-
PDF
- Split View
-
Views
-
Cite
Cite
Bernard Fisher, Joseph P. Costantino, D. Lawrence Wickerham, Reena S. Cecchini, Walter M. Cronin, Andre Robidoux, Therese B. Bevers, Maureen T. Kavanah, James N. Atkins, Richard G. Margolese, Carolyn D. Runowicz, Joan M. James, Leslie G. Ford, Norman Wolmark, Tamoxifen for the Prevention of Breast Cancer: Current Status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study, JNCI: Journal of the National Cancer Institute, Volume 97, Issue 22, 16 November 2005, Pages 1652–1662, https://doi.org/10.1093/jnci/dji372
Close - Share Icon Share
Abstract
Background: Initial findings from the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial (P-1) demonstrated that tamoxifen reduced the risk of estrogen receptor–positive tumors and osteoporotic fractures in women at increased risk for breast cancer. Side effects of varying clinical significance were observed. The trial was unblinded because of the positive results, and follow-up continued. This report updates our initial findings. Methods: Women (n = 13 388) were randomly assigned to receive placebo or tamoxifen for 5 years. Rates of breast cancer and other events were compared by the use of risk ratios (RRs) and 95% confidence intervals (CIs). Estimates of the net benefit from 5 years of tamoxifen therapy were compared by age, race, and categories of predicted breast cancer risk. Statistical tests were two-sided. Results: After 7 years of follow-up, the cumulative rate of invasive breast cancer was reduced from 42.5 per 1000 women in the placebo group to 24.8 per 1000 women in the tamoxifen group (RR = 0.57, 95% CI = 0.46 to 0.70) and the cumulative rate of noninvasive breast cancer was reduced from 15.8 per 1000 women in the placebo group to 10.2 per 1000 women in the tamoxifen group (RR = 0.63, 95% CI = 0.45 to 0.89). These reductions were similar to those seen in the initial report. Tamoxifen led to a 32% reduction in osteoporotic fractures (RR = 0.68, 95% CI = 0.51 to 0.92). Relative risks of stroke, deep-vein thrombosis, and cataracts (which increased with tamoxifen) and of ischemic heart disease and death (which were not changed with tamoxifen) were also similar to those initially reported. Risks of pulmonary embolism were approximately 11% lower than in the original report, and risks of endometrial cancer were about 29% higher, but these differences were not statistically significant. The net benefit achieved with tamoxifen varied according to age, race, and level of breast cancer risk. Conclusions: Despite the potential bias caused by the unblinding of the P-1 trial, the magnitudes of all beneficial and undesirable treatment effects of tamoxifen were similar to those initially reported, with notable reductions in breast cancer and increased risks of thromboembolic events and endometrial cancer. Readily identifiable subsets of individuals comprising 2.5 million women could derive a net benefit from the drug.
When the National Surgical Adjuvant Breast and Bowel Project (NSABP) Breast Cancer Prevention Trial (P-1) was begun in 1992, two principal clinical strategies were being used to improve breast cancer management. The first approach was aimed at devising progressively better regimens for the systemic treatment of detectable disease. The second was directed toward improving methods of detection so as to identify breast cancer at an earlier stage of development. There was, however, a need to implement a plan that would evaluate the worth of modalities that were presumed to be capable of interfering with the initiation and/or promotion of clinically nondetectable tumors. That approach has been referred to as prevention.
An extensive body of literature describing the pharmacokinetics, metabolism, antitumor effects, and toxicity of the antiestrogen tamoxifen in experimental animals and in humans was obtained during the 1970s and 1980s ( 1 – 4 ) . Other studies showed that tamoxifen-treated women had a statistically significantly lower incidence of contralateral breast cancer than did women who received placebo ( 5 – 9 ) . That information was judged to be sufficient to justify the formulation of a testable hypothesis that proposed that the administration of tamoxifen as a preventive agent to women at high risk for breast cancer would reduce their risk. The P-1 trial became the mechanism for testing the credibility of that thesis. Initial findings from the P-1 study ( 10 ) demonstrated that tamoxifen administration resulted in a statistically significant reduction in the risk of noninvasive and estrogen receptor (ER)–positive invasive breast cancer. This risk reduction occurred in women regardless of age; of history of noninvasive breast disease, including both lobular carcinoma in situ (LCIS) and atypical hyperplasia; and of other breast cancer risk factors. Although tamoxifen also reduced the risk of osteoporotic fractures, it increased the risk of endometrial cancer, thromboembolism, and other undesirable side effects but had no effect on the rate of heart disease. This article provides an update of the findings from the P-1 trial after 7 years of follow-up, with an average of 74 months of follow-up.
P ATIENTS AND M ETHODS
Accrual, Drug Administration, and Follow-up
From April 22, 1992, through May 20, 1997, risk assessments were performed for 98 018 women ( 10 ) . On the basis of their risk, 57 641 (58.8%) of these women were deemed eligible for participation in the trial, and 14 453 agreed to be medically evaluated to further determine their eligibility; a total of 13 954 participants met all eligibility requirements ( 10 ) . Between June 1, 1992, and September 30, 1997, 13 388 of those women were randomly assigned to receive either placebo (6707 participants) or tamoxifen at 20 mg/day (6681 participants) for 5 years. After the trial results were announced in 1998, all women and their physicians were informed as to whether they had received tamoxifen or placebo. Women in the tamoxifen group who wished to do so continued to receive that drug for a total of 5 years. Participants in the placebo group were given the opportunity either to receive a 5-year course of tamoxifen or to be randomized to the Study of Tamoxifen and Raloxifene (STAR) trial. Almost 32% of the women in the placebo group accepted one of those alternatives. Other women in the placebo group received tamoxifen or raloxifene by prescription, although the precise number of women who did so is unknown.
The original protocol for the P-1 study included a plan for follow-up through 7 years after randomization. After the trial was unblinded, the protocol was amended to continue follow-up, beyond 7 years, of only those women who had been randomly assigned to the tamoxifen group. Written informed consent was obtained for each participant according to federal and institutional guidelines; the consent allowed both for the collection of data about breast and other cancers and for obtaining information about the side effects that had been found to be associated with tamoxifen administration.
Participant Eligibility and Breast Cancer Risk Assessment
To be eligible for the trial, women had to have signed a consent form that had been witnessed and dated before randomization; had to be either 60 years of age or older or between 35 and 59 years of age with a 5-year predicted risk for breast cancer of at least 1.66% (as determined by the algorithm described below); or had to have a history of LCIS or atypical hyperplasia. Within 180 days before randomization, women had to have undergone a mammogram that was determined to be negative for breast cancer. Also, women were not eligible for participation in the study if they had taken estrogen or progesterone replacement therapy, oral contraceptives, or androgens within 3 months of randomization. Participants were also prohibited from taking those drugs while they were enrolled in the study. Women who had a history of deep-vein thrombosis or pulmonary embolism were ineligible. The algorithm used to determine the predicted risk of breast cancer was based on a modified version of the multivariable logistic regression model developed by Costantino et al. ( 11 ) . The variables included in the model were a woman's age, race, number of first-degree relatives with breast cancer, nulliparity or age at first live birth, number of benign breast biopsies, pathologic diagnosis of atypical hyperplasia, and age at menarche.
Statistical Methods
Randomization of participants was performed centrally in a double-blind fashion by the NSABP Biostatistical Center. Participants were stratified by age (35–49 years, 50–59 years, or ≥60 years), race (African American, white, or other), history of LCIS (yes or no), and 5-year predicted breast cancer risk (<2.5%, 2.5%–3.9%, or ≥4.0%). An adaptive randomization scheme using the biased coin method was used to avoid imbalances in group assignment within a clinical center ( 12 ) . The design of the study included formal interim monitoring for early stopping based on the primary end point of the trial, i.e., the incidence of invasive breast cancer ( 13 ) . To facilitate the monitoring of multiple potential beneficial and detrimental outcomes, a form of monitoring that used a global index modeled after the one proposed for the Women's Health Initiative trial was used ( 14 ) .
The data included in this article are based on information received and processed by the NSABP Biostatistical Center as of March 31, 2005. Because follow-up data were not collected for participants in the placebo group after 7 years, all analyses, which were based on the assignment of women at the time of their randomization, have been censored at 7 years. All randomly assigned participants who were at risk and for whom follow-up data were obtained were included in the analyses. Incidence rates for the study end points were calculated for each group by dividing the number of observed events by the number of observed event-specific person-years of follow-up. Two-sided P values for tests of differences between the groups for the rates of invasive breast cancer, noninvasive breast cancer, and invasive endometrial cancer were determined by use of the exact method ( 15 ). Event rates in the two groups were also compared by use of the risk ratios (RRs) and 95% confidence intervals (CIs). Confidence intervals were determined assuming that the events followed a Poisson distribution, conditioning on the total number of events and person-years at risk. Cumulative incidence rates by follow-up time were determined accounting for competing risk due to death ( 16 ) . Differences between cumulative incidence curves were assessed by the method of Pepe and Mori ( 17 ) .
R ESULTS
Participant Characteristics
Table 1 shows, by duration of follow-up time, the distributions of women in the placebo and tamoxifen groups who were included in the analyses. Of the 13 388 women who were randomly assigned, 13 207 were included in these analyses. One participant was discovered to have had a prior diagnosis of breast cancer, and follow-up information was not available for 180 of the women. In the initial report of the P-1 results ( 10 ) , follow-up was not available for 212 women, but it has since been obtained for 32 of those women. The number of person-years of follow-up included in this analysis was 40 648 for the placebo group and 40 844 for the tamoxifen group. These numbers of person-years are 56% greater than those included in the original publication for both treatment groups. Since the study was unblinded in March 1998, many women have decided to discontinue their participation. Because the withdrawal rate between the sixth and seventh years of follow-up was higher in the placebo group than in the tamoxifen group, the amount of information available for the two groups for this period was substantially different. In particular, 552 more women in the tamoxifen group than the placebo group had follow-up through 7 years (4379 and 4931, respectively).
Women included in the analyses and number followed up through 5, 6, and 7 years
| Accrual and follow-up status . | Placebo . | Tamoxifen . | Total . |
|---|---|---|---|
| Accrual | |||
| Women randomly assigned | 6707 | 6681 | 13 388 |
| Not at risk * | 0 | 1 | 1 |
| Without follow-up | 97 | 83 | 180 |
| Included in analysis | 6610 | 6597 | 13 207 |
| Follow-up time (y) | |||
| ≥5 | 5550 | 5602 | 11 152 |
| ≥6 | 5285 | 5372 | 10 657 |
| ≥7 | 4379 | 4931 | 9310 |
| Average follow-up time (mo) | 73.8 | 74.3 | 74.0 |
| Total person-years of follow-up included in this analysis † | 40 648 | 40 844 | 81 492 |
| Accrual and follow-up status . | Placebo . | Tamoxifen . | Total . |
|---|---|---|---|
| Accrual | |||
| Women randomly assigned | 6707 | 6681 | 13 388 |
| Not at risk * | 0 | 1 | 1 |
| Without follow-up | 97 | 83 | 180 |
| Included in analysis | 6610 | 6597 | 13 207 |
| Follow-up time (y) | |||
| ≥5 | 5550 | 5602 | 11 152 |
| ≥6 | 5285 | 5372 | 10 657 |
| ≥7 | 4379 | 4931 | 9310 |
| Average follow-up time (mo) | 73.8 | 74.3 | 74.0 |
| Total person-years of follow-up included in this analysis † | 40 648 | 40 844 | 81 492 |
History of invasive breast cancer prior to randomization.
Follow-up was censored at 7 years (see text for details).
Women included in the analyses and number followed up through 5, 6, and 7 years
| Accrual and follow-up status . | Placebo . | Tamoxifen . | Total . |
|---|---|---|---|
| Accrual | |||
| Women randomly assigned | 6707 | 6681 | 13 388 |
| Not at risk * | 0 | 1 | 1 |
| Without follow-up | 97 | 83 | 180 |
| Included in analysis | 6610 | 6597 | 13 207 |
| Follow-up time (y) | |||
| ≥5 | 5550 | 5602 | 11 152 |
| ≥6 | 5285 | 5372 | 10 657 |
| ≥7 | 4379 | 4931 | 9310 |
| Average follow-up time (mo) | 73.8 | 74.3 | 74.0 |
| Total person-years of follow-up included in this analysis † | 40 648 | 40 844 | 81 492 |
| Accrual and follow-up status . | Placebo . | Tamoxifen . | Total . |
|---|---|---|---|
| Accrual | |||
| Women randomly assigned | 6707 | 6681 | 13 388 |
| Not at risk * | 0 | 1 | 1 |
| Without follow-up | 97 | 83 | 180 |
| Included in analysis | 6610 | 6597 | 13 207 |
| Follow-up time (y) | |||
| ≥5 | 5550 | 5602 | 11 152 |
| ≥6 | 5285 | 5372 | 10 657 |
| ≥7 | 4379 | 4931 | 9310 |
| Average follow-up time (mo) | 73.8 | 74.3 | 74.0 |
| Total person-years of follow-up included in this analysis † | 40 648 | 40 844 | 81 492 |
History of invasive breast cancer prior to randomization.
Follow-up was censored at 7 years (see text for details).
The distributions of the 13 207 participants included in the analyses by participant characteristics are shown in Table 2 . Approximately 39% of the women were aged 35–49 years at the time of random assignment, 31% were aged 50–59 years, and 30% were aged 60 years or older. Approximately 3% of the participants were aged 35–39 years, and 6% were aged 70 years or older. Almost all of the participants were white (96%), more than one-third (37%) had had a hysterectomy prior to randomization, 6% had a history of LCIS, and 9% had a history of atypical hyperplasia. More than 75% of the participants had at least one first-degree relative with breast cancer; more than half (57%) of the participants had one such relative, 16% had two, and 3% had three or more. Approximately one-quarter of the women had a 5-year predicted breast cancer risk of 2.00% or less, almost 58% had a 5-year risk of between 2.01% and 5.00%, and 17% had a 5-year risk of more than 5.00%.
Participant characteristics at time of randomization for women included in the analyses *
| . | Placebo . | . | Tamoxifen . | . | ||
|---|---|---|---|---|---|---|
| Characteristic . | No. . | % . | No. . | % . | ||
| Age (y) | ||||||
| 35–39 | 186 | 2.8 | 160 | 2.4 | ||
| 40–49 | 2414 | 36.5 | 2429 | 36.8 | ||
| 50–59 | 2022 | 30.6 | 2037 | 30.9 | ||
| 60–69 | 1592 | 24.1 | 1577 | 23.9 | ||
| ≥70 | 396 | 6.0 | 394 | 6.0 | ||
| Race | ||||||
| White | 6368 | 96.3 | 6366 | 96.5 | ||
| African American | 112 | 1.7 | 111 | 1.7 | ||
| Other | 130 | 2.0 | 120 | 1.8 | ||
| No. of first-degree relatives with breast cancer | ||||||
| 0 | 1597 | 24.2 | 1548 | 23.5 | ||
| 1 | 3738 | 56.6 | 3763 | 57.0 | ||
| 2 | 1094 | 16.6 | 1072 | 16.2 | ||
| ≥3 | 181 | 2.7 | 214 | 3.2 | ||
| Prior hysterectomy | ||||||
| No | 4200 | 63.5 | 4111 | 62.3 | ||
| Yes | 2410 | 36.5 | 2486 | 37.7 | ||
| History of LCIS | ||||||
| No | 6197 | 93.8 | 6181 | 93.7 | ||
| Yes | 413 | 6.2 | 416 | 6.3 | ||
| History of AH | ||||||
| No | 5995 | 90.7 | 6016 | 91.2 | ||
| Yes | 615 | 9.3 | 581 | 8.8 | ||
| 5-y predicted breast cancer risk (%) † | ||||||
| ≤2.00 | 1661 | 25.1 | 1643 | 24.9 | ||
| 2.01–3.00 | 2035 | 30.8 | 2064 | 31.3 | ||
| 3.01–5.00 | 1794 | 27.1 | 1718 | 26.0 | ||
| ≥5.01 | 1120 | 16.9 | 1172 | 17.8 | ||
| Total | 6610 | 100.0 | 6597 | 100.0 | ||
| . | Placebo . | . | Tamoxifen . | . | ||
|---|---|---|---|---|---|---|
| Characteristic . | No. . | % . | No. . | % . | ||
| Age (y) | ||||||
| 35–39 | 186 | 2.8 | 160 | 2.4 | ||
| 40–49 | 2414 | 36.5 | 2429 | 36.8 | ||
| 50–59 | 2022 | 30.6 | 2037 | 30.9 | ||
| 60–69 | 1592 | 24.1 | 1577 | 23.9 | ||
| ≥70 | 396 | 6.0 | 394 | 6.0 | ||
| Race | ||||||
| White | 6368 | 96.3 | 6366 | 96.5 | ||
| African American | 112 | 1.7 | 111 | 1.7 | ||
| Other | 130 | 2.0 | 120 | 1.8 | ||
| No. of first-degree relatives with breast cancer | ||||||
| 0 | 1597 | 24.2 | 1548 | 23.5 | ||
| 1 | 3738 | 56.6 | 3763 | 57.0 | ||
| 2 | 1094 | 16.6 | 1072 | 16.2 | ||
| ≥3 | 181 | 2.7 | 214 | 3.2 | ||
| Prior hysterectomy | ||||||
| No | 4200 | 63.5 | 4111 | 62.3 | ||
| Yes | 2410 | 36.5 | 2486 | 37.7 | ||
| History of LCIS | ||||||
| No | 6197 | 93.8 | 6181 | 93.7 | ||
| Yes | 413 | 6.2 | 416 | 6.3 | ||
| History of AH | ||||||
| No | 5995 | 90.7 | 6016 | 91.2 | ||
| Yes | 615 | 9.3 | 581 | 8.8 | ||
| 5-y predicted breast cancer risk (%) † | ||||||
| ≤2.00 | 1661 | 25.1 | 1643 | 24.9 | ||
| 2.01–3.00 | 2035 | 30.8 | 2064 | 31.3 | ||
| 3.01–5.00 | 1794 | 27.1 | 1718 | 26.0 | ||
| ≥5.01 | 1120 | 16.9 | 1172 | 17.8 | ||
| Total | 6610 | 100.0 | 6597 | 100.0 | ||
LCIS = lobular carcinoma in situ; AH = atypical hyperplasia.
Determined with the Gail model ( 11 ) .
Participant characteristics at time of randomization for women included in the analyses *
| . | Placebo . | . | Tamoxifen . | . | ||
|---|---|---|---|---|---|---|
| Characteristic . | No. . | % . | No. . | % . | ||
| Age (y) | ||||||
| 35–39 | 186 | 2.8 | 160 | 2.4 | ||
| 40–49 | 2414 | 36.5 | 2429 | 36.8 | ||
| 50–59 | 2022 | 30.6 | 2037 | 30.9 | ||
| 60–69 | 1592 | 24.1 | 1577 | 23.9 | ||
| ≥70 | 396 | 6.0 | 394 | 6.0 | ||
| Race | ||||||
| White | 6368 | 96.3 | 6366 | 96.5 | ||
| African American | 112 | 1.7 | 111 | 1.7 | ||
| Other | 130 | 2.0 | 120 | 1.8 | ||
| No. of first-degree relatives with breast cancer | ||||||
| 0 | 1597 | 24.2 | 1548 | 23.5 | ||
| 1 | 3738 | 56.6 | 3763 | 57.0 | ||
| 2 | 1094 | 16.6 | 1072 | 16.2 | ||
| ≥3 | 181 | 2.7 | 214 | 3.2 | ||
| Prior hysterectomy | ||||||
| No | 4200 | 63.5 | 4111 | 62.3 | ||
| Yes | 2410 | 36.5 | 2486 | 37.7 | ||
| History of LCIS | ||||||
| No | 6197 | 93.8 | 6181 | 93.7 | ||
| Yes | 413 | 6.2 | 416 | 6.3 | ||
| History of AH | ||||||
| No | 5995 | 90.7 | 6016 | 91.2 | ||
| Yes | 615 | 9.3 | 581 | 8.8 | ||
| 5-y predicted breast cancer risk (%) † | ||||||
| ≤2.00 | 1661 | 25.1 | 1643 | 24.9 | ||
| 2.01–3.00 | 2035 | 30.8 | 2064 | 31.3 | ||
| 3.01–5.00 | 1794 | 27.1 | 1718 | 26.0 | ||
| ≥5.01 | 1120 | 16.9 | 1172 | 17.8 | ||
| Total | 6610 | 100.0 | 6597 | 100.0 | ||
| . | Placebo . | . | Tamoxifen . | . | ||
|---|---|---|---|---|---|---|
| Characteristic . | No. . | % . | No. . | % . | ||
| Age (y) | ||||||
| 35–39 | 186 | 2.8 | 160 | 2.4 | ||
| 40–49 | 2414 | 36.5 | 2429 | 36.8 | ||
| 50–59 | 2022 | 30.6 | 2037 | 30.9 | ||
| 60–69 | 1592 | 24.1 | 1577 | 23.9 | ||
| ≥70 | 396 | 6.0 | 394 | 6.0 | ||
| Race | ||||||
| White | 6368 | 96.3 | 6366 | 96.5 | ||
| African American | 112 | 1.7 | 111 | 1.7 | ||
| Other | 130 | 2.0 | 120 | 1.8 | ||
| No. of first-degree relatives with breast cancer | ||||||
| 0 | 1597 | 24.2 | 1548 | 23.5 | ||
| 1 | 3738 | 56.6 | 3763 | 57.0 | ||
| 2 | 1094 | 16.6 | 1072 | 16.2 | ||
| ≥3 | 181 | 2.7 | 214 | 3.2 | ||
| Prior hysterectomy | ||||||
| No | 4200 | 63.5 | 4111 | 62.3 | ||
| Yes | 2410 | 36.5 | 2486 | 37.7 | ||
| History of LCIS | ||||||
| No | 6197 | 93.8 | 6181 | 93.7 | ||
| Yes | 413 | 6.2 | 416 | 6.3 | ||
| History of AH | ||||||
| No | 5995 | 90.7 | 6016 | 91.2 | ||
| Yes | 615 | 9.3 | 581 | 8.8 | ||
| 5-y predicted breast cancer risk (%) † | ||||||
| ≤2.00 | 1661 | 25.1 | 1643 | 24.9 | ||
| 2.01–3.00 | 2035 | 30.8 | 2064 | 31.3 | ||
| 3.01–5.00 | 1794 | 27.1 | 1718 | 26.0 | ||
| ≥5.01 | 1120 | 16.9 | 1172 | 17.8 | ||
| Total | 6610 | 100.0 | 6597 | 100.0 | ||
LCIS = lobular carcinoma in situ; AH = atypical hyperplasia.
Determined with the Gail model ( 11 ) .
Comparison of the Initial and Updated Results
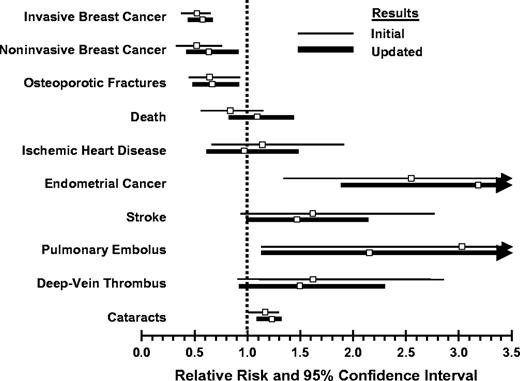
The updated estimates of the risk ratios and 95% confidence intervals for all of the beneficial and undesirable outcomes of tamoxifen administration are similar to those that were reported initially ( 10 ) ( Fig. 1 ). Both datasets showed similar decreases in invasive and noninvasive breast cancer and in osteoporotic fractures. The datasets also showed similar increases in endometrial cancer, thromboembolic events, and cataracts associated with tamoxifen administration.
Comparison of relative risks (with 95% confidence intervals) of benefits and undesirable effects of tamoxifen from the initial and updated results of NSABP P-1.
Reduction in Invasive and Noninvasive Breast Cancer Events
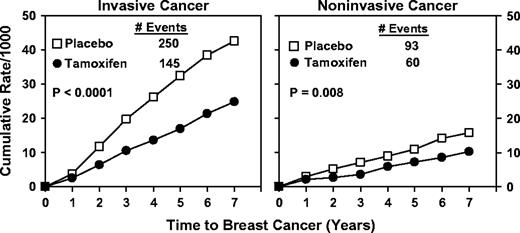
Through 7 years of follow-up, the cumulative rate of invasive breast cancer was reduced from 42.5 per 1000 women in the placebo group to 24.8 per 1000 women in the tamoxifen group ( P <.001; Fig. 2 ). The risk ratio was 0.57 (95% CI = 0.46 to 0.70), and the incidence rate of invasive breast cancer was 0.27% less in the tamoxifen group than in the placebo group ( Table 3 ). The cumulative rate of noninvasive breast cancer (ductal carcinoma in situ [DCIS] and LCIS) was reduced from 15.8 per 1000 women in the placebo group to 10.2 per 1000 women in the tamoxifen group ( P = .008; Fig. 2 ). The risk ratio was 0.63 (95% CI = 0.45 to 0.89), and the incidence rate of noninvasive breast cancer was 0.09% less in the placebo group than in the tamoxifen group (data not shown). Tamoxifen reduced the risk of invasive breast cancer in women in all subgroups defined by age, history of LCIS, history of atypical hyperplasia, or level of predicted risk of breast cancer ( Table 3 ). Although there were some variations in the point estimates of the risk ratio across the various categories of these subgroups, there were no statistically significant differences in the magnitudes of effect across categories.
Cumulative rates per 1000 women of invasive and noninvasive breast cancers in NSABP P-1 participants by treatment group.
Events and incidence rates of invasive breast cancer in the placebo and tamoxifen groups by selected participant characteristics *
| . | No. of events . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference † . | RR ‡ . | 95% CI . | |||
| All women | 250 | 145 | 6.29 | 3.59 | 2.70 | 0.57 | 0.46 to 0.70 | |||
| Age at Entry (y) | ||||||||||
| ≤49 | 98 | 63 | 6.32 | 4.04 | 2.28 | 0.64 | 0.46 to 0.89 | |||
| 50–59 | 72 | 42 | 5.87 | 3.33 | 2.54 | 0.57 | 0.38 to 0.84 | |||
| ≥60 | 80 | 40 | 6.68 | 3.30 | 3.38 | 0.49 | 0.33 to 0.73 | |||
| History of LCIS | ||||||||||
| No | 221 | 129 | 5.93 | 3.41 | 2.52 | 0.58 | 0.46 to 0.72 | |||
| Yes | 29 | 16 | 11.70 | 6.27 | 5.43 | 0.54 | 0.27 to 1.02 | |||
| History of AH | ||||||||||
| No | 212 | 136 | 5.87 | 3.69 | 2.18 | 0.63 | 0.50 to 0.78 | |||
| Yes | 38 | 9 | 10.42 | 2.55 | 7.87 | 0.25 | 0.10 to 0.52 | |||
| 5-y predicted breast cancer risk (%) § | ||||||||||
| ≤2.00 | 58 | 40 | 4.77 | 3.18 | 1.59 | 0.67 | 0.43 to 1.01 | |||
| 2.01–3.00 | 67 | 41 | 6.13 | 3.88 | 2.25 | 0.63 | 0.42 to 0.95 | |||
| 3.01–5.00 | 45 | 27 | 4.51 | 2.70 | 1.81 | 0.60 | 0.36 to 0.99 | |||
| ≥5.01 | 80 | 37 | 11.98 | 5.15 | 6.83 | 0.43 | 0.28 to 0.64 | |||
| No. of first-degree relatives with breast cancer | ||||||||||
| 0 | 62 | 33 | 6.47 | 3.48 | 2.99 | 0.54 | 0.34 to 0.83 | |||
| 1 | 124 | 73 | 5.52 | 3.16 | 2.36 | 0.57 | 0.42 to 0.77 | |||
| 2 | 52 | 32 | 7.84 | 4.91 | 2.93 | 0.63 | 0.39 to 0.99 | |||
| ≥3 | 12 | 7 | 11.24 | 5.48 | 5.76 | 0.49 | 0.16 to 1.34 | |||
| . | No. of events . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference † . | RR ‡ . | 95% CI . | |||
| All women | 250 | 145 | 6.29 | 3.59 | 2.70 | 0.57 | 0.46 to 0.70 | |||
| Age at Entry (y) | ||||||||||
| ≤49 | 98 | 63 | 6.32 | 4.04 | 2.28 | 0.64 | 0.46 to 0.89 | |||
| 50–59 | 72 | 42 | 5.87 | 3.33 | 2.54 | 0.57 | 0.38 to 0.84 | |||
| ≥60 | 80 | 40 | 6.68 | 3.30 | 3.38 | 0.49 | 0.33 to 0.73 | |||
| History of LCIS | ||||||||||
| No | 221 | 129 | 5.93 | 3.41 | 2.52 | 0.58 | 0.46 to 0.72 | |||
| Yes | 29 | 16 | 11.70 | 6.27 | 5.43 | 0.54 | 0.27 to 1.02 | |||
| History of AH | ||||||||||
| No | 212 | 136 | 5.87 | 3.69 | 2.18 | 0.63 | 0.50 to 0.78 | |||
| Yes | 38 | 9 | 10.42 | 2.55 | 7.87 | 0.25 | 0.10 to 0.52 | |||
| 5-y predicted breast cancer risk (%) § | ||||||||||
| ≤2.00 | 58 | 40 | 4.77 | 3.18 | 1.59 | 0.67 | 0.43 to 1.01 | |||
| 2.01–3.00 | 67 | 41 | 6.13 | 3.88 | 2.25 | 0.63 | 0.42 to 0.95 | |||
| 3.01–5.00 | 45 | 27 | 4.51 | 2.70 | 1.81 | 0.60 | 0.36 to 0.99 | |||
| ≥5.01 | 80 | 37 | 11.98 | 5.15 | 6.83 | 0.43 | 0.28 to 0.64 | |||
| No. of first-degree relatives with breast cancer | ||||||||||
| 0 | 62 | 33 | 6.47 | 3.48 | 2.99 | 0.54 | 0.34 to 0.83 | |||
| 1 | 124 | 73 | 5.52 | 3.16 | 2.36 | 0.57 | 0.42 to 0.77 | |||
| 2 | 52 | 32 | 7.84 | 4.91 | 2.93 | 0.63 | 0.39 to 0.99 | |||
| ≥3 | 12 | 7 | 11.24 | 5.48 | 5.76 | 0.49 | 0.16 to 1.34 | |||
LCIS = lobular carcinoma in situ; AH = atypical hyperplasia; RR = risk ratio; CI = confidence interval.
Rate in the placebo group minus rate in the tamoxifen group.
Risk ratio for women in the tamoxifen group relative to women in the placebo group.
Determined with the Gail model ( 11 ) .
Events and incidence rates of invasive breast cancer in the placebo and tamoxifen groups by selected participant characteristics *
| . | No. of events . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference † . | RR ‡ . | 95% CI . | |||
| All women | 250 | 145 | 6.29 | 3.59 | 2.70 | 0.57 | 0.46 to 0.70 | |||
| Age at Entry (y) | ||||||||||
| ≤49 | 98 | 63 | 6.32 | 4.04 | 2.28 | 0.64 | 0.46 to 0.89 | |||
| 50–59 | 72 | 42 | 5.87 | 3.33 | 2.54 | 0.57 | 0.38 to 0.84 | |||
| ≥60 | 80 | 40 | 6.68 | 3.30 | 3.38 | 0.49 | 0.33 to 0.73 | |||
| History of LCIS | ||||||||||
| No | 221 | 129 | 5.93 | 3.41 | 2.52 | 0.58 | 0.46 to 0.72 | |||
| Yes | 29 | 16 | 11.70 | 6.27 | 5.43 | 0.54 | 0.27 to 1.02 | |||
| History of AH | ||||||||||
| No | 212 | 136 | 5.87 | 3.69 | 2.18 | 0.63 | 0.50 to 0.78 | |||
| Yes | 38 | 9 | 10.42 | 2.55 | 7.87 | 0.25 | 0.10 to 0.52 | |||
| 5-y predicted breast cancer risk (%) § | ||||||||||
| ≤2.00 | 58 | 40 | 4.77 | 3.18 | 1.59 | 0.67 | 0.43 to 1.01 | |||
| 2.01–3.00 | 67 | 41 | 6.13 | 3.88 | 2.25 | 0.63 | 0.42 to 0.95 | |||
| 3.01–5.00 | 45 | 27 | 4.51 | 2.70 | 1.81 | 0.60 | 0.36 to 0.99 | |||
| ≥5.01 | 80 | 37 | 11.98 | 5.15 | 6.83 | 0.43 | 0.28 to 0.64 | |||
| No. of first-degree relatives with breast cancer | ||||||||||
| 0 | 62 | 33 | 6.47 | 3.48 | 2.99 | 0.54 | 0.34 to 0.83 | |||
| 1 | 124 | 73 | 5.52 | 3.16 | 2.36 | 0.57 | 0.42 to 0.77 | |||
| 2 | 52 | 32 | 7.84 | 4.91 | 2.93 | 0.63 | 0.39 to 0.99 | |||
| ≥3 | 12 | 7 | 11.24 | 5.48 | 5.76 | 0.49 | 0.16 to 1.34 | |||
| . | No. of events . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference † . | RR ‡ . | 95% CI . | |||
| All women | 250 | 145 | 6.29 | 3.59 | 2.70 | 0.57 | 0.46 to 0.70 | |||
| Age at Entry (y) | ||||||||||
| ≤49 | 98 | 63 | 6.32 | 4.04 | 2.28 | 0.64 | 0.46 to 0.89 | |||
| 50–59 | 72 | 42 | 5.87 | 3.33 | 2.54 | 0.57 | 0.38 to 0.84 | |||
| ≥60 | 80 | 40 | 6.68 | 3.30 | 3.38 | 0.49 | 0.33 to 0.73 | |||
| History of LCIS | ||||||||||
| No | 221 | 129 | 5.93 | 3.41 | 2.52 | 0.58 | 0.46 to 0.72 | |||
| Yes | 29 | 16 | 11.70 | 6.27 | 5.43 | 0.54 | 0.27 to 1.02 | |||
| History of AH | ||||||||||
| No | 212 | 136 | 5.87 | 3.69 | 2.18 | 0.63 | 0.50 to 0.78 | |||
| Yes | 38 | 9 | 10.42 | 2.55 | 7.87 | 0.25 | 0.10 to 0.52 | |||
| 5-y predicted breast cancer risk (%) § | ||||||||||
| ≤2.00 | 58 | 40 | 4.77 | 3.18 | 1.59 | 0.67 | 0.43 to 1.01 | |||
| 2.01–3.00 | 67 | 41 | 6.13 | 3.88 | 2.25 | 0.63 | 0.42 to 0.95 | |||
| 3.01–5.00 | 45 | 27 | 4.51 | 2.70 | 1.81 | 0.60 | 0.36 to 0.99 | |||
| ≥5.01 | 80 | 37 | 11.98 | 5.15 | 6.83 | 0.43 | 0.28 to 0.64 | |||
| No. of first-degree relatives with breast cancer | ||||||||||
| 0 | 62 | 33 | 6.47 | 3.48 | 2.99 | 0.54 | 0.34 to 0.83 | |||
| 1 | 124 | 73 | 5.52 | 3.16 | 2.36 | 0.57 | 0.42 to 0.77 | |||
| 2 | 52 | 32 | 7.84 | 4.91 | 2.93 | 0.63 | 0.39 to 0.99 | |||
| ≥3 | 12 | 7 | 11.24 | 5.48 | 5.76 | 0.49 | 0.16 to 1.34 | |||
LCIS = lobular carcinoma in situ; AH = atypical hyperplasia; RR = risk ratio; CI = confidence interval.
Rate in the placebo group minus rate in the tamoxifen group.
Risk ratio for women in the tamoxifen group relative to women in the placebo group.
Determined with the Gail model ( 11 ) .
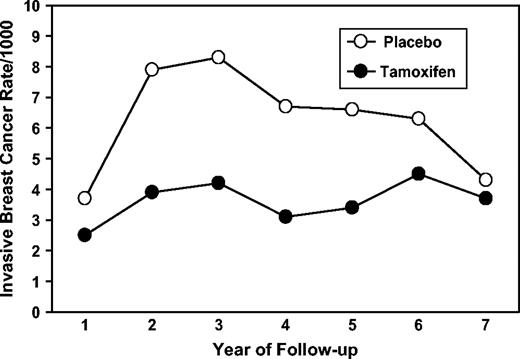
The effectiveness of tamoxifen was also assessed by comparing the rates of occurrence of invasive breast cancer during each of the seven yearly intervals of follow-up ( Fig. 3 ). In each of the years 2 through 5, the rates of tumors in women who received tamoxifen were reduced by approximately 50% compared with rates in women who received placebo; in year 6, the reduction was 29%, and in year 7, it was only 14%. However, it is important to note that the rate in the tamoxifen group remained relatively constant during the 7-year period; the decline in the magnitude of difference between the groups in the later years was due to a decrease in the rate of breast cancer in the placebo group rather than to an increase in the rate of breast cancer in the tamoxifen group.
Annual rates of invasive breast cancer per 1000 women by year of follow-up and treatment group in NSABP P-1.
Relation of Tumor Characteristics to Reduction in Invasive Breast Cancer
The size distributions of invasive tumors in women in the placebo and tamoxifen groups were similar. In each group, nearly 40% of the tumors were 1 cm or smaller, approximately 50% were 1.1–3.0 cm, and approximately 10% were 3.1 cm or larger ( Table 4 ). In women who received tamoxifen, the reduction in the rate of invasive cancer among the three groups was similar, i.e., 39%, 43%, and 49%, respectively. In the placebo and tamoxifen groups, the distribution of tumors according to nodal status was also similar; approximately two-thirds of the women with breast cancer in each group had negative nodes. Tamoxifen reduced the rate of node-negative cancer by 45% and of node-positive cancer by 32%, but the magnitudes of these reductions were not statistically significantly different from each other. The tumors in the placebo and tamoxifen groups had different distributions by ER status; 81% of the tumors that developed in the placebo group and 56% of those in the tamoxifen group were ER positive. Tamoxifen administration resulted in a 62% reduction in the rate of ER-positive invasive breast cancer but did not reduce the rate of ER-negative breast cancer.
Events and incidence rates of invasive cancer in the placebo and tamoxifen groups by selected tumor characteristics
| . | No. of events (%) . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference * . | RR † . | 95% CI . | |||
| Tumor size (cm) | ||||||||||
| ≤1.0 | 90 (36.0) | 56 (38.6) | 2.26 | 1.39 | 0.87 | 0.61 | 0.43 to 0.87 | |||
| 1.1–3.0 | 130 (52.0) | 75 (51.7) | 3.27 | 1.86 | 1.41 | 0.57 | 0.42 to 0.76 | |||
| ≥3.1 | 25 (10.0) | 13 (9.0) | 0.63 | 0.32 | 0.31 | 0.51 | 0.24 to 1.04 | |||
| Unknown | 5 (2.0) | 1 (0.7) | 0.13 | 0.02 | 0.11 | 0.20 | 0.01 to 1.76 | |||
| Pathologic nodal status | ||||||||||
| Negative | 162 (64.8) | 91 (62.8) | 4.08 | 2.26 | 1.82 | 0.55 | 0.42 to 0.72 | |||
| Positive | 70 (28.0) | 48 (33.1) | 1.76 | 1.19 | 0.57 | 0.68 | 0.46 to 0.99 | |||
| Unknown | 18 (7.2) | 6 (4.1) | 0.45 | 0.15 | 0.30 | 0.33 | 0.11 to 0.86 | |||
| Estrogen receptor status | ||||||||||
| Negative | 42 (16.8) | 56 (38.6) | 1.06 | 1.39 | −0.33 | 1.31 | 0.86 to 2.01 | |||
| Positive | 182 (72.8) | 70 (48.3) | 4.58 | 1.74 | 2.84 | 0.38 | 0.28 to 0.50 | |||
| Unknown | 26 (10.4) | 19 (13.1) | 0.65 | 0.47 | 0.18 | 0.72 | 0.38 to 1.35 | |||
| . | No. of events (%) . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference * . | RR † . | 95% CI . | |||
| Tumor size (cm) | ||||||||||
| ≤1.0 | 90 (36.0) | 56 (38.6) | 2.26 | 1.39 | 0.87 | 0.61 | 0.43 to 0.87 | |||
| 1.1–3.0 | 130 (52.0) | 75 (51.7) | 3.27 | 1.86 | 1.41 | 0.57 | 0.42 to 0.76 | |||
| ≥3.1 | 25 (10.0) | 13 (9.0) | 0.63 | 0.32 | 0.31 | 0.51 | 0.24 to 1.04 | |||
| Unknown | 5 (2.0) | 1 (0.7) | 0.13 | 0.02 | 0.11 | 0.20 | 0.01 to 1.76 | |||
| Pathologic nodal status | ||||||||||
| Negative | 162 (64.8) | 91 (62.8) | 4.08 | 2.26 | 1.82 | 0.55 | 0.42 to 0.72 | |||
| Positive | 70 (28.0) | 48 (33.1) | 1.76 | 1.19 | 0.57 | 0.68 | 0.46 to 0.99 | |||
| Unknown | 18 (7.2) | 6 (4.1) | 0.45 | 0.15 | 0.30 | 0.33 | 0.11 to 0.86 | |||
| Estrogen receptor status | ||||||||||
| Negative | 42 (16.8) | 56 (38.6) | 1.06 | 1.39 | −0.33 | 1.31 | 0.86 to 2.01 | |||
| Positive | 182 (72.8) | 70 (48.3) | 4.58 | 1.74 | 2.84 | 0.38 | 0.28 to 0.50 | |||
| Unknown | 26 (10.4) | 19 (13.1) | 0.65 | 0.47 | 0.18 | 0.72 | 0.38 to 1.35 | |||
Rate in the placebo group minus rate in the tamoxifen group.
Risk ratio for women in the tamoxifen group relative to women in the placebo group. RR = risk ratio; CI = confidence interval.
Events and incidence rates of invasive cancer in the placebo and tamoxifen groups by selected tumor characteristics
| . | No. of events (%) . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference * . | RR † . | 95% CI . | |||
| Tumor size (cm) | ||||||||||
| ≤1.0 | 90 (36.0) | 56 (38.6) | 2.26 | 1.39 | 0.87 | 0.61 | 0.43 to 0.87 | |||
| 1.1–3.0 | 130 (52.0) | 75 (51.7) | 3.27 | 1.86 | 1.41 | 0.57 | 0.42 to 0.76 | |||
| ≥3.1 | 25 (10.0) | 13 (9.0) | 0.63 | 0.32 | 0.31 | 0.51 | 0.24 to 1.04 | |||
| Unknown | 5 (2.0) | 1 (0.7) | 0.13 | 0.02 | 0.11 | 0.20 | 0.01 to 1.76 | |||
| Pathologic nodal status | ||||||||||
| Negative | 162 (64.8) | 91 (62.8) | 4.08 | 2.26 | 1.82 | 0.55 | 0.42 to 0.72 | |||
| Positive | 70 (28.0) | 48 (33.1) | 1.76 | 1.19 | 0.57 | 0.68 | 0.46 to 0.99 | |||
| Unknown | 18 (7.2) | 6 (4.1) | 0.45 | 0.15 | 0.30 | 0.33 | 0.11 to 0.86 | |||
| Estrogen receptor status | ||||||||||
| Negative | 42 (16.8) | 56 (38.6) | 1.06 | 1.39 | −0.33 | 1.31 | 0.86 to 2.01 | |||
| Positive | 182 (72.8) | 70 (48.3) | 4.58 | 1.74 | 2.84 | 0.38 | 0.28 to 0.50 | |||
| Unknown | 26 (10.4) | 19 (13.1) | 0.65 | 0.47 | 0.18 | 0.72 | 0.38 to 1.35 | |||
| . | No. of events (%) . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference * . | RR † . | 95% CI . | |||
| Tumor size (cm) | ||||||||||
| ≤1.0 | 90 (36.0) | 56 (38.6) | 2.26 | 1.39 | 0.87 | 0.61 | 0.43 to 0.87 | |||
| 1.1–3.0 | 130 (52.0) | 75 (51.7) | 3.27 | 1.86 | 1.41 | 0.57 | 0.42 to 0.76 | |||
| ≥3.1 | 25 (10.0) | 13 (9.0) | 0.63 | 0.32 | 0.31 | 0.51 | 0.24 to 1.04 | |||
| Unknown | 5 (2.0) | 1 (0.7) | 0.13 | 0.02 | 0.11 | 0.20 | 0.01 to 1.76 | |||
| Pathologic nodal status | ||||||||||
| Negative | 162 (64.8) | 91 (62.8) | 4.08 | 2.26 | 1.82 | 0.55 | 0.42 to 0.72 | |||
| Positive | 70 (28.0) | 48 (33.1) | 1.76 | 1.19 | 0.57 | 0.68 | 0.46 to 0.99 | |||
| Unknown | 18 (7.2) | 6 (4.1) | 0.45 | 0.15 | 0.30 | 0.33 | 0.11 to 0.86 | |||
| Estrogen receptor status | ||||||||||
| Negative | 42 (16.8) | 56 (38.6) | 1.06 | 1.39 | −0.33 | 1.31 | 0.86 to 2.01 | |||
| Positive | 182 (72.8) | 70 (48.3) | 4.58 | 1.74 | 2.84 | 0.38 | 0.28 to 0.50 | |||
| Unknown | 26 (10.4) | 19 (13.1) | 0.65 | 0.47 | 0.18 | 0.72 | 0.38 to 1.35 | |||
Rate in the placebo group minus rate in the tamoxifen group.
Risk ratio for women in the tamoxifen group relative to women in the placebo group. RR = risk ratio; CI = confidence interval.
Reduction in Osteoporotic Fractures
Women who received tamoxifen experienced reductions in hip, spine, and radius (Colles') fractures compared with women who received placebo (RR = 0.68, 95% CI = 0.51 to 0.92) ( Table 5 ). Most fractures (89%) occurred in women aged 50 years or older. Tamoxifen treatment reduced fractures in that age group by 29% (RR = 0.71, 95% CI = 0.52 to 0.97). Among women aged 49 years or younger, tamoxifen reduced fractures by 53% (RR = 0.47, 95% CI = 0.16 to 1.22).
Events and incidence rates of osteoporotic fractures in the placebo and tamoxifen groups by site of fracture and age at entry
| . | No. of events . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference * . | RR † . | 95% CI . | |||
| Fractures | 116 ‡ | 80 § | 2.88 | 1.97 | 0.91 | 0.68 | 0.51 to 0.92 | |||
| Hip | 35 | 24 | 0.86 | 0.59 | 0.27 | 0.68 | 0.39 to 1.18 | |||
| Spine | 53 | 40 | 1.31 | 0.98 | 0.33 | 0.75 | 0.48 to 1.15 | |||
| Radius (Colles') | 29 | 20 | 0.72 | 0.49 | 0.23 | 0.69 | 0.37 to 1.25 | |||
| Age at entry (y) | ||||||||||
| ≤49 | 15 | 7 | 0.95 | 0.44 | 0.51 | 0.47 | 0.16 to 1.22 | |||
| ≥50 | 101 | 73 | 4.13 | 2.95 | 1.18 | 0.71 | 0.52 to 0.97 | |||
| . | No. of events . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference * . | RR † . | 95% CI . | |||
| Fractures | 116 ‡ | 80 § | 2.88 | 1.97 | 0.91 | 0.68 | 0.51 to 0.92 | |||
| Hip | 35 | 24 | 0.86 | 0.59 | 0.27 | 0.68 | 0.39 to 1.18 | |||
| Spine | 53 | 40 | 1.31 | 0.98 | 0.33 | 0.75 | 0.48 to 1.15 | |||
| Radius (Colles') | 29 | 20 | 0.72 | 0.49 | 0.23 | 0.69 | 0.37 to 1.25 | |||
| Age at entry (y) | ||||||||||
| ≤49 | 15 | 7 | 0.95 | 0.44 | 0.51 | 0.47 | 0.16 to 1.22 | |||
| ≥50 | 101 | 73 | 4.13 | 2.95 | 1.18 | 0.71 | 0.52 to 0.97 | |||
Rate in the placebo group minus rate in the tamoxifen group.
Risk ratio for women in the tamoxifen group relative to women in the placebo group. RR = risk ratio; CI = confidence interval.
One woman in the placebo group had both a hip and a Colles' fracture.
In the tamoxifen group, one woman had a hip and a Colles' fracture; one woman had a hip and a spine fracture; and one woman had a hip, a spine, and a Colles' fracture.
Events and incidence rates of osteoporotic fractures in the placebo and tamoxifen groups by site of fracture and age at entry
| . | No. of events . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference * . | RR † . | 95% CI . | |||
| Fractures | 116 ‡ | 80 § | 2.88 | 1.97 | 0.91 | 0.68 | 0.51 to 0.92 | |||
| Hip | 35 | 24 | 0.86 | 0.59 | 0.27 | 0.68 | 0.39 to 1.18 | |||
| Spine | 53 | 40 | 1.31 | 0.98 | 0.33 | 0.75 | 0.48 to 1.15 | |||
| Radius (Colles') | 29 | 20 | 0.72 | 0.49 | 0.23 | 0.69 | 0.37 to 1.25 | |||
| Age at entry (y) | ||||||||||
| ≤49 | 15 | 7 | 0.95 | 0.44 | 0.51 | 0.47 | 0.16 to 1.22 | |||
| ≥50 | 101 | 73 | 4.13 | 2.95 | 1.18 | 0.71 | 0.52 to 0.97 | |||
| . | No. of events . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference * . | RR † . | 95% CI . | |||
| Fractures | 116 ‡ | 80 § | 2.88 | 1.97 | 0.91 | 0.68 | 0.51 to 0.92 | |||
| Hip | 35 | 24 | 0.86 | 0.59 | 0.27 | 0.68 | 0.39 to 1.18 | |||
| Spine | 53 | 40 | 1.31 | 0.98 | 0.33 | 0.75 | 0.48 to 1.15 | |||
| Radius (Colles') | 29 | 20 | 0.72 | 0.49 | 0.23 | 0.69 | 0.37 to 1.25 | |||
| Age at entry (y) | ||||||||||
| ≤49 | 15 | 7 | 0.95 | 0.44 | 0.51 | 0.47 | 0.16 to 1.22 | |||
| ≥50 | 101 | 73 | 4.13 | 2.95 | 1.18 | 0.71 | 0.52 to 0.97 | |||
Rate in the placebo group minus rate in the tamoxifen group.
Risk ratio for women in the tamoxifen group relative to women in the placebo group. RR = risk ratio; CI = confidence interval.
One woman in the placebo group had both a hip and a Colles' fracture.
In the tamoxifen group, one woman had a hip and a Colles' fracture; one woman had a hip and a spine fracture; and one woman had a hip, a spine, and a Colles' fracture.
Ischemic Heart Disease
There was no evidence that the administration of tamoxifen increased ischemic heart disease, either overall or by a specific type of event ( Table 6 ). Risk ratios comparing tamoxifen with placebo for fatal and nonfatal myocardial infarctions, severe angina, and acute ischemic syndrome ranged from 0.94 (95% CI = 0.55 to 1.58) to 1.12 (95% CI = 0.68 to 1.86). Overall, the risk ratio for ischemic heart disease was 1.03 (95% CI = 0.79 to 1.36).
Events and incidence rates of ischemic heart disease in the placebo and tamoxifen groups
| . | No. of events . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of event . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference * . | RR † . | 95% CI . | |||
| Myocardial infarction | 44 | 43 | 1.09 | 1.06 | 0.03 | 0.97 | 0.62 to 1.52 | |||
| Fatal | 11 | 12 | 0.27 | 0.29 | 0.02 | 1.09 | 0.44 to 2.72 | |||
| Nonfatal | 33 | 31 | 0.81 | 0.76 | 0.05 | 0.94 | 0.55 to 1.58 | |||
| Severe angina | 33 | 34 | 0.82 | 0.84 | −0.02 | 1.03 | 0.62 to 1.71 | |||
| Acute ischemic syndrome ‡ | 32 | 36 | 0.79 | 0.89 | −0.10 | 1.12 | 0.68 to 1.86 | |||
| Total | 109 | 113 | 2.70 | 2.79 | −0.09 | 1.03 | 0.79 to 1.36 | |||
| . | No. of events . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of event . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference * . | RR † . | 95% CI . | |||
| Myocardial infarction | 44 | 43 | 1.09 | 1.06 | 0.03 | 0.97 | 0.62 to 1.52 | |||
| Fatal | 11 | 12 | 0.27 | 0.29 | 0.02 | 1.09 | 0.44 to 2.72 | |||
| Nonfatal | 33 | 31 | 0.81 | 0.76 | 0.05 | 0.94 | 0.55 to 1.58 | |||
| Severe angina | 33 | 34 | 0.82 | 0.84 | −0.02 | 1.03 | 0.62 to 1.71 | |||
| Acute ischemic syndrome ‡ | 32 | 36 | 0.79 | 0.89 | −0.10 | 1.12 | 0.68 to 1.86 | |||
| Total | 109 | 113 | 2.70 | 2.79 | −0.09 | 1.03 | 0.79 to 1.36 | |||
Rate in the placebo group minus rate in the tamoxifen group.
Risk ratio for women in the tamoxifen group relative to women in the placebo group. RR = risk ratio; CI = confidence interval.
Diagnosis based upon new Q-wave on electrocardiogram without angina, elevation of serum enzymes, or angina requiring hospitalization without surgery.
Events and incidence rates of ischemic heart disease in the placebo and tamoxifen groups
| . | No. of events . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of event . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference * . | RR † . | 95% CI . | |||
| Myocardial infarction | 44 | 43 | 1.09 | 1.06 | 0.03 | 0.97 | 0.62 to 1.52 | |||
| Fatal | 11 | 12 | 0.27 | 0.29 | 0.02 | 1.09 | 0.44 to 2.72 | |||
| Nonfatal | 33 | 31 | 0.81 | 0.76 | 0.05 | 0.94 | 0.55 to 1.58 | |||
| Severe angina | 33 | 34 | 0.82 | 0.84 | −0.02 | 1.03 | 0.62 to 1.71 | |||
| Acute ischemic syndrome ‡ | 32 | 36 | 0.79 | 0.89 | −0.10 | 1.12 | 0.68 to 1.86 | |||
| Total | 109 | 113 | 2.70 | 2.79 | −0.09 | 1.03 | 0.79 to 1.36 | |||
| . | No. of events . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of event . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference * . | RR † . | 95% CI . | |||
| Myocardial infarction | 44 | 43 | 1.09 | 1.06 | 0.03 | 0.97 | 0.62 to 1.52 | |||
| Fatal | 11 | 12 | 0.27 | 0.29 | 0.02 | 1.09 | 0.44 to 2.72 | |||
| Nonfatal | 33 | 31 | 0.81 | 0.76 | 0.05 | 0.94 | 0.55 to 1.58 | |||
| Severe angina | 33 | 34 | 0.82 | 0.84 | −0.02 | 1.03 | 0.62 to 1.71 | |||
| Acute ischemic syndrome ‡ | 32 | 36 | 0.79 | 0.89 | −0.10 | 1.12 | 0.68 to 1.86 | |||
| Total | 109 | 113 | 2.70 | 2.79 | −0.09 | 1.03 | 0.79 to 1.36 | |||
Rate in the placebo group minus rate in the tamoxifen group.
Risk ratio for women in the tamoxifen group relative to women in the placebo group. RR = risk ratio; CI = confidence interval.
Diagnosis based upon new Q-wave on electrocardiogram without angina, elevation of serum enzymes, or angina requiring hospitalization without surgery.
Uterine Cancer
Women who received tamoxifen had a statistically significantly increased risk of invasive endometrial cancer (RR = 3.28, 95% CI = 1.87 to 6.03) ( Table 7 ). The risk was not increased in women aged 49 years or younger (RR = 1.42, 95% CI = 0.55 to 3.81), but there was a statistically significant increase in risk in women aged 50 years or older (RR = 5.33, 95% CI = 2.47 to 13.17). The cumulative rate of invasive endometrial cancer through 7 years of follow-up was 4.68 per 1000 women in the placebo group and 15.64 per 1000 women in the tamoxifen group, respectively ( P <.001). Of the 70 cases of endometrial cancer (17 in the placebo group and 53 in the tamoxifen group), 67 cases (15 in the placebo group and 52 in the tamoxifen group) were International Federation of Gynecology and Obstetrics (FIGO) stage I. Of the remaining two cases in the placebo group, one was stage III and one was stage IV. The remaining case in the tamoxifen group was stage III. Four cases of endometrial cancer in situ were observed: three in the placebo group and one in the tamoxifen group. In addition to these cases of endometrial cancer, there were four cases of uterine sarcoma, one in the placebo group and three in the tamoxifen group.
Events and incidence rates of invasive and in situ endometrial cancer in the placebo and tamoxifen groups by age at study entry *
| . | No. of events . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of cancer . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference † . | RR ‡ . | 95% CI . | |||
| Invasive | 17 | 53 | 0.68 | 2.24 | −1.56 | 3.28 | 1.87 to 6.03 | |||
| ≤49 y at entry | 9 | 12 | 0.82 | 1.16 | −0.34 | 1.42 | 0.55 to 3.81 | |||
| ≥50 y at entry | 8 | 41 | 0.58 | 3.08 | −2.50 | 5.33 | 2.47 to 13.17 | |||
| In situ cancer | 3 | 1 | 0.12 | 0.04 | 0.08 | 0.35 | 0.01 to 4.36 | |||
| . | No. of events . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of cancer . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference † . | RR ‡ . | 95% CI . | |||
| Invasive | 17 | 53 | 0.68 | 2.24 | −1.56 | 3.28 | 1.87 to 6.03 | |||
| ≤49 y at entry | 9 | 12 | 0.82 | 1.16 | −0.34 | 1.42 | 0.55 to 3.81 | |||
| ≥50 y at entry | 8 | 41 | 0.58 | 3.08 | −2.50 | 5.33 | 2.47 to 13.17 | |||
| In situ cancer | 3 | 1 | 0.12 | 0.04 | 0.08 | 0.35 | 0.01 to 4.36 | |||
Women at risk were those with an intact uterus. RR = risk ratio; CI = confidence interval.
Rate in the placebo group minus rate in the tamoxifen group.
Risk ratio for women in the tamoxifen group relative to women in the placebo group.
Events and incidence rates of invasive and in situ endometrial cancer in the placebo and tamoxifen groups by age at study entry *
| . | No. of events . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of cancer . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference † . | RR ‡ . | 95% CI . | |||
| Invasive | 17 | 53 | 0.68 | 2.24 | −1.56 | 3.28 | 1.87 to 6.03 | |||
| ≤49 y at entry | 9 | 12 | 0.82 | 1.16 | −0.34 | 1.42 | 0.55 to 3.81 | |||
| ≥50 y at entry | 8 | 41 | 0.58 | 3.08 | −2.50 | 5.33 | 2.47 to 13.17 | |||
| In situ cancer | 3 | 1 | 0.12 | 0.04 | 0.08 | 0.35 | 0.01 to 4.36 | |||
| . | No. of events . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of cancer . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference † . | RR ‡ . | 95% CI . | |||
| Invasive | 17 | 53 | 0.68 | 2.24 | −1.56 | 3.28 | 1.87 to 6.03 | |||
| ≤49 y at entry | 9 | 12 | 0.82 | 1.16 | −0.34 | 1.42 | 0.55 to 3.81 | |||
| ≥50 y at entry | 8 | 41 | 0.58 | 3.08 | −2.50 | 5.33 | 2.47 to 13.17 | |||
| In situ cancer | 3 | 1 | 0.12 | 0.04 | 0.08 | 0.35 | 0.01 to 4.36 | |||
Women at risk were those with an intact uterus. RR = risk ratio; CI = confidence interval.
Rate in the placebo group minus rate in the tamoxifen group.
Risk ratio for women in the tamoxifen group relative to women in the placebo group.
Thromboembolic Events
Women in the P-1 study who experienced more than one thromboembolic event were categorized according to the most severe event; e.g., if a woman had both a pulmonary embolism and deep-vein thrombosis, she was considered to have had a pulmonary embolism. There was evidence of an increased risk of stroke in women who received tamoxifen, but the increase was not statistically significant (RR = 1.42, 95% CI = 0.97 to 2.08) ( Table 8 ). The incidence rate of stroke was 0.05% greater in the tamoxifen group than in the placebo group. In analyses by age, women aged 49 years or younger were not at increased risk for stroke if they had received tamoxifen (RR = 1.13, 95% CI = 0.39 to 3.36). However, for women aged 50 years or older, there was some evidence that tamoxifen increased the risk of stroke (RR = 1.47, 95% CI = 0.97 to 2.22). The risk of transient ischemic attacks was similar in both groups (RR = 0.91, 95% CI = 0.54 to 1.52). The incidence of pulmonary embolism was statistically significantly greater in the tamoxifen group than in the placebo group (RR = 2.15, 95% CI = 1.08 to 4.51). There were six cases of pulmonary embolism in women aged 49 years or younger: Two occurred among women in the placebo group and four among women in the tamoxifen group (RR = 2.01, 95% CI = 0.29 to 22.19). Among women aged 50 years or more, those who received tamoxifen had an increased risk of pulmonary embolism (RR = 2.16, 95% CI = 1.02 to 4.89). The overall risk of deep-vein thrombosis was also greater in the tamoxifen group than in the placebo group, although the difference was not statistically significant (RR=1.44, 95% CI = 0.91 to 2.30). The risk increase was similar in both age groups.
Events and incidence rates of vascular-related events in the placebo and tamoxifen groups by age at study entry
| . | No. of events . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of event by age at study entry (y) . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference * . | RR † . | 95% CI . | |||
| Stroke | 50 | 71 | 1.23 | 1.75 | −0.52 | 1.42 | 0.97 to 2.08 | |||
| ≤49 | 8 | 9 | 0.50 | 0.57 | −0.07 | 1.13 | 0.39 to 3.36 | |||
| ≥50 | 42 | 62 | 1.70 | 2.50 | −0.80 | 1.47 | 0.97 to 2.22 | |||
| Transient ischemic attack | 34 | 31 | 0.84 | 0.76 | 0.08 | 0.91 | 0.54 to 1.52 | |||
| ≤49 | 7 | 4 | 0.44 | 0.25 | 0.19 | 0.57 | 0.12 to 2.25 | |||
| ≥50 | 27 | 27 | 1.10 | 1.09 | 0.01 | 0.99 | 0.56 to 1.76 | |||
| Pulmonary embolism | 13 | 28 | 0.32 | 0.69 | −0.37 | 2.15 | 1.08 to 4.51 | |||
| ≤49 | 2 | 4 | 0.13 | 0.25 | −0.12 | 2.01 | 0.29 to 22.19 | |||
| ≥50 | 11 | 24 | 0.44 | 0.96 | −0.52 | 2.16 | 1.02 to 4.89 | |||
| DVT ‡ | 34 | 49 | 0.84 | 1.21 | −0.37 | 1.44 | 0.91 to 2.30 | |||
| ≤49 | 12 | 16 | 0.76 | 1.01 | −0.25 | 1.34 | 0.59 to 3.10 | |||
| ≥50 | 22 | 33 | 0.89 | 1.33 | −0.44 | 1.49 | 0.84 to 2.68 | |||
| . | No. of events . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of event by age at study entry (y) . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference * . | RR † . | 95% CI . | |||
| Stroke | 50 | 71 | 1.23 | 1.75 | −0.52 | 1.42 | 0.97 to 2.08 | |||
| ≤49 | 8 | 9 | 0.50 | 0.57 | −0.07 | 1.13 | 0.39 to 3.36 | |||
| ≥50 | 42 | 62 | 1.70 | 2.50 | −0.80 | 1.47 | 0.97 to 2.22 | |||
| Transient ischemic attack | 34 | 31 | 0.84 | 0.76 | 0.08 | 0.91 | 0.54 to 1.52 | |||
| ≤49 | 7 | 4 | 0.44 | 0.25 | 0.19 | 0.57 | 0.12 to 2.25 | |||
| ≥50 | 27 | 27 | 1.10 | 1.09 | 0.01 | 0.99 | 0.56 to 1.76 | |||
| Pulmonary embolism | 13 | 28 | 0.32 | 0.69 | −0.37 | 2.15 | 1.08 to 4.51 | |||
| ≤49 | 2 | 4 | 0.13 | 0.25 | −0.12 | 2.01 | 0.29 to 22.19 | |||
| ≥50 | 11 | 24 | 0.44 | 0.96 | −0.52 | 2.16 | 1.02 to 4.89 | |||
| DVT ‡ | 34 | 49 | 0.84 | 1.21 | −0.37 | 1.44 | 0.91 to 2.30 | |||
| ≤49 | 12 | 16 | 0.76 | 1.01 | −0.25 | 1.34 | 0.59 to 3.10 | |||
| ≥50 | 22 | 33 | 0.89 | 1.33 | −0.44 | 1.49 | 0.84 to 2.68 | |||
Rate in the placebo group minus rate in the tamoxifen group.
Risk ratio for women in the tamoxifen group relative to women in the placebo group. RR = risk ratio; CI = confidence interval.
DVT = deep-vein thrombosis.
Events and incidence rates of vascular-related events in the placebo and tamoxifen groups by age at study entry
| . | No. of events . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of event by age at study entry (y) . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference * . | RR † . | 95% CI . | |||
| Stroke | 50 | 71 | 1.23 | 1.75 | −0.52 | 1.42 | 0.97 to 2.08 | |||
| ≤49 | 8 | 9 | 0.50 | 0.57 | −0.07 | 1.13 | 0.39 to 3.36 | |||
| ≥50 | 42 | 62 | 1.70 | 2.50 | −0.80 | 1.47 | 0.97 to 2.22 | |||
| Transient ischemic attack | 34 | 31 | 0.84 | 0.76 | 0.08 | 0.91 | 0.54 to 1.52 | |||
| ≤49 | 7 | 4 | 0.44 | 0.25 | 0.19 | 0.57 | 0.12 to 2.25 | |||
| ≥50 | 27 | 27 | 1.10 | 1.09 | 0.01 | 0.99 | 0.56 to 1.76 | |||
| Pulmonary embolism | 13 | 28 | 0.32 | 0.69 | −0.37 | 2.15 | 1.08 to 4.51 | |||
| ≤49 | 2 | 4 | 0.13 | 0.25 | −0.12 | 2.01 | 0.29 to 22.19 | |||
| ≥50 | 11 | 24 | 0.44 | 0.96 | −0.52 | 2.16 | 1.02 to 4.89 | |||
| DVT ‡ | 34 | 49 | 0.84 | 1.21 | −0.37 | 1.44 | 0.91 to 2.30 | |||
| ≤49 | 12 | 16 | 0.76 | 1.01 | −0.25 | 1.34 | 0.59 to 3.10 | |||
| ≥50 | 22 | 33 | 0.89 | 1.33 | −0.44 | 1.49 | 0.84 to 2.68 | |||
| . | No. of events . | . | Rate per 1000 women . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of event by age at study entry (y) . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | Difference * . | RR † . | 95% CI . | |||
| Stroke | 50 | 71 | 1.23 | 1.75 | −0.52 | 1.42 | 0.97 to 2.08 | |||
| ≤49 | 8 | 9 | 0.50 | 0.57 | −0.07 | 1.13 | 0.39 to 3.36 | |||
| ≥50 | 42 | 62 | 1.70 | 2.50 | −0.80 | 1.47 | 0.97 to 2.22 | |||
| Transient ischemic attack | 34 | 31 | 0.84 | 0.76 | 0.08 | 0.91 | 0.54 to 1.52 | |||
| ≤49 | 7 | 4 | 0.44 | 0.25 | 0.19 | 0.57 | 0.12 to 2.25 | |||
| ≥50 | 27 | 27 | 1.10 | 1.09 | 0.01 | 0.99 | 0.56 to 1.76 | |||
| Pulmonary embolism | 13 | 28 | 0.32 | 0.69 | −0.37 | 2.15 | 1.08 to 4.51 | |||
| ≤49 | 2 | 4 | 0.13 | 0.25 | −0.12 | 2.01 | 0.29 to 22.19 | |||
| ≥50 | 11 | 24 | 0.44 | 0.96 | −0.52 | 2.16 | 1.02 to 4.89 | |||
| DVT ‡ | 34 | 49 | 0.84 | 1.21 | −0.37 | 1.44 | 0.91 to 2.30 | |||
| ≤49 | 12 | 16 | 0.76 | 1.01 | −0.25 | 1.34 | 0.59 to 3.10 | |||
| ≥50 | 22 | 33 | 0.89 | 1.33 | −0.44 | 1.49 | 0.84 to 2.68 | |||
Rate in the placebo group minus rate in the tamoxifen group.
Risk ratio for women in the tamoxifen group relative to women in the placebo group. RR = risk ratio; CI = confidence interval.
DVT = deep-vein thrombosis.
Cataracts
The rates of both cataracts and cataract surgery were statistically significantly elevated in women in the tamoxifen group. The incidence rate of cataract development was 27.75 per 1000 women in the tamoxifen group and 22.85 per 1000 women in the placebo group (RR = 1.21, 95% CI = 1.10 to 1.34). In women who developed cataracts, the incidence rate of cataract surgery was 10.54 per 1000 women in the tamoxifen group and 7.58 per 1000 women in the placebo group (RR = 1.39, 95% CI = 1.19 to 1.63).
Cancers Other Than Those of the Breast and Endometrium
There were 155 cancers at 18 sites other than the breast and endometrium among women who received placebo and 178 cancers at 21 other sites among those who received tamoxifen ( Table 9 ). None of the differences by site was statistically significant. The largest absolute difference in numbers of events between the placebo and tamoxifen groups was six cases, which occurred for both skin cancer and for lymphatic and hematopoietic cancers. The most frequently occurring cancer was lung cancer, which developed in 30 women in the placebo group and 33 in the tamoxifen group.
Events and incidence rates for invasive cancer cases other than breast and uterine cancer in the placebo and tamoxifen groups
| . | No. of events . | . | Rate per 1000 women . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|
| Site of cancer . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | RR * . | 95% CI † . | ||
| Mouth, pharynx, larynx | 2 | 6 | 0.05 | 0.15 | 2.99 | 0.53 to 30.27 | ||
| Stomach | 2 | 2 | 0.05 | 0.05 | 1.00 | 0.07 to 13.74 | ||
| Gallbladder | 4 | 1 | 0.10 | 0.02 | 0.25 | 0.01 to 2.52 | ||
| Pancreas | 11 | 8 | 0.27 | 0.20 | 0.72 | 0.25 to 1.98 | ||
| Retroperitoneum | 3 | 1 | 0.07 | 0.02 | 0.33 | 0.01 to 4.14 | ||
| Colon | 15 | 20 | 0.37 | 0.49 | 1.33 | 0.65 to 2.79 | ||
| Rectum | 4 | 6 | 0.10 | 0.15 | 1.49 | 0.35 to 7.20 | ||
| Liver | 0 | 1 | 0 | 0.02 | — | — | ||
| Lung, trachea, bronchus | 30 | 33 | 0.74 | 0.82 | 1.10 | 0.65 to 1.86 | ||
| Lymphatic and hematopoietic systems | 20 | 26 | 0.50 | 0.64 | 1.29 | 0.70 to 2.45 | ||
| Uterus, ovary, fallopian tube | 18 | 17 | 0.45 | 0.42 | 0.94 | 0.46 to 1.93 | ||
| Other genital | 3 | 3 | 0.07 | 0.07 | 1.00 | 0.13 to 7.44 | ||
| Urinary bladder | 3 | 5 | 0.07 | 0.12 | 1.66 | 0.32 to 10.69 | ||
| Kidney | 6 | 8 | 0.15 | 0.20 | 1.33 | 0.40 to 4.64 | ||
| Connective tissue | 2 | 1 | 0.05 | 0.02 | 0.50 | 0.01 to 9.57 | ||
| Skin | 13 | 19 | 0.32 | 0.47 | 1.46 | 0.68 to 3.21 | ||
| Nervous system | 5 | 3 | 0.12 | 0.07 | 0.60 | 0.09 to 3.07 | ||
| Thyroid gland | 7 | 8 | 0.17 | 0.20 | 1.14 | 0.36 to 3.69 | ||
| Adrenal gland | 0 | 1 | 0 | 0.02 | — | — | ||
| Other digestive system | 0 | 1 | 0 | 0.02 | — | — | ||
| Unknown | 7 | 8 | 0.17 | 0.20 | 1.14 | 0.36 to 3.69 | ||
| . | No. of events . | . | Rate per 1000 women . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|
| Site of cancer . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | RR * . | 95% CI † . | ||
| Mouth, pharynx, larynx | 2 | 6 | 0.05 | 0.15 | 2.99 | 0.53 to 30.27 | ||
| Stomach | 2 | 2 | 0.05 | 0.05 | 1.00 | 0.07 to 13.74 | ||
| Gallbladder | 4 | 1 | 0.10 | 0.02 | 0.25 | 0.01 to 2.52 | ||
| Pancreas | 11 | 8 | 0.27 | 0.20 | 0.72 | 0.25 to 1.98 | ||
| Retroperitoneum | 3 | 1 | 0.07 | 0.02 | 0.33 | 0.01 to 4.14 | ||
| Colon | 15 | 20 | 0.37 | 0.49 | 1.33 | 0.65 to 2.79 | ||
| Rectum | 4 | 6 | 0.10 | 0.15 | 1.49 | 0.35 to 7.20 | ||
| Liver | 0 | 1 | 0 | 0.02 | — | — | ||
| Lung, trachea, bronchus | 30 | 33 | 0.74 | 0.82 | 1.10 | 0.65 to 1.86 | ||
| Lymphatic and hematopoietic systems | 20 | 26 | 0.50 | 0.64 | 1.29 | 0.70 to 2.45 | ||
| Uterus, ovary, fallopian tube | 18 | 17 | 0.45 | 0.42 | 0.94 | 0.46 to 1.93 | ||
| Other genital | 3 | 3 | 0.07 | 0.07 | 1.00 | 0.13 to 7.44 | ||
| Urinary bladder | 3 | 5 | 0.07 | 0.12 | 1.66 | 0.32 to 10.69 | ||
| Kidney | 6 | 8 | 0.15 | 0.20 | 1.33 | 0.40 to 4.64 | ||
| Connective tissue | 2 | 1 | 0.05 | 0.02 | 0.50 | 0.01 to 9.57 | ||
| Skin | 13 | 19 | 0.32 | 0.47 | 1.46 | 0.68 to 3.21 | ||
| Nervous system | 5 | 3 | 0.12 | 0.07 | 0.60 | 0.09 to 3.07 | ||
| Thyroid gland | 7 | 8 | 0.17 | 0.20 | 1.14 | 0.36 to 3.69 | ||
| Adrenal gland | 0 | 1 | 0 | 0.02 | — | — | ||
| Other digestive system | 0 | 1 | 0 | 0.02 | — | — | ||
| Unknown | 7 | 8 | 0.17 | 0.20 | 1.14 | 0.36 to 3.69 | ||
RR = risk ratio for women in the tamoxifen group relative to women in the placebo group.
CI = confidence interval.
Events and incidence rates for invasive cancer cases other than breast and uterine cancer in the placebo and tamoxifen groups
| . | No. of events . | . | Rate per 1000 women . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|
| Site of cancer . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | RR * . | 95% CI † . | ||
| Mouth, pharynx, larynx | 2 | 6 | 0.05 | 0.15 | 2.99 | 0.53 to 30.27 | ||
| Stomach | 2 | 2 | 0.05 | 0.05 | 1.00 | 0.07 to 13.74 | ||
| Gallbladder | 4 | 1 | 0.10 | 0.02 | 0.25 | 0.01 to 2.52 | ||
| Pancreas | 11 | 8 | 0.27 | 0.20 | 0.72 | 0.25 to 1.98 | ||
| Retroperitoneum | 3 | 1 | 0.07 | 0.02 | 0.33 | 0.01 to 4.14 | ||
| Colon | 15 | 20 | 0.37 | 0.49 | 1.33 | 0.65 to 2.79 | ||
| Rectum | 4 | 6 | 0.10 | 0.15 | 1.49 | 0.35 to 7.20 | ||
| Liver | 0 | 1 | 0 | 0.02 | — | — | ||
| Lung, trachea, bronchus | 30 | 33 | 0.74 | 0.82 | 1.10 | 0.65 to 1.86 | ||
| Lymphatic and hematopoietic systems | 20 | 26 | 0.50 | 0.64 | 1.29 | 0.70 to 2.45 | ||
| Uterus, ovary, fallopian tube | 18 | 17 | 0.45 | 0.42 | 0.94 | 0.46 to 1.93 | ||
| Other genital | 3 | 3 | 0.07 | 0.07 | 1.00 | 0.13 to 7.44 | ||
| Urinary bladder | 3 | 5 | 0.07 | 0.12 | 1.66 | 0.32 to 10.69 | ||
| Kidney | 6 | 8 | 0.15 | 0.20 | 1.33 | 0.40 to 4.64 | ||
| Connective tissue | 2 | 1 | 0.05 | 0.02 | 0.50 | 0.01 to 9.57 | ||
| Skin | 13 | 19 | 0.32 | 0.47 | 1.46 | 0.68 to 3.21 | ||
| Nervous system | 5 | 3 | 0.12 | 0.07 | 0.60 | 0.09 to 3.07 | ||
| Thyroid gland | 7 | 8 | 0.17 | 0.20 | 1.14 | 0.36 to 3.69 | ||
| Adrenal gland | 0 | 1 | 0 | 0.02 | — | — | ||
| Other digestive system | 0 | 1 | 0 | 0.02 | — | — | ||
| Unknown | 7 | 8 | 0.17 | 0.20 | 1.14 | 0.36 to 3.69 | ||
| . | No. of events . | . | Rate per 1000 women . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|
| Site of cancer . | Placebo . | Tamoxifen . | Placebo . | Tamoxifen . | RR * . | 95% CI † . | ||
| Mouth, pharynx, larynx | 2 | 6 | 0.05 | 0.15 | 2.99 | 0.53 to 30.27 | ||
| Stomach | 2 | 2 | 0.05 | 0.05 | 1.00 | 0.07 to 13.74 | ||
| Gallbladder | 4 | 1 | 0.10 | 0.02 | 0.25 | 0.01 to 2.52 | ||
| Pancreas | 11 | 8 | 0.27 | 0.20 | 0.72 | 0.25 to 1.98 | ||
| Retroperitoneum | 3 | 1 | 0.07 | 0.02 | 0.33 | 0.01 to 4.14 | ||
| Colon | 15 | 20 | 0.37 | 0.49 | 1.33 | 0.65 to 2.79 | ||
| Rectum | 4 | 6 | 0.10 | 0.15 | 1.49 | 0.35 to 7.20 | ||
| Liver | 0 | 1 | 0 | 0.02 | — | — | ||
| Lung, trachea, bronchus | 30 | 33 | 0.74 | 0.82 | 1.10 | 0.65 to 1.86 | ||
| Lymphatic and hematopoietic systems | 20 | 26 | 0.50 | 0.64 | 1.29 | 0.70 to 2.45 | ||
| Uterus, ovary, fallopian tube | 18 | 17 | 0.45 | 0.42 | 0.94 | 0.46 to 1.93 | ||
| Other genital | 3 | 3 | 0.07 | 0.07 | 1.00 | 0.13 to 7.44 | ||
| Urinary bladder | 3 | 5 | 0.07 | 0.12 | 1.66 | 0.32 to 10.69 | ||
| Kidney | 6 | 8 | 0.15 | 0.20 | 1.33 | 0.40 to 4.64 | ||
| Connective tissue | 2 | 1 | 0.05 | 0.02 | 0.50 | 0.01 to 9.57 | ||
| Skin | 13 | 19 | 0.32 | 0.47 | 1.46 | 0.68 to 3.21 | ||
| Nervous system | 5 | 3 | 0.12 | 0.07 | 0.60 | 0.09 to 3.07 | ||
| Thyroid gland | 7 | 8 | 0.17 | 0.20 | 1.14 | 0.36 to 3.69 | ||
| Adrenal gland | 0 | 1 | 0 | 0.02 | — | — | ||
| Other digestive system | 0 | 1 | 0 | 0.02 | — | — | ||
| Unknown | 7 | 8 | 0.17 | 0.20 | 1.14 | 0.36 to 3.69 | ||
RR = risk ratio for women in the tamoxifen group relative to women in the placebo group.
CI = confidence interval.
Causes of Death
Death rates were similar in the two groups (RR = 1.10, 95% CI = 0.85 to 1.43). No cause-specific category of death exhibited a statistically significant difference between the groups ( Table 10 ). The most frequent cause of death was lung cancer, with 17 such deaths occurring in each group. There were 11 deaths due to breast cancer in the placebo group and 12 such deaths in the tamoxifen group. In the tamoxifen group, three deaths were related to pulmonary embolism and nine to stroke. In the placebo group, one death occurred from pulmonary embolism and three occurred from stroke.
Deaths in the placebo and tamoxifen groups
| Cause of death . | Placebo . | Tamoxifen . | |
|---|---|---|---|
| Cancer | |||
| Bladder | 0 | 1 | |
| Brain | 5 | 2 | |
| Breast | 11 | 12 | |
| Colon | 2 | 2 | |
| Gallbladder and extrahepatic bile duct | 4 | 0 | |
| Kidney | 3 | 2 | |
| Lung | 17 | 17 | |
| Lymphatic and hematopoietic | 8 | 5 | |
| Melanoma | 0 | 1 | |
| Ovary | 3 | 7 | |
| Pancreas | 9 | 4 | |
| Stomach | 1 | 1 | |
| Thyroid gland | 1 | 0 | |
| Uterus | 1 | 0 | |
| Primary site unknown | 6 | 3 | |
| Cardiac and vascular disease | |||
| Disorder of arteries | 0 | 1 | |
| Ischemic heart disease | 11 | 11 | |
| Other heart disease | 7 | 12 | |
| Pulmonary embolism | 1 | 3 | |
| Stroke | 3 | 9 | |
| Other | |||
| Auto accident | 2 | 1 | |
| Other disease of the digestive system | 3 | 1 | |
| Kidney/urinary tract | 2 | 2 | |
| Other lung disease | 0 | 3 | |
| Septicemia and other infection | 1 | 2 | |
| Miscellaneous | 6 | 7 | |
| Unknown | 7 | 17 | |
| Total No. of deaths | 114 | 126 | |
| Incidence rate per 1000 women | 2.80 | 3.08 | |
| RR (95% CI) * | 1.10 (0.85 to 1.43) | ||
| Cause of death . | Placebo . | Tamoxifen . | |
|---|---|---|---|
| Cancer | |||
| Bladder | 0 | 1 | |
| Brain | 5 | 2 | |
| Breast | 11 | 12 | |
| Colon | 2 | 2 | |
| Gallbladder and extrahepatic bile duct | 4 | 0 | |
| Kidney | 3 | 2 | |
| Lung | 17 | 17 | |
| Lymphatic and hematopoietic | 8 | 5 | |
| Melanoma | 0 | 1 | |
| Ovary | 3 | 7 | |
| Pancreas | 9 | 4 | |
| Stomach | 1 | 1 | |
| Thyroid gland | 1 | 0 | |
| Uterus | 1 | 0 | |
| Primary site unknown | 6 | 3 | |
| Cardiac and vascular disease | |||
| Disorder of arteries | 0 | 1 | |
| Ischemic heart disease | 11 | 11 | |
| Other heart disease | 7 | 12 | |
| Pulmonary embolism | 1 | 3 | |
| Stroke | 3 | 9 | |
| Other | |||
| Auto accident | 2 | 1 | |
| Other disease of the digestive system | 3 | 1 | |
| Kidney/urinary tract | 2 | 2 | |
| Other lung disease | 0 | 3 | |
| Septicemia and other infection | 1 | 2 | |
| Miscellaneous | 6 | 7 | |
| Unknown | 7 | 17 | |
| Total No. of deaths | 114 | 126 | |
| Incidence rate per 1000 women | 2.80 | 3.08 | |
| RR (95% CI) * | 1.10 (0.85 to 1.43) | ||
RR = risk ratio; CI = confidence interval.
Deaths in the placebo and tamoxifen groups
| Cause of death . | Placebo . | Tamoxifen . | |
|---|---|---|---|
| Cancer | |||
| Bladder | 0 | 1 | |
| Brain | 5 | 2 | |
| Breast | 11 | 12 | |
| Colon | 2 | 2 | |
| Gallbladder and extrahepatic bile duct | 4 | 0 | |
| Kidney | 3 | 2 | |
| Lung | 17 | 17 | |
| Lymphatic and hematopoietic | 8 | 5 | |
| Melanoma | 0 | 1 | |
| Ovary | 3 | 7 | |
| Pancreas | 9 | 4 | |
| Stomach | 1 | 1 | |
| Thyroid gland | 1 | 0 | |
| Uterus | 1 | 0 | |
| Primary site unknown | 6 | 3 | |
| Cardiac and vascular disease | |||
| Disorder of arteries | 0 | 1 | |
| Ischemic heart disease | 11 | 11 | |
| Other heart disease | 7 | 12 | |
| Pulmonary embolism | 1 | 3 | |
| Stroke | 3 | 9 | |
| Other | |||
| Auto accident | 2 | 1 | |
| Other disease of the digestive system | 3 | 1 | |
| Kidney/urinary tract | 2 | 2 | |
| Other lung disease | 0 | 3 | |
| Septicemia and other infection | 1 | 2 | |
| Miscellaneous | 6 | 7 | |
| Unknown | 7 | 17 | |
| Total No. of deaths | 114 | 126 | |
| Incidence rate per 1000 women | 2.80 | 3.08 | |
| RR (95% CI) * | 1.10 (0.85 to 1.43) | ||
| Cause of death . | Placebo . | Tamoxifen . | |
|---|---|---|---|
| Cancer | |||
| Bladder | 0 | 1 | |
| Brain | 5 | 2 | |
| Breast | 11 | 12 | |
| Colon | 2 | 2 | |
| Gallbladder and extrahepatic bile duct | 4 | 0 | |
| Kidney | 3 | 2 | |
| Lung | 17 | 17 | |
| Lymphatic and hematopoietic | 8 | 5 | |
| Melanoma | 0 | 1 | |
| Ovary | 3 | 7 | |
| Pancreas | 9 | 4 | |
| Stomach | 1 | 1 | |
| Thyroid gland | 1 | 0 | |
| Uterus | 1 | 0 | |
| Primary site unknown | 6 | 3 | |
| Cardiac and vascular disease | |||
| Disorder of arteries | 0 | 1 | |
| Ischemic heart disease | 11 | 11 | |
| Other heart disease | 7 | 12 | |
| Pulmonary embolism | 1 | 3 | |
| Stroke | 3 | 9 | |
| Other | |||
| Auto accident | 2 | 1 | |
| Other disease of the digestive system | 3 | 1 | |
| Kidney/urinary tract | 2 | 2 | |
| Other lung disease | 0 | 3 | |
| Septicemia and other infection | 1 | 2 | |
| Miscellaneous | 6 | 7 | |
| Unknown | 7 | 17 | |
| Total No. of deaths | 114 | 126 | |
| Incidence rate per 1000 women | 2.80 | 3.08 | |
| RR (95% CI) * | 1.10 (0.85 to 1.43) | ||
RR = risk ratio; CI = confidence interval.
D ISCUSSION
The updated data from the P-1 trial confirm the reduction in the risk of invasive breast cancer with tamoxifen treatment that we reported in the initial publication ( 10 ) . That conclusion is supported by the observation that the incidence rate of breast cancer among women who received tamoxifen was relatively constant through 7 years of follow-up and the fact that the rate remained stable for at least 2 years past the maximum time that the women received tamoxifen ( Fig. 3 ).
It should be noted that reduction in mortality was not the primary end point of the P-1 trial because, when the study was designed, it was anticipated that the follow-up time required to obtain definitive information about reduction in mortality was likely to be 15–20 years. Instead, reduction in incidence of breast cancer was used as a marker of outcome. In 1998, when an overall 49% reduction in the risk of breast cancer ( P <.001) was observed, the independent data monitoring committee that regularly reviewed the P-1 data decided that the primary aim of the trial had been attained beyond all reasonable doubt. The committee recommended, therefore, that the study be unblinded, the findings be disclosed, and participants be informed of whether or not they had received placebo so that they could decide whether to take tamoxifen to reduce their risk of breast cancer ( 18 ) . Although the study had been designed to determine reduction in incidence of breast cancer rather than improved survival, and despite the recommendation of the data monitoring committee, some critics suggested that the participants should not have been informed of the marked benefit achieved with tamoxifen until a survival benefit was demonstrated ( 19 – 22 ). This position is an unrealistic one that fails to take into account the ethical considerations under which medical research is conducted, i.e., adhering to commitments in the consent form regarding data sharing and notifying participants of the findings once the independent data monitoring committee has recommended doing so.
Our findings and those of other investigators indicated the relevance of using incidence rather than mortality as the primary end point in a breast cancer prevention study. Of the three other prevention trials that have reported findings ( 20 , 23 , 24 ) , only one study ( 23 ) defined breast cancer mortality as a primary end point, and information on this end point will probably never be forthcoming. The investigators of the recently implemented International Breast Cancer Intervention Study (IBIS-II [Prevention]) have identified breast cancer incidence as the primary end point in their trial and have indicated that evidence to test that hypothesis will be obtained after a median follow-up of 5 years ( 25 ) . Although those investigators have designated breast cancer mortality as a secondary end point, they have also recognized that reduction in the incidence of breast cancer is likely to be determined well before the findings with regard to survival are ascertained. They have stated that the power to detect a survival end point within 10 years is marginal ( 25 ) .
Although criticism of the decision to unblind the P-1 trial was unjustified, the current findings do provide justification for questioning whether it was appropriate to continue to obtain follow-up information from participants once they had been informed of whether they had received placebo or tamoxifen. After the unblinding, almost one-third of the placebo participants began taking a selective estrogen-receptor modifier (SERM) for chemoprevention. Therefore, the proportion of women in the placebo group with follow-up was 8.5% less than that in the tamoxifen group. Thus, the potential for bias and confounding in the long-term findings was substantial. The observation that the rate of breast cancer in the placebo group dropped in the sixth and seventh years of follow-up to a level that was close to the rate seen in the tamoxifen group ( Fig. 3 ) supports the conclusion that there is a bias causing an attenuation of the relative risk toward 1.0. As a result of these potential biases, the true magnitude of the effects from tamoxifen cannot be estimated well from the updated data. The data presented in the original report, which were obtained before the P-1 trial was unblinded, thus provide the best estimate of the true magnitude of tamoxifen effects.
Although the updated P-1 data fail to provide evidence of a reduction in mortality, findings from NSABP B-14, a randomized trial that compared the outcome of patients with ER-positive tumors and negative nodes who received either placebo or tamoxifen ( 26 ) , indicate that the reduction in breast cancer incidence in the P-1 trial will likely be accompanied by a decrease in breast cancer mortality. In the B-14 study, the recurrence-free survival benefit from tamoxifen that was noted after 5 years continued to increase through 15 years of follow-up. Although an overall survival benefit was not observed until after about 10 years of follow-up, it, too, progressively increased through 15 years.
Other information that can be gained from the updated P-1 data relates to the reliability of conclusions about the beneficial and undesirable effects that have previously been associated with tamoxifen administration. All of the previously reported findings and conclusions about these effects ( 10 ) have persisted with longer follow-up. Some of the more important of those findings were ones demonstrating that effects that were originally hypothesized did not exist. For example, when the P-1 trial was designed, there was evidence to indicate that tamoxifen altered lipid and lipoprotein metabolism ( 27 – 31 ) . Consequently, it was postulated that tamoxifen might reduce the rate of ischemic heart disease. Although a reduced risk of heart disease with tamoxifen has been reported ( 32 – 35 ) and tamoxifen reduced total cholesterol, fibrinogen, and C-reactive protein in the P-1 cohort ( 36 ) , the current findings continue to demonstrate that tamoxifen administration does not reduce the risk of ischemic heart disease.
Because an increased risk of endometrial cancer had been associated with tamoxifen use among breast cancer patients ( 37 , 38 ) by the time the P-1 study was conceived, the risk of that event was included as a secondary end point. The increased risk of endometrial cancer noted in the initial report of the P-1 findings ( 10 ) persists in the updated data. Findings from the IBIS-I prevention trial demonstrated a non–statistically significant ( P = .2) twofold excess of endometrial cancer in women who received tamoxifen and, as with the P-1 findings, showed that the majority of such cancers were FIGO stage I ( 24 ). The early stage of this disease associated with tamoxifen discounts the contention that endometrial cancer in women who received tamoxifen was associated with an “extremely” bad prognosis ( 39 ) . In an earlier report of data from the P-1 trial, we noted four cases of uterine sarcoma in tamoxifen-treated women and none in women who received placebo ( 40 ). Numbers in this report differ from those previously reported because one woman with a tumor that occurred after more than 7 years of follow-up was not included in the current analysis. Also, one case of uterine sarcoma has been noted in a woman in the placebo group since our initial report.
Pulmonary embolism and deep-vein thrombosis were included as secondary end points in the P-1 trial. In both the initial ( 10 ) and current findings from P-1, the frequency of those outcomes was elevated in the tamoxifen group; however, the difference reached statistical significance only for pulmonary embolism. Stroke, another thromboembolic event that has been observed in women who received tamoxifen, was not a predefined secondary end point, but we included it as an outcome in the initial report because it was elevated, although not statistically significantly. The current findings continue to demonstrate a non–statistically significant increase in the incidence of stroke. However, it is not clear whether this finding should be interpreted as a true elevation in risk because other investigators who have evaluated the risk of stroke associated with tamoxifen have obtained various findings. Recent results from a meta-analysis estimated that the risk of ischemic stroke was increased among breast cancer patients who were treated with tamoxifen ( 41 ) . Results from a large, nested case–control study showed that, after adjustment for other factors associated with stroke, breast cancer patients who used tamoxifen for at least 1 year had an increase in the risk of stroke that was of borderline statistical significance ( 42 ) . Although the results from the IBIS-I prevention study showed a statistically significant increased risk of thromboembolic events with tamoxifen, the study did not demonstrate a statistically significantly increased risk of stroke ( 24 , 43 ) .
In the initial report of P-1, a slight but statistically significant excess in posterior subcapsular lens opacities (cataracts) was observed in women who had received tamoxifen. The current data continue to demonstrate such an increase in the incidence rate of cataract occurrence, as well as in the rate of cataract surgery. Evaluation of the frequency of other adverse eye-related events from tamoxifen failed to demonstrate vision-threatening toxicity ( 44 ) . More recently, investigators concluded that there was no evidence that retinal small-vessel occlusive disease occurred at an increased rate in patients taking tamoxifen ( 45 ) . No increase in either the frequency of cataracts or eye complaints was observed in tamoxifen recipients in either the IBIS-I prevention trial ( 24 ) or in a recent nested, matched case–control study ( 46 ) . It should be noted that eye morbidity was included as one of the planned end points in the P-1 trial. Consequently, as for all end points included in that study, the reporting requirements were comprehensive. Whether the other studies were as thorough in their reporting of ocular events is unclear.
Another aspect of the current findings that has clinical significance relates to the estimation of the net benefit resulting from the use of tamoxifen. The methodology for conducting such an estimate has been developed by Gail et al. ( 47 ) . The process is complex, involving 10 different outcomes relative to beneficial and undesirable effects. As a consequence of this complexity, it has not been fully recognized that there are large populations of women who could likely benefit from taking tamoxifen ( 48 – 53 ) ; thus, tamoxifen has been underused as a breast cancer prevention agent. Using the data from the Gail et al. report ( 47 ) , we have constructed Fig. 4 , which quantifies the benefits and risks from tamoxifen in groups identified by predicted breast cancer risk, age, and race. Although the graphic presentation does not include all of the beneficial and undesirable events associated with the drug, and although not all types of events have equal medical significance, the presentation is adequate to illustrate the differences in net effect among populations. It is apparent from the observations in Fig. 4 that the potential benefit from tamoxifen therapy is related to a woman's predicted 5-year risk of breast cancer; i.e., the benefit increases with an increase in the level of predicted risk. It is also evident that the potential risks of thromboembolic events and endometrial cancer are related to a woman's age and race. There are categories of women for whom the net effect of chemoprevention is highly positive, e.g., all women younger than 50 years whose 5-year predicted breast cancer risk is at least 2.5% and white women younger than 60 years whose 5-year predicted risk is at least 4.5%. Because the potential for undesirable effects is greater among older African American women, a smaller proportion of such women than of white women would gain a net beneficial effect from tamoxifen chemoprevention.
Benefits and risks associated with tamoxifen use for breast cancer risk reduction. Numbers of breast cancers prevented by tamoxifen in cases per 10 000 women over 5 years by 10-year age group and by level of predicted risk (left) . Numbers of thromboembolic events and endometrial cancers caused by tamoxifen in cases per 10 000 women over 5 years, by ethnicity (right) .
Because women with a history of LCIS or atypical hyperplasia have a high risk of developing invasive breast cancer, women in this group also have the potential for demonstrating a positive benefit–risk ratio from tamoxifen. The high breast cancer risk among women with such a history is evident from the P-1 population, for whom the initial report found a breast cancer incidence rate of 12.99 per 1000 in women with LCIS and 10.11 per 1000 for those with atypical hyperplasia. This rate extrapolates to 5-year observed breast cancer rates of about 6.5% and 5.0%, respectively. Because the incidence of LCIS has more than doubled during the past 25 years, with the peak incidence occurring during menopause ( 54 ) , and because of the frequent occurrence of atypical hyperplasia, a substantial population of women with a history of either of these conditions might be expected to benefit from tamoxifen. Because women with a history of DCIS are at even higher risk for subsequent invasive breast cancer than those with a history of LCIS or atypical hyperplasia ( 10 , 55 ) , they should also be considered for tamoxifen chemoprevention. Findings from the NSABP B-17 and NSABP B-24 trials, which evaluated the management of DCIS, provide additional justification for including women with a history of DCIS within the context of breast cancer prevention ( 55 ) .
Freedman et al. ( 56 ) have used Gail's benefit/risk assessment methodology to determine the total number of women in the United States who would have a positive benefit–risk ratio from chemopreventive therapy with tamoxifen. After considering the distribution of women in the United States by age, race, hysterectomy status, and 5-year breast cancer risk, they determined that approximately 2.5 million women in the age range of 35–70 years could obtain a net benefit from chemopreventive therapy with tamoxifen. This number represents about 25% of the women in that age range who are considered to be at high risk for breast cancer (i.e., women with a 5-year risk of ≥1.66%). The current data from the P-1 trial are in accord with those findings.
Because this article is apt to be the final report of findings from P-1, it is appropriate to conclude it by indicating that, from its inception, we viewed the study not as a trial to evaluate the worth of a drug ( 57 ) but rather as a scientific inquiry aimed at testing the hypothesis that occult pathologic aberrations could be altered so that they fail to become clinically detectable. Positive findings have been obtained from the P-1 trial that have established proof of that principle. The P-1 trial should therefore be viewed as the starting point from which a new paradigm for breast cancer management can evolve. In that regard, a series of new breast cancer prevention trials are currently being conducted in postmenopausal women to evaluate other agents that could be more effective than tamoxifen in decreasing the risk of breast tumors and reducing the frequency of undesirable side effects noted with the drug. One study, NSABP P-2, also known as the STAR trial, has compared tamoxifen with another SERM, raloxifene, an agent that was originally developed to prevent osteoporosis ( 58 ) . Breast cancer incidence was evaluated as a secondary end point in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial that raloxifene reduced the incidence of invasive breast cancer without increasing the risk of endometrial cancer ( 59 ) . IBIS-II ( 24 ) and MAP3 ( 60 ) are comparing the efficacy of the aromatase inhibitors anastrozole and exemestane, respectively, with placebo. Evidence from trials supporting the efficacy of those agents for the treatment of primary breast cancer justifies evaluating them in the prevention setting ( 61 – 64 ) . Until one of these trials demonstrates a greater net benefit from an alternative therapy, tamoxifen remains the only proven chemopreventive treatment for breast cancer risk reduction.
Supported by Public Health Service grants (U10-CA-37377 and U10-CA-69974) from the National Cancer Institute and the Department of Health and Human Services.
We continue to acknowledge the physicians, statisticians, and women who participated in the P-1 study. The names of the institutions, principal investigators, and program coordinators have been listed in the initial report of P-1 ( 10 ) . We thank Tanya Spewock for editorial assistance, Mary Hof for preparation of the manuscript, and Jean Coyle for the graphic representations. Lynne Anderson and Lynn Holman are also acknowledged for their assistance in data management.
References
Furr BJ, Patterson JS, Richardson DN, Slater SR, Wakeling AE. Tamoxifen (review). In: Goldberg ME, editor. Pharmacological and biochemical properties of drug substances. Vol. 2. Washington (DC): American Pharmaceutical Association;
Adam HK. Pharmacokinetic studies with Nolvadex.
Wakeling AE, Valcaccia B, Newboult E, Green LR. Non-steroidal antioestrogens—receptor binding and biological response in rat uterus, rat mammary carcinoma and human breast cancer cells.
Jordan VC, Fritz NF, Tormey DC. Long-term adjuvant therapy with tamoxifen: effects on sex hormone binding globulin and antithrombin III.
Adjuvant tamoxifen in the management of operable breast cancer: the Scottish Trial. Report from the Breast Cancer Trials Committee, Scottish Cancer Trials Office (MRC), Edinburgh.
Fisher B, Costantino J, Redmond C, Poisson R, Bowman D, Couture J, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors.
CRC Adjuvant Breast Trial Working Party. Cyclophosphamide and tamoxifen as adjuvant therapies in the management of breast cancer.
Rutqvist LE, Cedermark B, Glas U, Mattsson A, Skoog L, Somell A, et al. Contralateral primary tumors in breast cancer patients in a randomized trial of adjuvant tamoxifen therapy.
Fisher B, Redmond C. New perspective on cancer of the contralateral breast: a marker for assessing tamoxifen as a preventive agent.
Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study.
Costantino JP, Gail MH, Pee D, Anderson S, Redmond CK, Benichou J, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence.
Fleming TR, Harrington DP, O'Brien PC. Designs for group sequential tests.
Freedman L, Anderson G, Kipnis V, Prentice R, Wang CY, Rossouw J, et al. Approaches to monitoring the results of long-term disease prevention trials: examples from the Women's Health Initiative.
Pepe MS, Mori M. Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data?
Fisher B, Costantino J. Highlights of the NSABP Breast Cancer Prevention Trial.
Pritchard KI. Is tamoxifen effective in prevention of breast cancer?
Powles T, Eeles R, Ashley S, Easton D, Chang J, Dowsett M, et al. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial.
Cuzick J, Powles T, Veronesi U, Forbes J, Edwards R, Ashley S, et al. Overview of the main outcomes in breast-cancer prevention trials.
Veronesi U, Maisonneuve P, Costa A, Sacchini V, Maltoni C, Robertson C, et al. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomised trial among hysterectomised women.
IBIS investigators. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial.
IBIS-II (Prevention) Protocol: An international multi-centre study of anastrozole vs. placebo in postmenopausal women at increased risk of breast cancer. June
Fisher B, Jeong JH, Bryant J, Anderson S, Dignam J, Fisher ER, et al. Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials.
Rossner S, Wallgren A. Serum lipoproteins and proteins after breast cancer surgery and effects of tamoxifen.
Bertelli G, Pronzato P, Amoroso D, Cusimano MP, Conte PF, Montagna G, et al. Adjuvant tamoxifen in primary breast cancer: influence on plasma lipids and antithrombin III levels.
Bruning PF, Bonfrer JM, Hart AA, de Jong-Bakker M, Linders D, van Loon J, et al. Tamoxifen, serum lipoproteins and cardiovascular risk.
Love RR, Newcomb PA, Wiebe DA, Surawicz TS, Jordan VC. Carbone PP, et al. Effects of tamoxifen therapy on lipid and lipoprotein levels in postmenopausal patients with node-negative breast cancer.
Bagdade JD, Wolter J, Subbaiah PV, Ryan W. Effects of tamoxifen treatment on plasma lipids and lipoprotein lipid composition.
Rutqvist LE, Mattsson A. Cardiac and thromboembolic morbidity among postmenopausal women with early-stage breast cancer in a randomized trial of adjuvant tamoxifen. The Stockholm Breast Cancer Study Group.
McDonald CC, Alexander FE, Whyte BW, Forrest AP, Stewart HJ. Cardiac and vascular morbidity in women receiving adjuvant tamoxifen for breast cancer in a randomise trial. The Scottish Cancer Trials Breast Group.
Costantino JP, Kuller LH, Ives DG, Fisher B, Dignam J. Coronary heart disease mortality and adjuvant tamoxifen therapy.
Nordenskjold B, Rosell J, Rutqvist LE, Malmstrom P, Bergh J, Bengtsson NO, et al. The Swedish Breast Cancer Group trial of two versus five years of adjuvant tamoxifen: reduced coronary heart disease death rate in the five year group.
Cushman M, Costantino JP, Tracy RP, Song K, Buckley L, Roberts JD, et al. Tamoxifen and cardiac risk factors in healthy women: suggestion of an anti-inflammatory effect.
MacMahon B. Epidemiologic studies on endometrial, gastrointestinal, and other cancers in humans. Overview of studies on endometrial cancer and other types of cancer in humans: perspectives of an epidemiologist.
Creasman WT. Endometrial cancer: incidence, prognostic factors, diagnosis, and treatment.
Magriples U, Naftolin F, Swartz PE. High-grade endometrial carcinoma in tamoxifen-treated breast cancer patients.
Wickerham DL, Fisher B, Wolmark N, Bryant J, Costantino J, Bernstein L, et al. Association of tamoxifen and uterine sarcoma.
Bushnell CD, Goldstein LB. Risk of ischemic stroke with tamoxifen treatment for breast cancer: a meta-analysis.
Geiger AM, Fischberg GM, Chen W, Bernstein L. Stroke risk and tamoxifen therapy for breast cancer.
Duggan C, Marriott K, Edwards R, Cuzick J. Inherited and acquired risk factors for venous thromboembolic disease among women taking tamoxifen to prevent breast cancer.
Gorin MB, Day R, Costantino JP, Fisher B, Redmond CK, Wickerham L, et al. Long-term tamoxifen citrate use and potential ocular toxicity.
Gorin MB, Costantino JP, Kulacoglu DN, Demirci FY, Wickerham DL, Fisher B, et al. Is tamoxifen a risk factor for retinal vaso-occlusive disease?
Bradbury BD, Lash TL, Kaye JA, Jick SS. Tamoxifen and cataracts: a null association.
Gail MH, Costantino JP, Bryant J, Croyle R, Freedman L, Helzlsouer K, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer.
Taylor AL, Adams-Campbell LL, Wright JT Jr. Risk/benefit assessment of tamoxifen to prevent breast cancer—still a work in progress?
Cuzick J. Side effect with tamoxifen and solutions with aromatase inhibiters.
Bober SL, Hoke LA, Duda RB, Regan MM, Tung NM. Decision-making about tamoxifen in women at high risk for breast cancer: clinical and psychological factors.
Tchou J, Hou N, Rademaker A, Jordan VC, Morrow M. Acceptance of tamoxifen chemoprevention by physicians and women at risk.
Port ER, Montgomery LL, Heerdt AS, Borgen PI. Patient reluctance toward tamoxifen use for breast cancer primary prevention.
Anderson BO, Rinn K, Georgian-Smith D, Lawton T, Li CI, Moe RE. Lobular carcinoma in situ. In: Silverstein MJ, Recht A, Lagios M, editors. Ductal carcinoma in situ of the breast, 2nd ed. New York (NY): Lippincott, Williams & Wilkins;
Fisher B, Land S, Mamounas E, Dignam J, Fisher ER, Wolmark N. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the National Surgical Adjuvant Breast and Bowel Project experience.
Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. Estimates of the number of U.S. women who could benefit from tamoxifen for breast cancer prevention.
Fisher B. National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial: a reflective commentary.
Dunn BK, Ford LG. From adjuvant therapy to breast cancer prevention: BCPT and STAR.
Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial.
Goss PE. Targeting estrogen synthesis: a rational approach to preventing breast cancer. Cancer Prevention: New York-Presbyterian Cancer Centers Prevention Newsletter, Spring
The ATAC (Arimidex, Tamoxifen Alone or in Combination) Trialists' Group. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial.
Love RR, Hutson PR, Havighurst TC, Cleary JF. Endocrine effects of tamoxifen plus exemestane in postmenopausal women with breast cancer.
Carpenter R, Miller WR. Role of aromatase inhibitors in breast cancer.