-
PDF
- Split View
-
Views
-
Cite
Cite
Hiroharu Yamashita, Joji Kitayama, Hirokazu Nagawa, Hyperfibrinogenemia is a Useful Predictor for Lymphatic Metastasis in Human Gastric Cancer, Japanese Journal of Clinical Oncology, Volume 35, Issue 10, October 2005, Pages 595–600, https://doi.org/10.1093/jjco/hyi150
Close - Share Icon Share
Abstract
Background: Although abnormal hemostasis has been described in cancer patients, the precise association between the plasma fibrinogen level and lymphatic metastasis has not been reported in a large-scale clinical study.
Methods: Preoperative plasma levels of fibrinogen as well as C-reactive protein (CRP) and carcinoembryonic antigen (CEA) were retrospectively examined in 649 patients who underwent surgery for gastric cancer, and the correlation between these factors and nodal status was evaluated.
Results: Plasma fibrinogen level in patients with gastric cancer showed a positive association with nodal classification (P < 0.0001). Hyperfibrinogenemia (>310 mg/dl) as well as high CEA (>5 ng/ml) and CRP (>0.3 mg/dl) showed a significant association with nodal metastasis in univariate analysis. Multivariate analysis revealed that hyperfibrinogenemia had an independent association with nodal metastasis (odds ratio, 2.004 (1.140–3.521); P = 0.0157), whereas CEA and CRP were not independent factors. Hyperfibrinogenemia showed an independent association even in advanced cancer [odds ratio 2.611 (1.404–4.854), P = 0.0024, n = 319]. When the 649 gastric cancers were classified into intestinal-type and gastric-type adenocarcinomas, plasma fibrinogen level was correlated with nodal metastasis only in the intestinal-type.
Conclusions: Our results suggest that hyperfibrinogenemia may provide favorable circumstances for cancer cells to metastasize via the lymphatic system. Preoperative plasma fibrinogen level is a useful predictor of lymphatic metastasis in intestinal-type gastric cancer.
INTRODUCTION
Many abnormalities of platelets or blood coagulation factors, such as fragment 1+2, thrombin–antithrombin III complexes, fibrinopeptide A and D-dimer, have been described in malignancy for more than a century (1–4). Thrombocytosis is thought to be associated with poor prognosis in gastric cancer (5), as well as esophageal cancer (6), lung cancer (7), colon cancer (7), renal cell carcinoma (8) and gynecological malignancies (9). D-dimer is also reported to be associated with poor prognosis in patients with lung cancer (10,11) and colorectal cancer (12,13), as well as being a good predictor of survival and disease progression.
Fibrinogen, an essential hemostatic factor, is converted to fibrin (a final product of the hemostatic pathway) by activated thrombin. Dvorak (14) suggested that elevated plasma fibrinogen levels were frequently observed in patients with malignant disease. However, a report by Di Micco et al. (15) showed elevated plasma fibrinogen levels in 11 patients with non-metastatic gastric cancer. The relationship between fibrinogen level and metastasis in malignancy has not been fully examined.
Recently, it was reported that plasma fibrinogen level was correlated with tumor size, depth of tumor invasion and metastasis of gastric cancer (16). Although hyperfibrinogenemia was considered to result from spreading of cancer, there is evidence that the loss of fibrinogen is the cause of reduction of metastasis. For example, in fibrinogen-deficient mice, lymphatic and hematogenous metastases were greatly reduced, indicating a positive role of fibrinogen in the metastatic progression of cancer (17,18). In this study, therefore, we evaluated clinicopathological significance of hyperfibrinogenemia more in detail using a larger number of clinical cases and demonstrated an independent significance of hyperfibrinogenemia on lymphatic spread of gastric cancer.
PATIENTS AND METHODS
Among the 1083 patients with gastric cancer who underwent gastrectomy between January 1987 and December 2003 in the First Department of Surgery, University of Tokyo Hospital, Tokyo, plasma fibrinogen, serum C-reactive protein (CRP) and carcinoembryonic antigen (CEA) levels were determined before surgery in 653 patients. Because plasma fibrinogen and serum CRP levels are critically affected by the presence of inflammation, four patients with apparent acute inflammatory disease were excluded from the study and the remaining 649 patients were enrolled in this study. As a control group, we also evaluated preoperative plasma fibrinogen level of 126 patients with benign disease (inguinal hernia).
In this study, we referred to the classification established by the Japanese Research Society for Gastric Cancer (19), which defines early gastric cancer as a lesion confined to the mucosal or submucosal layer, and advanced gastric cancer as a lesion invading the proper muscle layer or deeper. Histologically, well and moderately differentiated tubular adenocarcinoma, papillary adenocarcinoma and solid-type poorly differentiated adenocarcinoma were classified as differentiated type (intestinal-type) carcinoma; non-solid-type poorly differentiated adenocarcinoma and signet ring cell carcinoma as undifferentiated type (gastric-type); and mucinous carcinoma and adenosquamous cell carcinoma as other type. The nodal classification was determined according to the International Union Against Cancer (UICC) TNM staging system for gastric cancer (20), in which lymph node metastasis is classified into four groups: pN0, no metastasis; pN1, 1–6 positive lymph nodes; pN2, 7–15 positive lymph nodes; pN3, 16 or more positive lymph nodes.
The preoperative values were measured in early morning samples taken before breakfast 5–10 days before surgery. The normal range of plasma fibrinogen level was defined as between 210 and 310 mg/dl, and plasma fibrinogen levels >310 mg/dl were defined as hyperfibrinogenemia. Serum CRP level <0.3 mg/dl and CEA level <5 ng/ml were taken as the normal range.
Statistical analysis was carried out using STATView (SAS Institute, Cary, NC). Plasma fibrinogen levels were compared by one-way ANOVA followed by the Student–Newman–Keuls test. The association of fibrinogen level with clinicopathological factors was assessed by Fisher's exact test. Multivariate forward stepwise logistic regression analysis was performed to identify variables that had independent associations with lymph node metastasis. Values of P < 0.05 were considered significant for all statistical analyses.
RESULTS
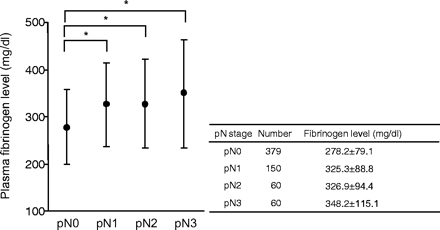
The mean ± SD of plasma fibrinogen level in the 649 patients studied was 300.1 ± 84.5 mg/dl, which was not statistically different from that in control patients with inguinal hernia (279.2 ± 44.2 mg/dl). As shown in Table 1, high fibrinogen level (≥311 mg/dl) was positively correlated with nodal or hematogeneous metastasis as well as other clinical or pathological factors. In this study, we evaluated the association of lymphatic metastasis with hyperfibrinogenemia in more detail. When patients were classified into four groups according to the extent of lymphatic metastasis, the fibrinogen level showed a positive association with nodal classification (P < 0.0001) (Fig. 1).
Plasma fibrinogen level in 649 patients with gastric cancer classified according to UICC pN classification. Note that mean values of plasma fibrinogen level gradually increase in accordance with the elevation of the extent of lymph node metastasis. *The mean values of plasma fibrinogen were significantly different between the groups of pN0 and pN1 (P < 0.01), between the groups of pN0 and pN2 (P < 0.01) and between the groups of pN0 and pN3 (P < 0.01).
Relationship between clinicopathological factors and plasma fibrinogen level in gastric cancer
| . | Plasma fibrinogen level . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Variables . | Total . | ≤310 . | ≥311 . | P-value . | ||||
| Number of cases | 649 | 407 | 242 | |||||
| Age (years) | ||||||||
| ≤59 | 315 | 234 | 81 | <0.0001 | ||||
| ≥60 | 334 | 173 | 161 | |||||
| Gender | ||||||||
| Male | 468 | 278 | 190 | 0.0051 | ||||
| Female | 181 | 129 | 52 | |||||
| Tumor location | ||||||||
| Upper | 136 | 74 | 62 | 0.0067 | ||||
| Middle | 302 | 208 | 94 | |||||
| Lower | 211 | 125 | 86 | |||||
| Histology | ||||||||
| Intestinal type | 382 | 219 | 163 | 0.0028 | ||||
| Gastric type | 247 | 175 | 72 | |||||
| Other type | 20 | 13 | 7 | |||||
| Lymphatic involvement | ||||||||
| Negative | 382 | 266 | 116 | <0.0001 | ||||
| Positive | 267 | 141 | 126 | |||||
| Venous involvement | ||||||||
| Negative | 413 | 288 | 125 | <0.0001 | ||||
| Positive | 236 | 119 | 117 | |||||
| Lymph node metastasis | ||||||||
| Negative | 379 | 275 | 104 | <0.0001 | ||||
| Positive | 270 | 132 | 138 | |||||
| Liver metastasis | ||||||||
| Negative | 634 | 405 | 229 | 0.0001 | ||||
| Positive | 15 | 2 | 13 | |||||
| . | Plasma fibrinogen level . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Variables . | Total . | ≤310 . | ≥311 . | P-value . | ||||
| Number of cases | 649 | 407 | 242 | |||||
| Age (years) | ||||||||
| ≤59 | 315 | 234 | 81 | <0.0001 | ||||
| ≥60 | 334 | 173 | 161 | |||||
| Gender | ||||||||
| Male | 468 | 278 | 190 | 0.0051 | ||||
| Female | 181 | 129 | 52 | |||||
| Tumor location | ||||||||
| Upper | 136 | 74 | 62 | 0.0067 | ||||
| Middle | 302 | 208 | 94 | |||||
| Lower | 211 | 125 | 86 | |||||
| Histology | ||||||||
| Intestinal type | 382 | 219 | 163 | 0.0028 | ||||
| Gastric type | 247 | 175 | 72 | |||||
| Other type | 20 | 13 | 7 | |||||
| Lymphatic involvement | ||||||||
| Negative | 382 | 266 | 116 | <0.0001 | ||||
| Positive | 267 | 141 | 126 | |||||
| Venous involvement | ||||||||
| Negative | 413 | 288 | 125 | <0.0001 | ||||
| Positive | 236 | 119 | 117 | |||||
| Lymph node metastasis | ||||||||
| Negative | 379 | 275 | 104 | <0.0001 | ||||
| Positive | 270 | 132 | 138 | |||||
| Liver metastasis | ||||||||
| Negative | 634 | 405 | 229 | 0.0001 | ||||
| Positive | 15 | 2 | 13 | |||||
Relationship between clinicopathological factors and plasma fibrinogen level in gastric cancer
| . | Plasma fibrinogen level . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Variables . | Total . | ≤310 . | ≥311 . | P-value . | ||||
| Number of cases | 649 | 407 | 242 | |||||
| Age (years) | ||||||||
| ≤59 | 315 | 234 | 81 | <0.0001 | ||||
| ≥60 | 334 | 173 | 161 | |||||
| Gender | ||||||||
| Male | 468 | 278 | 190 | 0.0051 | ||||
| Female | 181 | 129 | 52 | |||||
| Tumor location | ||||||||
| Upper | 136 | 74 | 62 | 0.0067 | ||||
| Middle | 302 | 208 | 94 | |||||
| Lower | 211 | 125 | 86 | |||||
| Histology | ||||||||
| Intestinal type | 382 | 219 | 163 | 0.0028 | ||||
| Gastric type | 247 | 175 | 72 | |||||
| Other type | 20 | 13 | 7 | |||||
| Lymphatic involvement | ||||||||
| Negative | 382 | 266 | 116 | <0.0001 | ||||
| Positive | 267 | 141 | 126 | |||||
| Venous involvement | ||||||||
| Negative | 413 | 288 | 125 | <0.0001 | ||||
| Positive | 236 | 119 | 117 | |||||
| Lymph node metastasis | ||||||||
| Negative | 379 | 275 | 104 | <0.0001 | ||||
| Positive | 270 | 132 | 138 | |||||
| Liver metastasis | ||||||||
| Negative | 634 | 405 | 229 | 0.0001 | ||||
| Positive | 15 | 2 | 13 | |||||
| . | Plasma fibrinogen level . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Variables . | Total . | ≤310 . | ≥311 . | P-value . | ||||
| Number of cases | 649 | 407 | 242 | |||||
| Age (years) | ||||||||
| ≤59 | 315 | 234 | 81 | <0.0001 | ||||
| ≥60 | 334 | 173 | 161 | |||||
| Gender | ||||||||
| Male | 468 | 278 | 190 | 0.0051 | ||||
| Female | 181 | 129 | 52 | |||||
| Tumor location | ||||||||
| Upper | 136 | 74 | 62 | 0.0067 | ||||
| Middle | 302 | 208 | 94 | |||||
| Lower | 211 | 125 | 86 | |||||
| Histology | ||||||||
| Intestinal type | 382 | 219 | 163 | 0.0028 | ||||
| Gastric type | 247 | 175 | 72 | |||||
| Other type | 20 | 13 | 7 | |||||
| Lymphatic involvement | ||||||||
| Negative | 382 | 266 | 116 | <0.0001 | ||||
| Positive | 267 | 141 | 126 | |||||
| Venous involvement | ||||||||
| Negative | 413 | 288 | 125 | <0.0001 | ||||
| Positive | 236 | 119 | 117 | |||||
| Lymph node metastasis | ||||||||
| Negative | 379 | 275 | 104 | <0.0001 | ||||
| Positive | 270 | 132 | 138 | |||||
| Liver metastasis | ||||||||
| Negative | 634 | 405 | 229 | 0.0001 | ||||
| Positive | 15 | 2 | 13 | |||||
Univariate analysis showed that lymph node metastasis was significantly associated with many factors such as tumor size, lymphatic and venous involvement as well as the levels of serum CEA, CRP and plasma fibrinogen (Table 2). However, a multivariate analysis revealed that lymphatic involvement, tumor size, advanced stage and hyperfibrinogenemia were identified as independent risk factors for lymph node metastasis, with an odds ratio [95% confidence interval (CI)] of [9.180 (5.486–15.360), 2.320 (1.395–3.861), 7.353 (4.082–13.333) and 2.004 (1.140–3.521), respectively] (Table 2). In contrast, serum levels of CRP and CEA did not show an independent association with nodal metastasis.
Relationship between clinicopathological factors and metastasis in gastric cancer (univariate and multivariate analysis)
. | Total . | Node negative . | Node positive . | P-value . | ||||
|---|---|---|---|---|---|---|---|---|
| Number of cases | 649 | 379 | 270 | |||||
| Univariate analysis variables | ||||||||
| Age (years) | ||||||||
| ≤59 | 315 | 192 | 123 | 0.2035 | ||||
| ≥60 | 334 | 187 | 147 | |||||
| Gender | ||||||||
| Male | 468 | 283 | 185 | 0.0919 | ||||
| Female | 181 | 96 | 85 | |||||
| Tumor location* | ||||||||
| Upper | 136 | 58 | 78 | <0.0001 | ||||
| Middle | 302 | 200 | 102 | |||||
| Lower | 211 | 121 | 90 | |||||
| Histology | ||||||||
| Intestinal type | 382 | 228 | 154 | 0.0922 | ||||
| Gastric type | 247 | 144 | 103 | |||||
| Other type | 20 | 7 | 13 | |||||
| Lymphatic involvement | ||||||||
| Negative | 382 | 330 | 52 | <0.0001 | ||||
| Positive | 267 | 49 | 218 | |||||
| Venous involvement | ||||||||
| Negative | 413 | 315 | 98 | <0.0001 | ||||
| Positive | 236 | 64 | 172 | |||||
| Tumor stage | ||||||||
| Early | 330 | 298 | 32 | <0.0001 | ||||
| Advanced | 319 | 81 | 238 | |||||
| Tumor size | ||||||||
| ≤4 | 317 | 259 | 58 | <0.0001 | ||||
| >4 | 332 | 120 | 212 | |||||
| CEA | ||||||||
| Negative | 522 | 325 | 197 | <0.0001 | ||||
| Positive | 127 | 54 | 73 | |||||
| CRP | ||||||||
| Negative | 521 | 327 | 194 | <0.0001 | ||||
| Positive | 128 | 52 | 76 | |||||
| Plasma fibrinogen level (mg/dl) | ||||||||
| ≤310 | 407 | 275 | 132 | <0.0001 | ||||
| ≥311 | 242 | 104 | 138 | |||||
. | Total . | Node negative . | Node positive . | P-value . | ||||
|---|---|---|---|---|---|---|---|---|
| Number of cases | 649 | 379 | 270 | |||||
| Univariate analysis variables | ||||||||
| Age (years) | ||||||||
| ≤59 | 315 | 192 | 123 | 0.2035 | ||||
| ≥60 | 334 | 187 | 147 | |||||
| Gender | ||||||||
| Male | 468 | 283 | 185 | 0.0919 | ||||
| Female | 181 | 96 | 85 | |||||
| Tumor location* | ||||||||
| Upper | 136 | 58 | 78 | <0.0001 | ||||
| Middle | 302 | 200 | 102 | |||||
| Lower | 211 | 121 | 90 | |||||
| Histology | ||||||||
| Intestinal type | 382 | 228 | 154 | 0.0922 | ||||
| Gastric type | 247 | 144 | 103 | |||||
| Other type | 20 | 7 | 13 | |||||
| Lymphatic involvement | ||||||||
| Negative | 382 | 330 | 52 | <0.0001 | ||||
| Positive | 267 | 49 | 218 | |||||
| Venous involvement | ||||||||
| Negative | 413 | 315 | 98 | <0.0001 | ||||
| Positive | 236 | 64 | 172 | |||||
| Tumor stage | ||||||||
| Early | 330 | 298 | 32 | <0.0001 | ||||
| Advanced | 319 | 81 | 238 | |||||
| Tumor size | ||||||||
| ≤4 | 317 | 259 | 58 | <0.0001 | ||||
| >4 | 332 | 120 | 212 | |||||
| CEA | ||||||||
| Negative | 522 | 325 | 197 | <0.0001 | ||||
| Positive | 127 | 54 | 73 | |||||
| CRP | ||||||||
| Negative | 521 | 327 | 194 | <0.0001 | ||||
| Positive | 128 | 52 | 76 | |||||
| Plasma fibrinogen level (mg/dl) | ||||||||
| ≤310 | 407 | 275 | 132 | <0.0001 | ||||
| ≥311 | 242 | 104 | 138 | |||||
. | Odds ratio . | 95% CI . | . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Multivariate analysis variables | ||||||||
| Location (middle) | 1.270 | 0.672 | 2.398 | 0.4621 | ||||
| Location (lower) | 1.300 | 0.666 | 2.536 | 0.4419 | ||||
| Lymphatic involvement | 9.180 | 5.486 | 15.36 | <0.0001 | ||||
| Venous involvement | 1.178 | 0.674 | 2.057 | 0.5657 | ||||
| Advanced cancer | 7.353 | 4.082 | 13.333 | <0.0001 | ||||
| Large tumor | 2.320 | 1.395 | 3.861 | 0.0012 | ||||
| CEA positive | 1.572 | 0.858 | 2.882 | 0.1428 | ||||
| CRP positive | 1.057 | 0.536 | 2.083 | 0.8732 | ||||
| Hyperfibrinogenemia | 2.004 | 1.140 | 3.521 | 0.0157 | ||||
. | Odds ratio . | 95% CI . | . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Multivariate analysis variables | ||||||||
| Location (middle) | 1.270 | 0.672 | 2.398 | 0.4621 | ||||
| Location (lower) | 1.300 | 0.666 | 2.536 | 0.4419 | ||||
| Lymphatic involvement | 9.180 | 5.486 | 15.36 | <0.0001 | ||||
| Venous involvement | 1.178 | 0.674 | 2.057 | 0.5657 | ||||
| Advanced cancer | 7.353 | 4.082 | 13.333 | <0.0001 | ||||
| Large tumor | 2.320 | 1.395 | 3.861 | 0.0012 | ||||
| CEA positive | 1.572 | 0.858 | 2.882 | 0.1428 | ||||
| CRP positive | 1.057 | 0.536 | 2.083 | 0.8732 | ||||
| Hyperfibrinogenemia | 2.004 | 1.140 | 3.521 | 0.0157 | ||||
Tumor of a large size in two or three different areas was classified to be located at the most predominant area.
Relationship between clinicopathological factors and metastasis in gastric cancer (univariate and multivariate analysis)
. | Total . | Node negative . | Node positive . | P-value . | ||||
|---|---|---|---|---|---|---|---|---|
| Number of cases | 649 | 379 | 270 | |||||
| Univariate analysis variables | ||||||||
| Age (years) | ||||||||
| ≤59 | 315 | 192 | 123 | 0.2035 | ||||
| ≥60 | 334 | 187 | 147 | |||||
| Gender | ||||||||
| Male | 468 | 283 | 185 | 0.0919 | ||||
| Female | 181 | 96 | 85 | |||||
| Tumor location* | ||||||||
| Upper | 136 | 58 | 78 | <0.0001 | ||||
| Middle | 302 | 200 | 102 | |||||
| Lower | 211 | 121 | 90 | |||||
| Histology | ||||||||
| Intestinal type | 382 | 228 | 154 | 0.0922 | ||||
| Gastric type | 247 | 144 | 103 | |||||
| Other type | 20 | 7 | 13 | |||||
| Lymphatic involvement | ||||||||
| Negative | 382 | 330 | 52 | <0.0001 | ||||
| Positive | 267 | 49 | 218 | |||||
| Venous involvement | ||||||||
| Negative | 413 | 315 | 98 | <0.0001 | ||||
| Positive | 236 | 64 | 172 | |||||
| Tumor stage | ||||||||
| Early | 330 | 298 | 32 | <0.0001 | ||||
| Advanced | 319 | 81 | 238 | |||||
| Tumor size | ||||||||
| ≤4 | 317 | 259 | 58 | <0.0001 | ||||
| >4 | 332 | 120 | 212 | |||||
| CEA | ||||||||
| Negative | 522 | 325 | 197 | <0.0001 | ||||
| Positive | 127 | 54 | 73 | |||||
| CRP | ||||||||
| Negative | 521 | 327 | 194 | <0.0001 | ||||
| Positive | 128 | 52 | 76 | |||||
| Plasma fibrinogen level (mg/dl) | ||||||||
| ≤310 | 407 | 275 | 132 | <0.0001 | ||||
| ≥311 | 242 | 104 | 138 | |||||
. | Total . | Node negative . | Node positive . | P-value . | ||||
|---|---|---|---|---|---|---|---|---|
| Number of cases | 649 | 379 | 270 | |||||
| Univariate analysis variables | ||||||||
| Age (years) | ||||||||
| ≤59 | 315 | 192 | 123 | 0.2035 | ||||
| ≥60 | 334 | 187 | 147 | |||||
| Gender | ||||||||
| Male | 468 | 283 | 185 | 0.0919 | ||||
| Female | 181 | 96 | 85 | |||||
| Tumor location* | ||||||||
| Upper | 136 | 58 | 78 | <0.0001 | ||||
| Middle | 302 | 200 | 102 | |||||
| Lower | 211 | 121 | 90 | |||||
| Histology | ||||||||
| Intestinal type | 382 | 228 | 154 | 0.0922 | ||||
| Gastric type | 247 | 144 | 103 | |||||
| Other type | 20 | 7 | 13 | |||||
| Lymphatic involvement | ||||||||
| Negative | 382 | 330 | 52 | <0.0001 | ||||
| Positive | 267 | 49 | 218 | |||||
| Venous involvement | ||||||||
| Negative | 413 | 315 | 98 | <0.0001 | ||||
| Positive | 236 | 64 | 172 | |||||
| Tumor stage | ||||||||
| Early | 330 | 298 | 32 | <0.0001 | ||||
| Advanced | 319 | 81 | 238 | |||||
| Tumor size | ||||||||
| ≤4 | 317 | 259 | 58 | <0.0001 | ||||
| >4 | 332 | 120 | 212 | |||||
| CEA | ||||||||
| Negative | 522 | 325 | 197 | <0.0001 | ||||
| Positive | 127 | 54 | 73 | |||||
| CRP | ||||||||
| Negative | 521 | 327 | 194 | <0.0001 | ||||
| Positive | 128 | 52 | 76 | |||||
| Plasma fibrinogen level (mg/dl) | ||||||||
| ≤310 | 407 | 275 | 132 | <0.0001 | ||||
| ≥311 | 242 | 104 | 138 | |||||
. | Odds ratio . | 95% CI . | . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Multivariate analysis variables | ||||||||
| Location (middle) | 1.270 | 0.672 | 2.398 | 0.4621 | ||||
| Location (lower) | 1.300 | 0.666 | 2.536 | 0.4419 | ||||
| Lymphatic involvement | 9.180 | 5.486 | 15.36 | <0.0001 | ||||
| Venous involvement | 1.178 | 0.674 | 2.057 | 0.5657 | ||||
| Advanced cancer | 7.353 | 4.082 | 13.333 | <0.0001 | ||||
| Large tumor | 2.320 | 1.395 | 3.861 | 0.0012 | ||||
| CEA positive | 1.572 | 0.858 | 2.882 | 0.1428 | ||||
| CRP positive | 1.057 | 0.536 | 2.083 | 0.8732 | ||||
| Hyperfibrinogenemia | 2.004 | 1.140 | 3.521 | 0.0157 | ||||
. | Odds ratio . | 95% CI . | . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Multivariate analysis variables | ||||||||
| Location (middle) | 1.270 | 0.672 | 2.398 | 0.4621 | ||||
| Location (lower) | 1.300 | 0.666 | 2.536 | 0.4419 | ||||
| Lymphatic involvement | 9.180 | 5.486 | 15.36 | <0.0001 | ||||
| Venous involvement | 1.178 | 0.674 | 2.057 | 0.5657 | ||||
| Advanced cancer | 7.353 | 4.082 | 13.333 | <0.0001 | ||||
| Large tumor | 2.320 | 1.395 | 3.861 | 0.0012 | ||||
| CEA positive | 1.572 | 0.858 | 2.882 | 0.1428 | ||||
| CRP positive | 1.057 | 0.536 | 2.083 | 0.8732 | ||||
| Hyperfibrinogenemia | 2.004 | 1.140 | 3.521 | 0.0157 | ||||
Tumor of a large size in two or three different areas was classified to be located at the most predominant area.
Since the frequencies of nodal metastasis and hyperfibrinogenemia were much higher in patients with advanced cancer (9.7% in early cancer and 74.6% in advanced cancer), we evaluated the association of these factors in patients with advanced cancer (Table 3). Tumor size, venous or lymphatic invasion, high CEA and hyperfibrinogenemia showed a positive association with lymphatic metastasis in univariate analysis. CRP did not show a significant association in this population. In contrast, hyperfibrinogenemia showed an independent association with nodal metastasis, with an increased odds ratio of 2.611 (1.404–4.854) (P < 0.01), whereas high CEA did not show an independent association in cases of advanced cancer (Table 3).
Relationship between clinicopathological factors and metastasis in advanced gastric cancer (univariate and multivariate analysis)
. | Total . | Node negative . | Node positive . | P-value . | ||||
|---|---|---|---|---|---|---|---|---|
| Number of cases | 319 | 81 | 238 | |||||
| Univariate analysis variables | ||||||||
| Age (years) | ||||||||
| ≤59 | 141 | 38 | 103 | 0.6055 | ||||
| ≥60 | 178 | 43 | 135 | |||||
| Gender | ||||||||
| Male | 223 | 58 | 165 | 0.7796 | ||||
| Female | 96 | 23 | 73 | |||||
| Tumor location | ||||||||
| Upper | 100 | 23 | 77 | 0.5871 | ||||
| Middle | 115 | 33 | 82 | |||||
| Lower | 104 | 25 | 79 | |||||
| Histology | ||||||||
| Intestinal type | 182 | 44 | 138 | 0.7505 | ||||
| Gastric type | 118 | 31 | 87 | |||||
| Other type | 19 | 6 | 13 | |||||
| Lymphatic involvement | ||||||||
| Negative | 87 | 49 | 38 | <0.0001 | ||||
| Positive | 232 | 32 | 200 | |||||
| Venous involvement | ||||||||
| Negative | 110 | 36 | 74 | 0.0313 | ||||
| Positive | 209 | 45 | 164 | |||||
| Tumor size | ||||||||
| ≤6 | 148 | 57 | 91 | <0.0001 | ||||
| >6 | 171 | 24 | 147 | |||||
| CEA | ||||||||
| Negative | 240 | 69 | 171 | 0.017 | ||||
| Positive | 79 | 12 | 67 | |||||
| CRP | ||||||||
| Negative | 227 | 63 | 164 | 0.1557 | ||||
| Positive | 92 | 18 | 74 | |||||
| Plasma fibrinogen | ||||||||
| ≤310 | 166 | 56 | 110 | 0.0005 | ||||
| ≥311 | 153 | 25 | 128 | |||||
. | Total . | Node negative . | Node positive . | P-value . | ||||
|---|---|---|---|---|---|---|---|---|
| Number of cases | 319 | 81 | 238 | |||||
| Univariate analysis variables | ||||||||
| Age (years) | ||||||||
| ≤59 | 141 | 38 | 103 | 0.6055 | ||||
| ≥60 | 178 | 43 | 135 | |||||
| Gender | ||||||||
| Male | 223 | 58 | 165 | 0.7796 | ||||
| Female | 96 | 23 | 73 | |||||
| Tumor location | ||||||||
| Upper | 100 | 23 | 77 | 0.5871 | ||||
| Middle | 115 | 33 | 82 | |||||
| Lower | 104 | 25 | 79 | |||||
| Histology | ||||||||
| Intestinal type | 182 | 44 | 138 | 0.7505 | ||||
| Gastric type | 118 | 31 | 87 | |||||
| Other type | 19 | 6 | 13 | |||||
| Lymphatic involvement | ||||||||
| Negative | 87 | 49 | 38 | <0.0001 | ||||
| Positive | 232 | 32 | 200 | |||||
| Venous involvement | ||||||||
| Negative | 110 | 36 | 74 | 0.0313 | ||||
| Positive | 209 | 45 | 164 | |||||
| Tumor size | ||||||||
| ≤6 | 148 | 57 | 91 | <0.0001 | ||||
| >6 | 171 | 24 | 147 | |||||
| CEA | ||||||||
| Negative | 240 | 69 | 171 | 0.017 | ||||
| Positive | 79 | 12 | 67 | |||||
| CRP | ||||||||
| Negative | 227 | 63 | 164 | 0.1557 | ||||
| Positive | 92 | 18 | 74 | |||||
| Plasma fibrinogen | ||||||||
| ≤310 | 166 | 56 | 110 | 0.0005 | ||||
| ≥311 | 153 | 25 | 128 | |||||
. | Odds ratio . | 95% CI . | . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Multivariate analysis variables | ||||||||
| Lymphatic involvement | 7.138 | 3.783 | 13.467 | <0.0001 | ||||
| Venous involvement | 0.921 | 0.49 | 1.729 | 0.797 | ||||
| Large tumor | 2.632 | 1.433 | 4.831 | 0.0018 | ||||
| CEA positive | 2.183 | 0.997 | 4.785 | 0.051 | ||||
| Hyperfibrinogenemia | 2.611 | 1.404 | 4.854 | 0.0024 | ||||
. | Odds ratio . | 95% CI . | . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Multivariate analysis variables | ||||||||
| Lymphatic involvement | 7.138 | 3.783 | 13.467 | <0.0001 | ||||
| Venous involvement | 0.921 | 0.49 | 1.729 | 0.797 | ||||
| Large tumor | 2.632 | 1.433 | 4.831 | 0.0018 | ||||
| CEA positive | 2.183 | 0.997 | 4.785 | 0.051 | ||||
| Hyperfibrinogenemia | 2.611 | 1.404 | 4.854 | 0.0024 | ||||
Relationship between clinicopathological factors and metastasis in advanced gastric cancer (univariate and multivariate analysis)
. | Total . | Node negative . | Node positive . | P-value . | ||||
|---|---|---|---|---|---|---|---|---|
| Number of cases | 319 | 81 | 238 | |||||
| Univariate analysis variables | ||||||||
| Age (years) | ||||||||
| ≤59 | 141 | 38 | 103 | 0.6055 | ||||
| ≥60 | 178 | 43 | 135 | |||||
| Gender | ||||||||
| Male | 223 | 58 | 165 | 0.7796 | ||||
| Female | 96 | 23 | 73 | |||||
| Tumor location | ||||||||
| Upper | 100 | 23 | 77 | 0.5871 | ||||
| Middle | 115 | 33 | 82 | |||||
| Lower | 104 | 25 | 79 | |||||
| Histology | ||||||||
| Intestinal type | 182 | 44 | 138 | 0.7505 | ||||
| Gastric type | 118 | 31 | 87 | |||||
| Other type | 19 | 6 | 13 | |||||
| Lymphatic involvement | ||||||||
| Negative | 87 | 49 | 38 | <0.0001 | ||||
| Positive | 232 | 32 | 200 | |||||
| Venous involvement | ||||||||
| Negative | 110 | 36 | 74 | 0.0313 | ||||
| Positive | 209 | 45 | 164 | |||||
| Tumor size | ||||||||
| ≤6 | 148 | 57 | 91 | <0.0001 | ||||
| >6 | 171 | 24 | 147 | |||||
| CEA | ||||||||
| Negative | 240 | 69 | 171 | 0.017 | ||||
| Positive | 79 | 12 | 67 | |||||
| CRP | ||||||||
| Negative | 227 | 63 | 164 | 0.1557 | ||||
| Positive | 92 | 18 | 74 | |||||
| Plasma fibrinogen | ||||||||
| ≤310 | 166 | 56 | 110 | 0.0005 | ||||
| ≥311 | 153 | 25 | 128 | |||||
. | Total . | Node negative . | Node positive . | P-value . | ||||
|---|---|---|---|---|---|---|---|---|
| Number of cases | 319 | 81 | 238 | |||||
| Univariate analysis variables | ||||||||
| Age (years) | ||||||||
| ≤59 | 141 | 38 | 103 | 0.6055 | ||||
| ≥60 | 178 | 43 | 135 | |||||
| Gender | ||||||||
| Male | 223 | 58 | 165 | 0.7796 | ||||
| Female | 96 | 23 | 73 | |||||
| Tumor location | ||||||||
| Upper | 100 | 23 | 77 | 0.5871 | ||||
| Middle | 115 | 33 | 82 | |||||
| Lower | 104 | 25 | 79 | |||||
| Histology | ||||||||
| Intestinal type | 182 | 44 | 138 | 0.7505 | ||||
| Gastric type | 118 | 31 | 87 | |||||
| Other type | 19 | 6 | 13 | |||||
| Lymphatic involvement | ||||||||
| Negative | 87 | 49 | 38 | <0.0001 | ||||
| Positive | 232 | 32 | 200 | |||||
| Venous involvement | ||||||||
| Negative | 110 | 36 | 74 | 0.0313 | ||||
| Positive | 209 | 45 | 164 | |||||
| Tumor size | ||||||||
| ≤6 | 148 | 57 | 91 | <0.0001 | ||||
| >6 | 171 | 24 | 147 | |||||
| CEA | ||||||||
| Negative | 240 | 69 | 171 | 0.017 | ||||
| Positive | 79 | 12 | 67 | |||||
| CRP | ||||||||
| Negative | 227 | 63 | 164 | 0.1557 | ||||
| Positive | 92 | 18 | 74 | |||||
| Plasma fibrinogen | ||||||||
| ≤310 | 166 | 56 | 110 | 0.0005 | ||||
| ≥311 | 153 | 25 | 128 | |||||
. | Odds ratio . | 95% CI . | . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Multivariate analysis variables | ||||||||
| Lymphatic involvement | 7.138 | 3.783 | 13.467 | <0.0001 | ||||
| Venous involvement | 0.921 | 0.49 | 1.729 | 0.797 | ||||
| Large tumor | 2.632 | 1.433 | 4.831 | 0.0018 | ||||
| CEA positive | 2.183 | 0.997 | 4.785 | 0.051 | ||||
| Hyperfibrinogenemia | 2.611 | 1.404 | 4.854 | 0.0024 | ||||
. | Odds ratio . | 95% CI . | . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Multivariate analysis variables | ||||||||
| Lymphatic involvement | 7.138 | 3.783 | 13.467 | <0.0001 | ||||
| Venous involvement | 0.921 | 0.49 | 1.729 | 0.797 | ||||
| Large tumor | 2.632 | 1.433 | 4.831 | 0.0018 | ||||
| CEA positive | 2.183 | 0.997 | 4.785 | 0.051 | ||||
| Hyperfibrinogenemia | 2.611 | 1.404 | 4.854 | 0.0024 | ||||
Gastric-type gastric adenocarcinoma often shows biologically different characteristics from intestinal-type gastric adenocarcinoma. Therefore, we evaluated the difference in significance of plasma hyperfibrinogenemia between these two adenocarcinoma types. Interestingly, the fibrinogen level in gastric-type adenocarcinoma did not show any relationship with nodal metastasis in either total cases or advanced stage cancer, while the association was more prominent in intestinal-type adenocarcinoma (Table 4). In the patients with intestinal-type gastric adenocarcinoma, lymph node metastasis was detected in 58.9% (96 of 163) of hyperfibrinogenemic patients, whereas the metastasis was detected in 26.5% (58 of 219) of non-hyperfibrinogenemic patients (P < 0.0001). In contrast, in the patients with gastric-type gastric adenocarcinoma, lymph node metastasis was detected in 51.4% (37 of 72) of hyperfibrinogenemic patients, whereas the metastasis was detected in 37.7% (66 of 175) of non-hyperfibrinogenemic patients (P = 0.0645, not significant). This suggests that gastric-type cancer may have somewhat different mechanisms of development of nodal metastasis.
Relationship between clinicopathological factors and lymph node metastasis in gastric type and intestinal type cancer
| Variables . | Lymph node metastases . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Gastric type . | . | P-value . | Intestinal type . | . | P-value . | ||||||
. | Negative . | Positive . | . | Negative . | Positive . | . | ||||||
| Number of cases | 144 | 103 | 228 | 154 | ||||||||
| Age (years) | ||||||||||||
| ≤59 | 103 | 68 | 0.4022 | 88 | 52 | 0.3867 | ||||||
| ≥60 | 41 | 35 | 140 | 102 | ||||||||
| Gender | ||||||||||||
| Male | 81 | 59 | 0.8970 | 198 | 117 | 0.0088 | ||||||
| Female | 63 | 44 | 30 | 37 | ||||||||
| Tumor location | ||||||||||||
| Upper | 20 | 27 | 0.0091 | 34 | 47 | 0.0008 | ||||||
| Middle | 88 | 44 | 112 | 56 | ||||||||
| Lower | 36 | 32 | 82 | 51 | ||||||||
| Lymphatic involvement | ||||||||||||
| Negative | 131 | 25 | <0.0001 | 196 | 27 | <0.0001 | ||||||
| Positive | 13 | 78 | 32 | 127 | ||||||||
| Venous involvement | ||||||||||||
| Negative | 119 | 46 | <0.0001 | 191 | 49 | <0.0001 | ||||||
| Positive | 25 | 57 | 37 | 105 | ||||||||
| Tumor stage | ||||||||||||
| Early | 113 | 16 | <0.0001 | 184 | 16 | <0.0001 | ||||||
| Advanced | 31 | 87 | 44 | 138 | ||||||||
| Tumor size | ||||||||||||
| <4 | 90 | 13 | <0.0001 | 165 | 43 | <0.0001 | ||||||
| ≥4 | 54 | 90 | 63 | 111 | ||||||||
| CEA | ||||||||||||
| Negative | 132 | 90 | 0.2909 | 187 | 100 | 0.0002 | ||||||
| Positive | 12 | 13 | 41 | 54 | ||||||||
| CRP | ||||||||||||
| Negative | 129 | 89 | 0.5482 | 192 | 96 | <0.0001 | ||||||
| Positive | 15 | 14 | 36 | 58 | ||||||||
| Plasma fibrinogen level (mg/dl) | ||||||||||||
| ≤310 | 109 | 66 | 0.0645 | 161 | 58 | <0.0001 | ||||||
| ≥311 | 35 | 37 | 67 | 96 | ||||||||
| Variables . | Lymph node metastases . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Gastric type . | . | P-value . | Intestinal type . | . | P-value . | ||||||
. | Negative . | Positive . | . | Negative . | Positive . | . | ||||||
| Number of cases | 144 | 103 | 228 | 154 | ||||||||
| Age (years) | ||||||||||||
| ≤59 | 103 | 68 | 0.4022 | 88 | 52 | 0.3867 | ||||||
| ≥60 | 41 | 35 | 140 | 102 | ||||||||
| Gender | ||||||||||||
| Male | 81 | 59 | 0.8970 | 198 | 117 | 0.0088 | ||||||
| Female | 63 | 44 | 30 | 37 | ||||||||
| Tumor location | ||||||||||||
| Upper | 20 | 27 | 0.0091 | 34 | 47 | 0.0008 | ||||||
| Middle | 88 | 44 | 112 | 56 | ||||||||
| Lower | 36 | 32 | 82 | 51 | ||||||||
| Lymphatic involvement | ||||||||||||
| Negative | 131 | 25 | <0.0001 | 196 | 27 | <0.0001 | ||||||
| Positive | 13 | 78 | 32 | 127 | ||||||||
| Venous involvement | ||||||||||||
| Negative | 119 | 46 | <0.0001 | 191 | 49 | <0.0001 | ||||||
| Positive | 25 | 57 | 37 | 105 | ||||||||
| Tumor stage | ||||||||||||
| Early | 113 | 16 | <0.0001 | 184 | 16 | <0.0001 | ||||||
| Advanced | 31 | 87 | 44 | 138 | ||||||||
| Tumor size | ||||||||||||
| <4 | 90 | 13 | <0.0001 | 165 | 43 | <0.0001 | ||||||
| ≥4 | 54 | 90 | 63 | 111 | ||||||||
| CEA | ||||||||||||
| Negative | 132 | 90 | 0.2909 | 187 | 100 | 0.0002 | ||||||
| Positive | 12 | 13 | 41 | 54 | ||||||||
| CRP | ||||||||||||
| Negative | 129 | 89 | 0.5482 | 192 | 96 | <0.0001 | ||||||
| Positive | 15 | 14 | 36 | 58 | ||||||||
| Plasma fibrinogen level (mg/dl) | ||||||||||||
| ≤310 | 109 | 66 | 0.0645 | 161 | 58 | <0.0001 | ||||||
| ≥311 | 35 | 37 | 67 | 96 | ||||||||
Relationship between clinicopathological factors and lymph node metastasis in gastric type and intestinal type cancer
| Variables . | Lymph node metastases . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Gastric type . | . | P-value . | Intestinal type . | . | P-value . | ||||||
. | Negative . | Positive . | . | Negative . | Positive . | . | ||||||
| Number of cases | 144 | 103 | 228 | 154 | ||||||||
| Age (years) | ||||||||||||
| ≤59 | 103 | 68 | 0.4022 | 88 | 52 | 0.3867 | ||||||
| ≥60 | 41 | 35 | 140 | 102 | ||||||||
| Gender | ||||||||||||
| Male | 81 | 59 | 0.8970 | 198 | 117 | 0.0088 | ||||||
| Female | 63 | 44 | 30 | 37 | ||||||||
| Tumor location | ||||||||||||
| Upper | 20 | 27 | 0.0091 | 34 | 47 | 0.0008 | ||||||
| Middle | 88 | 44 | 112 | 56 | ||||||||
| Lower | 36 | 32 | 82 | 51 | ||||||||
| Lymphatic involvement | ||||||||||||
| Negative | 131 | 25 | <0.0001 | 196 | 27 | <0.0001 | ||||||
| Positive | 13 | 78 | 32 | 127 | ||||||||
| Venous involvement | ||||||||||||
| Negative | 119 | 46 | <0.0001 | 191 | 49 | <0.0001 | ||||||
| Positive | 25 | 57 | 37 | 105 | ||||||||
| Tumor stage | ||||||||||||
| Early | 113 | 16 | <0.0001 | 184 | 16 | <0.0001 | ||||||
| Advanced | 31 | 87 | 44 | 138 | ||||||||
| Tumor size | ||||||||||||
| <4 | 90 | 13 | <0.0001 | 165 | 43 | <0.0001 | ||||||
| ≥4 | 54 | 90 | 63 | 111 | ||||||||
| CEA | ||||||||||||
| Negative | 132 | 90 | 0.2909 | 187 | 100 | 0.0002 | ||||||
| Positive | 12 | 13 | 41 | 54 | ||||||||
| CRP | ||||||||||||
| Negative | 129 | 89 | 0.5482 | 192 | 96 | <0.0001 | ||||||
| Positive | 15 | 14 | 36 | 58 | ||||||||
| Plasma fibrinogen level (mg/dl) | ||||||||||||
| ≤310 | 109 | 66 | 0.0645 | 161 | 58 | <0.0001 | ||||||
| ≥311 | 35 | 37 | 67 | 96 | ||||||||
| Variables . | Lymph node metastases . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Gastric type . | . | P-value . | Intestinal type . | . | P-value . | ||||||
. | Negative . | Positive . | . | Negative . | Positive . | . | ||||||
| Number of cases | 144 | 103 | 228 | 154 | ||||||||
| Age (years) | ||||||||||||
| ≤59 | 103 | 68 | 0.4022 | 88 | 52 | 0.3867 | ||||||
| ≥60 | 41 | 35 | 140 | 102 | ||||||||
| Gender | ||||||||||||
| Male | 81 | 59 | 0.8970 | 198 | 117 | 0.0088 | ||||||
| Female | 63 | 44 | 30 | 37 | ||||||||
| Tumor location | ||||||||||||
| Upper | 20 | 27 | 0.0091 | 34 | 47 | 0.0008 | ||||||
| Middle | 88 | 44 | 112 | 56 | ||||||||
| Lower | 36 | 32 | 82 | 51 | ||||||||
| Lymphatic involvement | ||||||||||||
| Negative | 131 | 25 | <0.0001 | 196 | 27 | <0.0001 | ||||||
| Positive | 13 | 78 | 32 | 127 | ||||||||
| Venous involvement | ||||||||||||
| Negative | 119 | 46 | <0.0001 | 191 | 49 | <0.0001 | ||||||
| Positive | 25 | 57 | 37 | 105 | ||||||||
| Tumor stage | ||||||||||||
| Early | 113 | 16 | <0.0001 | 184 | 16 | <0.0001 | ||||||
| Advanced | 31 | 87 | 44 | 138 | ||||||||
| Tumor size | ||||||||||||
| <4 | 90 | 13 | <0.0001 | 165 | 43 | <0.0001 | ||||||
| ≥4 | 54 | 90 | 63 | 111 | ||||||||
| CEA | ||||||||||||
| Negative | 132 | 90 | 0.2909 | 187 | 100 | 0.0002 | ||||||
| Positive | 12 | 13 | 41 | 54 | ||||||||
| CRP | ||||||||||||
| Negative | 129 | 89 | 0.5482 | 192 | 96 | <0.0001 | ||||||
| Positive | 15 | 14 | 36 | 58 | ||||||||
| Plasma fibrinogen level (mg/dl) | ||||||||||||
| ≤310 | 109 | 66 | 0.0645 | 161 | 58 | <0.0001 | ||||||
| ≥311 | 35 | 37 | 67 | 96 | ||||||||
DISCUSSION
In the present study, we found that the plasma fibrinogen level gradually increased with the extent of lymphatic metastasis, and hyperfibrinogenemia was identified as an independent factor associated with nodal metastasis in gastric cancer. This finding is in agreement with the generally accepted concept that the coagulation cascade is upregulated with the progression of malignant disease. A recent study by Lee et al. (16) presented a similar link between the fibrinogen level and tumor stage in gastric cancer. However, they did not find an independent association between hyperfibrinogenemia and nodal metastasis, suggesting the possibility that the elevated fibrinogen level was simply the result of increased tumor mass.
Fibrinogen is one of the major acute phase proteins produced by the liver, and is greatly enhanced in response to infection and other inflammatory disorders, although the mechanisms regulating its production in vivo remain unclear. Since advanced cancer is often associated with an inflammatory response, it may be possible that the high fibrinogen level in patients with lymphatic metastasis is a secondary event resulting from the increased systemic inflammatory response caused by tumor progression. In fact, other inflammatory proteins, such as IL-6 and CRP, are reported to be higher in cancer patients compared with non-cancer patients (21,22). In our patients, CRP also showed a positive association with nodal status in total patients with univariate analysis. However, CRP did not have an independent association with nodal metastasis in multivariate analysis. Moreover, fibrinogen, but not CRP level, showed a positive association with nodal metastasis in patients with advanced stage cancer. These findings strongly suggest that a high fibrinogen level, different from a high CRP level, is not a simple by-product of tumor progression, but may have some additional relevance for lymphatic metastasis.
Recent studies by Palumbo et al. (18) provide clear evidence that fibrinogen plays a crucial role in lymphatic as well as hematogenous metastasis of cancer cells. They showed that the number of metastases in regional lymph nodes and the lungs was markedly reduced when Lewis lung cell carcinoma was subcutaneously inoculated in fibrinogen (Aα-chain)-deficient mice (18). Their results suggest a possibility that fibrinogen might play a crucial role in the development of metastasis in human as well.
Fibrinogen is a dimeric molecule with multiple integrin or non-integrin binding motifs, and malignant cells often express high levels of fibrinogen receptors, such as α5β1 and αvβ3 integrins, or the ICAM-1 molecule. Therefore, high plasma fibrinogen can enhance the adhesive interaction between tumor cells and platelets or endothelial cells that may result in enhanced hematogenous metastasis. In fact, we detected a positive relationship between hyperfibrinogenemia and liver metastasis, although an independent association was not obtained in multivariate analysis, presumably because of the low number of cases. However, the mechanism of augmentation of lymphatic metastasis is not identical to that of hematogenous metastasis, because the lymphatic fluid does not contain platelets (23) and the lymphatic endothelium shows different molecular characteristics from vascular endothelium (24). Moreover, the concentration of fibrinogen in lymphatic fluid is reported to be much lower than that in plasma (25), although a recent report has shown that the fibrinogen content in rat gastrointestinal lymph is elevated after intraduodenal administration of triglycerides (26).
From those findings, a high plasma fibrinogen level does not appear to be directly involved in the process of lymphatic metastasis. However, hyperfibrinogenemia can modify the stromal constituents in the primary tumor, which may possibly lead to augmentation of lymphatic metastasis. Fibrin or fibrinogen was identified as one of the major components of the tumor stroma that envelops tumor cells (27,28). Tumor cells secrete a large amount of vascular endothelial growth factor, which causes hyperpermeability of the tumor vasculature (29,30). As a consequence, various plasma components, including fibrinogen, can accumulate in the tumor stroma, and fibrinogen can be converted to cross-linked fibrin by the coagulant/procoagulant activities generated by malignant cells (31) and/or by tumor-infiltrating macrophages (32). In fact, recent studies have confirmed that fibrin deposition in the stroma is mostly derived from plasma fibrinogen (33,34). Kerlin et al. (35) found enhanced fibrin deposition in certain organs of hyperfibrinogenemic transgenic mice, suggesting that high plasma fibrinogen can augment fibrin deposition in the tumor stroma, even in humans. Since fibrin is well known to facilitate tumor progression by various mechanisms (36), hyperfibrinogenemia can enhance fibrin deposition in the primary tumor, which might be a preferable circumstance for the metastasis through the lymphatic system.
Another interesting finding in this study was that the association between high plasma fibrinogen and lymphatic metastasis was only detected in intestinal-type gastric carcinoma, and this association was not detected in gastric-type adenocarcinoma. It has been reported that a positive association between tumor progression and expression of angiogenesis-related factors can be observed in intestinal-type gastric adenocarcinoma, but not in gastric-type gastric adenocarcinoma, especially in signet ring cell carcinoma (37). These results suggested that the mechanisms of development of nodal metastasis may be somewhat different between intestinal-type and gastric-type adenocarcinomas. The progression of gastric-type gastric adenocarcinoma may be less dependent on angiogenesis. The lack of significant association between high plasma fibrinogen level and metastasis in gastric-type adenocarcinoma raises a hypothesis that the plasma fibrinogen may be related with metastasis in angiogenic situation.
In summary, our data, together with the results of basic experiments in previous studies, support the idea that hyperfibrinogenemia may be associated with an increased risk of lymph node metastasis in patients with gastric cancer, except those with gastric-type histology. Preoperative plasma fibrinogen level may be a useful clinical marker to predict lymphatic metastasis in gastric cancer. Functional inhibition of fibrinogen may be a new therapeutic strategy to control metastasis.
References
Trousseau A. Phlegmasia alba dolens. In: Clinique Medicale de L'Hotel Dieu de Paris, 2nd edn. Paris: Balliere
Sun NC, McAfee WM, Hum GJ, Weiner JM. Hemostatic abnormalities in malignancy, a prospective study of one hundred eight patients. Part I. Coagulation studies.
Lopez Y, Paloma MJ, Rifon J, Cuesta B, Paramo JA. Measurement of prethrombotic markers in the assessment of acquired hypercoagulable states.
Ikeda M, Furukawa H, Imamura H, Shimizu J, Ishida H, Masutani S, et al. Poor prognosis associated with thrombocytosis in patients with gastric cancer.
Shimada H, Oohira G, Okazumi S, Matsubara H, Nabeya Y, Hayashi H, et al. Thrombocytosis associated with poor prognosis in patients with esophageal carcinoma.
Costantini V, Zacharski LR, Moritz TE, Edwards RL. The platelet count in carcinoma of the lung and colon.
Symbas NP, Townsend MF, El-Galley R, Keane TE, Graham SD, Petros JA. Poor prognosis associated with thrombocytosis in patients with renal cell carcinoma.
Hernandez E, Donohue KA, Anderson LL, Heller PB, Stehman FB. The significance of thrombocytosis in patients with locally advanced cervical carcinoma: a Gynecologic Oncology Group study.
Unsal E, Atalay F, Atikcan S, Yilmaz A. Prognostic significance of hemostatic parameters in patients with lung cancer.
Buccheri G, Torchio P, Ferrigno D. Plasma levels of D-dimer in lung carcinoma: clinical and prognostic significance.
Oya M, Akiyama Y, Okuyama T, Ishikawa H. High preoperative plasma D-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer.
Blackwell K, Hurwitz H, Lieberman G, Novotny W, Snyder S, Dewhirst M, et al. Circulating D-dimer levels are better predictors of overall survival and disease progression than carcinoembryonic antigen levels in patients with metastatic colorectal carcinoma.
Di Micco P, Romano M, Niglio A, Nozzolillo P, Federico A, Petronella P, et al. Alteration of haemostasis in non-metastatic gastric cancer.
Lee JH, Ryu KW, Kim S, Bae JM. Preoperative plasma fibrinogen levels in gastric cancer patients correlate with extent of tumor.
Palumbo JS, Kombrinck KW, Drew AF, Grimes TS, Kiser JH, Degen JL, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells.
Palumbo JS, Potter JM, Kaplan LS, Talmage K, Jackson DG, Degen JL. Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen-deficient mice.
Japanese Gastric Cancer A. Japanese Classification of Gastric Carcinoma. 2nd English Edition.
TNM classification of malignant tumors. In: Sobin LH, Wittekind CH, editors. International Union Against Cancer, 5th edn. New York: John Willey & Sons
Wu CW, Wang SR, Chao MF, Wu TC, Lui WY, P'Eng FK, et al. Serum interleukin-6 levels reflect disease status of gastric cancer.
Kabir S, Daar GA. Serum levels of interleukin-1, interleukin-6 and tumour necrosis factor-alpha in patients with gastric carcinoma.
Radochova D, Chrobak L, Bartos V, Brzek V, Strnad L. The human thoracic duct lymph. Quantitative and qualitative analysis of the cellular composition of the lymph.
Podgrabinska S, Braun P, Velasco P, Kloos B, Pepper MS, Skobe M. Molecular characterization of lymphatic endothelial cells.
Miller GJ, Howarth DJ, Attfield JC, Cooke CJ, Nanjee MN, Olszewski WL, et al. Haemostatic factors in human peripheral afferent lymph.
DeAnglis AP, Einhaus CM, Sombun AD, Ee LC, Retzinger GS. Fibrinogen in rat gastrointestinal lymph before, during and after intraduodenal administration of emulsified triglyceride: fibrinogen bound to chylomicrons in gastrointestinal lymph is functional.
Day ED, Planinsek JA, Pressman D. Localization of radioiodinated rat fibrinogen in transplanted rat tumors.
Brown LF, Van de Water L, Harvey VS, Dvorak HF. Fibrinogen influx and accumulation of cross-linked fibrin in healing wounds and in tumor stroma.
Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis.
Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy.
Rickles FR, Hancock WW, Edwards RL, Zacharski LR. Antimetastatic agents. I. Role of cellular procoagulants in the pathogenesis of fibrin deposition in cancer and the use of anticoagulants and/or antiplatelet drugs in cancer treatment.
Nagy JA, Brown LF, Senger DR, Lanir N, Van de Water L, Dvorak AM, et al. Pathogenesis of tumor stroma generation: a critical role for leaky blood vessels and fibrin deposition.
Bardos H, Juhasz A, Repassy G, Adany R. Fibrin deposition in squamous cell carcinomas of the larynx and hypopharynx.
Kerlin B, Cooley BC, Isermann BH, Hernandez I, Sood R, Zogg M, et al. Cause-effect relation between hyperfibrinogenemia and vascular disease.
Costantini V, Zacharski LR. The role of fibrin in tumor metastasis.
- fibrinogen
- blood coagulation disorders
- cancer
- adenocarcinoma
- gastric cancer
- intestines
- lymphatic metastasis
- lymphatic system
- neoplasm metastasis
- plasma
- preoperative care
- surgical procedures, operative
- carcinoembryonic antigen
- c-reactive protein
- fibrinogen assay
- surgery specialty
- lymph node metastasis
- cancer, advanced
- tumor cells, malignant
- univariate analysis