Missense mutations cluster into 4 domains of the coding region and are thought to represent functional domains of the enzyme. Two clusters are within CuA and CuB binding sites, one is at the 5′ end and the last is at the 3′ end of the CuB region (Oetting and King 1994). Other TYR mutation studies include cattle (Schmutz et al. 2004), mink (Anistoroaei et al. 2008), and cats (Schmidt-Kûntzel et al. 2005; Imes et al. 2006).
In 1991, an all white humpback whale (Megaptera novaeangliae) was observed near Byron Bay, New South Wales and has since been referred to in the media and scientific literature as “Migaloo.” This is the only documented occurrence of an anomalously white humpback whale (Forestell et al. 2001). In this study, exon 1 of the whale TYR gene was investigated as a functional candidate gene for albinism in this species.
Materials and Methods
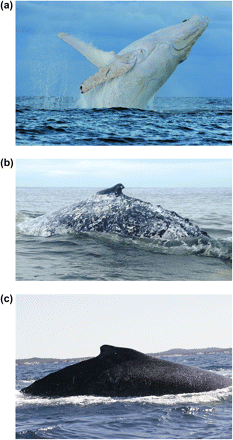
Sloughed skin and skin biopsy samples were collected from whales migrating north past the region of Evan's Head on the east coast of Australia (29°7′S, 153°27′E) in June–July 2009. Photographs were taken for each whale, and 2 unusual and obvious pigmentation phenotypes were observed among a population of otherwise wild-type whales (Figure 1). Biopsy samples were kept on ice for several hours, then split for DNA preservation in 75% ethanol, and RNA preservation in RNAlater. Total DNA was extracted from the skin portion of the whale biopsies using the Maxwell tissue DNA extraction kit (Promega). Thirty milligrams of tissue was homogenized and added to the automated DNA purification cartridge, and DNA was eluted in 250 μl of Tris–ethylenediaminetetraacetic acid.
Photographs of humpback whales representing 3 color phenotypes. (a) White (Migaloo) (Copyright D.P.), (b) mottled (c), and black dorsal.
To design primers for the experiment, a BLAST database created from humpback whale transcripts (Polanowski et al. 2011) was aligned with mRNA of the cow TYR gene (GenBank AF445639; Supplementary Table 1). The alignments started at nucleotide 346 in exon 1 and ended at nucleotide 1612 in exon 5, with numbering starting from the first nucleotide of the transcription initiation site of the cow TYR gene. PCR primers were designed to amplify a 573-bp fragment from exon 1 of the TYR gene. The forward primer 5′-GAGGAARAATGCTCCTGGCTG-3′ was designed by alignment of cow (AF445639), sheep (NM_001130027), pig (NM_001025212), and human (NM_000372) TYR sequences obtained from GenBank. The reverse primer 5′-TACTTGGGGGCTCTGAAATC-3′ was designed from the alignment of the humpback whale transcripts. Twenty-microliter PCR reactions contained 5–10 ng DNA, 0.2 mM dNTP, 0.25 mM each primer, 1 U Taq (New England BioLabs Inc.), and 1× PCR reaction buffer. Cycling profile was 2 min at 94 °C, followed by 35 cycles of 30 s at 94 °C, 30 s at 59 °C, 40 s at 72 °C, and a final step of 5 min at 72 °C. Products were sequenced using BigDye Terminator V 3.1 chemistry on an ABI 3100 Genetic Analyzer and aligned in Sequencher 4.8 (Gene Codes Corp.).
Results and Discussion
A 573 bp product of exon 1 of the TYR gene was aligned for 66 humpback whale samples representing 3 color phenotypes: 64 wild type (dark dorsal), 1 mottled, and 1 white. Three variable sites, 2 transitions and 1 deletion, were identified in a total of 6 whales. Three dark dorsal whales were heterozygous (C/T) and the mottled whale was homozygous (T/T) for the synonymous transition (126 C>T) at codon 42. The white whale Migaloo was homozygous for a cytosine deletion (264 del C) at codon 88. An individual dark dorsal whale was heterozygous (G/T) for a nonsynonymous transition (367 A>G) at codon 123, which results in an amino acid change from isoleucine to valine (Table 1). No variations were found in the remaining 60 samples.
Sequence variation in TYR exon 1 fragment for humpback whales
| Sample code | Phenotype | Total n | Sequence variants | GenBank number | |||
| Nucleotide: | 126 | 264 | 367 | ||||
| C>T | C del | A>G | |||||
| Amino acid: | D42D | del88C | I123V | ||||
| 1–66 | Black dorsal | 60 | C/C | C/C | A/A | — | |
| 2, 17, and 51 | Black dorsal | 3 | C/T | C/C | A/A | JF768702 | |
| 29 | Mottled | 1 | T/T | C/C | A/A | JF768703 | |
| 58 | White | 1 | C/C | –/– | A/A | JF768704 | |
| 63 | Black dorsal | 1 | C/C | C/C | A/G | JF768705 | |
| Sample code | Phenotype | Total n | Sequence variants | GenBank number | |||
| Nucleotide: | 126 | 264 | 367 | ||||
| C>T | C del | A>G | |||||
| Amino acid: | D42D | del88C | I123V | ||||
| 1–66 | Black dorsal | 60 | C/C | C/C | A/A | — | |
| 2, 17, and 51 | Black dorsal | 3 | C/T | C/C | A/A | JF768702 | |
| 29 | Mottled | 1 | T/T | C/C | A/A | JF768703 | |
| 58 | White | 1 | C/C | –/– | A/A | JF768704 | |
| 63 | Black dorsal | 1 | C/C | C/C | A/G | JF768705 | |
Nucleotide positions are in relation to the first nucleotide of the transcription initiation site of the cow TYR gene (GenBank AF445639). N, total number of individuals sharing a particular variant.
Sequence variation in TYR exon 1 fragment for humpback whales
| Sample code | Phenotype | Total n | Sequence variants | GenBank number | |||
| Nucleotide: | 126 | 264 | 367 | ||||
| C>T | C del | A>G | |||||
| Amino acid: | D42D | del88C | I123V | ||||
| 1–66 | Black dorsal | 60 | C/C | C/C | A/A | — | |
| 2, 17, and 51 | Black dorsal | 3 | C/T | C/C | A/A | JF768702 | |
| 29 | Mottled | 1 | T/T | C/C | A/A | JF768703 | |
| 58 | White | 1 | C/C | –/– | A/A | JF768704 | |
| 63 | Black dorsal | 1 | C/C | C/C | A/G | JF768705 | |
| Sample code | Phenotype | Total n | Sequence variants | GenBank number | |||
| Nucleotide: | 126 | 264 | 367 | ||||
| C>T | C del | A>G | |||||
| Amino acid: | D42D | del88C | I123V | ||||
| 1–66 | Black dorsal | 60 | C/C | C/C | A/A | — | |
| 2, 17, and 51 | Black dorsal | 3 | C/T | C/C | A/A | JF768702 | |
| 29 | Mottled | 1 | T/T | C/C | A/A | JF768703 | |
| 58 | White | 1 | C/C | –/– | A/A | JF768704 | |
| 63 | Black dorsal | 1 | C/C | C/C | A/G | JF768705 | |
Nucleotide positions are in relation to the first nucleotide of the transcription initiation site of the cow TYR gene (GenBank AF445639). N, total number of individuals sharing a particular variant.
The cytosine deletion, identified in Migaloo, results in a frameshift and introduces a premature stop codon, 30 codons downstream at c118. The truncated TYR protein would lack both of the putative copper-binding sites and the putative transmembrane segment and therefore would have no tyrosinase enzymatic activity. This result suggests that the truncated TYR protein is the likely cause of Migaloo's albinism. Functional studies in humans have shown that some frameshift mutations disrupt copper binding, and thus, the metal ion–protein interaction necessary for enzyme function. A single base insertion that shifts the reading frame and introduces a premature termination signal at residue 298 has been confirmed as the molecular basis of OCA1A in an albino patient (Tomita et al. 1989). Functional analysis of the mutated gene suggested that the truncated tyrosinase, which lacked CuB, was catalytically inactive. Mammalian tyrosinase directly binds copper and the CuA and CuB sites are both required for copper binding and for catalytic activity (Spritz et al. 1997). Other association studies, including humans (Giebel et al. 1991; Oetting et al. 1991; Camand et al. 2001), cattle (Schmutz et al. 2004), mink (Anistoroaei et al. 2008), and cat (Imes et al. 2006), have suggested that frameshift mutations in the TYR gene are responsible for the albino phenotype.
Migaloo is an adult male humpback whale believed to be between 23 and 25 years of age, who is frequently photographed while traveling along the east coast of Australia. The whale's eye is not clearly visible in any photograph, however, there is an unusual amount of pink pigmentation around the blowhole. Photographs from 1992 to 1998 also show abnormal swelling and cyst-like protuberances around the blowhole region (Forestell et al. 2001). In OCA1A, in humans, the absence of melanin pigment in the skin results in an increased sensitivity to UV radiation and a predisposition to skin cancer (Oetting and King 1993). A recent study, on sunburn and photoprotection in whales, found that individuals with fewer melanocytes have more lesions that are commonly associated with acute severe sun damage in humans. The prevalence of lesions also increased significantly over time as would be expected under increasing UV exposure (Martinez-Levasseur et al. 2010).
Melanin plays a key role in the survival ability of animals that live in the sun. It provides protection from UV damage; camouflage from predators and prey; and is selected for sexually in many species. Anomalously white individuals have been observed in 22 cetacean species, and the disadvantages associated with complete absence of pigment may include reduced heat absorption in colder waters, increased conspicuousness to predators, increased eye and skin sensitivity, and visual impairment (Fertl et al. 2004). Despite these potential disadvantages, Migaloo has reached adult age, with the most recent sighting in August 2011 (http://www.migaloo.com.au/Sightings.htm).
Funding
Australian Antarctic Division; Australian Marine Mammal Centre.
We would like to thank everyone involved in organizing the following permits—Environment Protection and Biodiversity Act 1999, 2007-0007; New South Wales Scientific License S12887; New South Wales Police Commissioner's permit 409979066; and the Antarctic Animal Ethics Committee Project 2941. A special thanks to Scott Baker for his comments on the manuscript.
References
Author notes
Corresponding Editor: Scott Baker