-
PDF
- Split View
-
Views
-
Cite
Cite
Charles A. Sklar, Ann C. Mertens, Pauline Mitby, John Whitton, Marilyn Stovall, Catherine Kasper, Jean Mulder, Daniel Green, H. Stacy Nicholson, Yutaka Yasui, Leslie L. Robison, Premature Menopause in Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study, JNCI: Journal of the National Cancer Institute, Volume 98, Issue 13, 5 July 2006, Pages 890–896, https://doi.org/10.1093/jnci/djj243
Close - Share Icon Share
Abstract
Background: Childhood cancer survivors who retain ovarian function after completing cancer treatment are at increased risk of developing premature menopause, defined as cessation of menses before age 40 years. However, published data pertaining to the risk and frequency of premature menopause are limited. Methods: We assessed the incidence of and risk factors for premature menopause in 2819 survivors of childhood cancer who were older than 18 years and were participants in the multicenter Childhood Cancer Survivor Study (CCSS). The comparison group was 1065 female siblings of participants in the CCSS. A multiple Poisson regression model was constructed to determine risk factors for nonsurgical premature menopause. All statistical tests were two-sided. Results: A total of 126 childhood cancer survivors and 33 control siblings developed premature menopause. Of these women, 61 survivors (48%) and 31 siblings (94%) had surgically induced menopause (rate ratio [RR] = 0.8, 95% confidence interval [CI] = 0.52 to 1.23). However, the cumulative incidence of nonsurgical premature menopause was higher for survivors than for siblings (8% versus 0.8%; RR = 13.21, 95% CI = 3.26 to 53.51; P <.001). A multiple Poisson regression model showed that risk factors for nonsurgical premature menopause included attained age, exposure to increasing doses of radiation to the ovaries, increasing alkylating agent score (based on number of alkylating agents and cumulative dose), and a diagnosis of Hodgkin lymphoma. For survivors who were treated with alkylating agents plus abdominopelvic radiation, the cumulative incidence of nonsurgical premature menopause approached 30%. Conclusions: The results of this study will facilitate counseling current survivors about their future risk of premature menopause and aid in designing new regimens that seek to diminish late ovarian toxicity.
Advances in treating childhood cancer have resulted in markedly improved survival rates; currently, more than 70% of children and adolescents diagnosed with cancer can expect to be long-term survivors ( 1 ) . However, with these advancements, survivors now face the consequences of exposure to intensive multimodality therapies. Both males and females who are treated with chemotherapy, surgery, and/or radiation can potentially suffer the loss of reproductive function. Acute ovarian failure or the loss of ovarian function during or shortly after completing cancer therapy is known to occur in a minority of females diagnosed with childhood cancer, primarily those who are exposed to high-dose pelvic irradiation and/or intensive alkylating agent–based regimens that are used for cytoreduction for stem cell transplantation ( 2 – 9 ) .
Nevertheless, data from a variety of sources, including autopsy studies of subjects who died in the peritreatment period ( 10 , 11 ) and ultrasound evaluations performed years after completing cancer therapy ( 12 ) , reveal that survivors of childhood cancer, including those who were still menstruating, have fewer primordial and antral follicles than age-matched control subjects. Because menopause appears to occur once the number of primordial follicles declines below a certain threshold ( 13 ) , one might predict that oocyte depletion due to cancer therapy would result in an earlier timing of menopause, at least for a subset of survivors of childhood and adolescent cancers.
Premature menopause leads to the early and often unexpected loss of reproductive potential as well as the cessation of ovarian sex hormone production. Thus, survivors who experience premature menopause are at increased risk of developing a variety of adverse health outcomes, including osteoporosis ( 14 , 15 ) , death from cardiovascular diseases ( 16 ) , and psychosexual dysfunction ( 17 , 18 ) compared with women who do not undergo premature menopause.
To provide young adult female survivors with reliable information about their risk of developing a premature menopause—information that would facilitate family planning and timing of future pregnancies—precise risk estimates are needed. However, current data on the incidence of premature menopause and on the patient and treatment factors associated with the development of premature menopause in survivors of childhood cancer are limited ( 19 , 20 ) . Furthermore, published studies suffer from several limitations, including small sample size, lack of detailed information on treatment exposures, failure to exclude individuals with a probable central cause for cessation of menses (e.g., gonadotropin deficiency), and failure to separate surgical from nonsurgical cases of premature menopause ( 19 , 20 ) .
In this study, we assessed menopausal status in a large and well-characterized cohort of childhood cancer survivors who continued to menstruate more than 5 years after their cancer diagnosis. A unique feature of this cohort is the availability of extensive treatment data ( 21 ) , including cumulative doses of key chemotherapeutic agents and detailed information on radiation dose and treatment field, which has allowed us to determine, more precisely than previous studies have done, the risk factors associated with premature menopause.
S UBJECTS AND M ETHODS
Childhood Cancer Survivor Study
The cohort of subjects assessed here is a subset of participants in the Childhood Cancer Survivor Study (CCSS), also known to study participants as the Long-term Follow-up Study. The methods, objectives, eligibility criteria, and characteristics of study participants of the CCSS have been published previously ( 21 ) . In brief, the CCSS is an ongoing multi-institutional epidemiologic follow-up study of 5-year survivors of childhood cancer who were diagnosed before age 21 years, between January 1, 1970, and December 31, 1986. The primary aim of the study is to determine late adverse outcomes that follow treatment for childhood and adolescent cancer. All CCSS studies, questionnaires, and documents have been approved by the internal review board at the University of Minnesota (the study coordinating center) and each of the participating centers. Each participant (or his/her proxy if the patient was younger than 18 years at interview or if they died after achieving 5-year survivorship but before being interviewed), provided written informed consent for the study and separate consent to allow release and use of medical records, including treatment records.
Each participant was required to complete an extensive baseline questionnaire (complete questionnaire is available at http://www.cancer.umn.edu/ccss ). A proxy completed the baseline questionnaire if the participant was younger than 18 years at study entry or was deceased after achieving 5-year survivorship but before study entry. Also, detailed medical information was taken from the medical record of each participant. Data collected included all treatments for the primary cancer diagnosis, including the initial treatment, treatment for any relapse, and preparatory regimens for bone marrow transplant. Information about cancer treatment included qualitative data on 42 chemotherapeutic agents, quantitative data on 22 of these agents, surgeries performed from the time of initial diagnosis, and quantitative data on radiation field, size, site, and dose.
Premature Menopause Study
Of the 14 372 participants in the CCSS, 6079 females were older than 18 years and known to be alive as of November 30, 2000. Of those 6079 survivors, 4620 (76%) completed a follow-up questionnaire (complete follow-up questionnaire is available at http://www.cancer.umn.edu/ccss ) during 2000 and 2001. This questionnaire requested information on age at menarche, current menstrual status, age at last menstrual period, and, for those who were currently menopausal, the etiology of menopause, i.e., surgical versus nonsurgical. Subjects were considered to be menopausal if they had not experienced a spontaneous menses for at least 6 months and other causes, e.g., pregnancy; use of agents such as injectable progesterone and gonadotropin-releasing hormone analogs had been excluded ( 22 , 23 ) . Subjects who indicated that they had undergone menopause after surgery were asked to provide more information about the specific surgical procedure that had been performed. However, the quality of the data that was provided did not allow us to determine the precise type of surgical procedure performed (i.e., hysterectomy, oophorectomy, hysterectomy plus oophorectomy) in most survivors with a surgical menopause.
From among these 4620 subjects, we excluded subjects who met any of the following criteria: had a diagnosis associated with ovarian dysfunction (n = 7), e.g., Turner syndrome; never had a spontaneous menses or ceased spontaneous menstruation within the first 5 years following the initial cancer diagnosis (n = 321); i.e., individuals with acute ovarian failure; received more than 30 Gy of radiation to the brain and/or had a primary tumor in the region of the hypothalamus–pituitary gland (n = 592); questionnaire completed by someone other than the participant (n = 712); developed a second malignancy before the onset of menopause (n = 391); or radiation treatment data incomplete or not available (n = 349). A total of 1801 patients were excluded; some subjects met multiple exclusion criteria. The incidence of acute ovarian failure in the CCSS is 6% ( 9 ) .
Therefore, a total of 2819 subjects were deemed eligible for this study. We used the sibling cohort as a comparison group. The sibling cohort included 1065 siblings of a subset of all the CCSS case patients, including those who were not included in the cohort for the premature menopause study. All participants in the sibling cohort were older than 18 years and had undergone menarche spontaneously.
Radiation Dosimetry
Radiation dose to the ovaries and pituitary was quantified by a radiation dosimetrist (C. Kasper) who evaluated radiation therapy records collected by all participating centers. Complete records included photographs of patients in treatment position or diagrams of treatment fields, beam energy, field size, beam blocking information, and daily treatment doses. When diagrams were not available, a written description of the treatment from the radiation therapy record or medical record was used to estimate the extent of the treatment and the dose administered. For treatments very near the sites of anatomic interest, records were reviewed to determine oophoropexy status, special gonadal shielding, beam shaping blocks, and field location. Doses from all treatment fields were summed and included the contribution of primary and scatter radiation ( 24 ) . If the surgical notes indicated an oophoropexy, the estimated radiation dose to the ovary was reduced to approximately 10% of the in-beam dose. Doses to right and left ovaries were estimated separately.
Chemotherapy
Seven broad classes of chemotherapeutic drugs were identified from treatment records: alkylating agents, alkaloids, platinum-containing agents, antimetabolites, topoisomerase inhibitors, antibiotics, and steroids. The total exposure to alkylating agents was measured by calculating an alkylating agent score that took into account both the number of alkylating agents and the cumulative doses of each drug administered. For each alkylating agent, the total dose per square meter of body surface area was calculated for each subject. A distribution of the doses received by all subjects was determined for each alkylating agent. Each subject was assigned a score of 0, 1, 2, or 3 for each drug, according to whether the individual received no drug or fell into the lower, middle, or upper tertiles of dose distribution of that drug. Individual scores were summed and each subject was assigned an overall alkylating agent score of 0, 1, 2, or 3, according to where the individual fell in the overall distribution ( 25 , 26 ) . Exposures to individual alkylating agents, e.g., cyclophosphamide and nitrogen mustard, were considered as separate dichotomous variables (yes or no).
Statistical Analysis
Descriptive, univariate statistics with t test and chi-square test were used to evaluate the relationship between premature menopause status and selected demographic and disease variables. The number of person-years at risk was determined as the time between menarche and the most recent menstrual period; women with surgically induced menopause were censored in the analysis of nonsurgical premature menopause. The person-years data were analyzed using Poisson regression ( 27 ) , which approximates Cox regression with a piecewise exponential baseline hazard to estimate the relative rate of having nonsurgical premature menopause. The standard large-sample inference for Poisson regression was used. In comparisons of case patients and siblings, generalized estimating equations models ( 28 ) with robust-inference procedures were used to adjust for the nonindependence of the two populations, which was caused by some case patients and siblings being members of the same family. Cumulative incidence curves were also drawn to illustrate the occurrence of premature menopause by age in the two groups ( 29 ) .
The multiple Poisson regression model of treatment and other effects on the development of nonsurgical premature menopause in survivors was constructed by selecting and deleting candidate variables, using the Akaike information criterion ( 30 ) to determine the effectiveness of adding or removing each term. The final model was the one with the highest Akaike information criterion. All statistical tests were two-sided, and P <.05 was considered as statistically significant.
R ESULTS
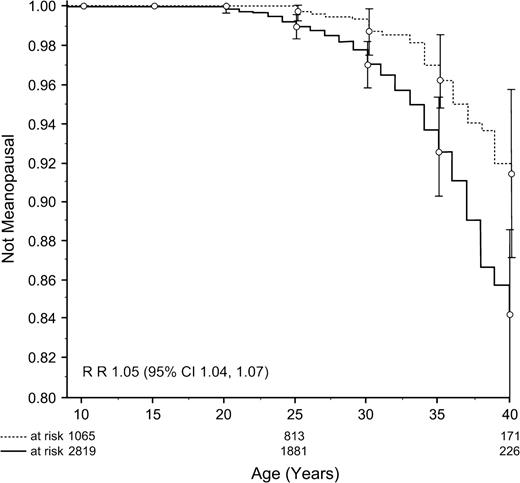
A total of 2819 subjects were included in the analyses ( Table 1 ). Median age at cancer diagnosis was 7 years (range = 0–20), and median age at study was 29 years (range = 18–50); 69% of survivors had reached age 25 years, 47%, age 30 years; 26%, age 35 years; and 10%, age 40 years; 8% were older than 40 years. A total of 126 survivors and 33 siblings had ceased menstruating before age 40 years and were considered to have developed premature menopause. The cumulative incidence of premature menopause in survivors was 15%; rate ratio (RR) = 1.05 (95% confidence interval [CI] = 1.04 to 1.07, P <.001) compared with siblings ( Fig. 1 ).
Cumulative incidence curves of premature menopause in survivors ( solid line ) compared with siblings ( broken line ). Vertical bars indicate 95% confidence intervals.
Characteristics of patients in the CCSS cohort included in this study *
| Characteristic . | Value . |
|---|---|
| Total No. | 2819 |
| Median age at diagnosis (range), y | 7 (0–20) |
| Median age at study (range), y | 29 (18–50) |
| Diagnosis, n (%) | |
| Leukemia | 1025 (36) |
| Hodgkin lymphoma | 404 (14) |
| Bone tumors | 324 (11) |
| Kidney tumors | 297 (11) |
| Sarcomas | 271 (10) |
| Neuroblastoma | 207 (7) |
| Non-Hodgkin lymphoma | 154 (5) |
| Brain tumors | 137 (5) |
| Treatment, n (%) | |
| Surgery only | 287 (10) |
| Chemotherapy only | 287 (10) |
| Radiation only | 2 (<1) |
| Chemotherapy + radiation | 487 (17) |
| Surgery + chemotherapy | 573 (20) |
| Surgery + radiation | 238 (8) |
| Surgery + chemotherapy + radiation | 942 (33) |
| Stem cell transplant | 32 (1) |
| Characteristic . | Value . |
|---|---|
| Total No. | 2819 |
| Median age at diagnosis (range), y | 7 (0–20) |
| Median age at study (range), y | 29 (18–50) |
| Diagnosis, n (%) | |
| Leukemia | 1025 (36) |
| Hodgkin lymphoma | 404 (14) |
| Bone tumors | 324 (11) |
| Kidney tumors | 297 (11) |
| Sarcomas | 271 (10) |
| Neuroblastoma | 207 (7) |
| Non-Hodgkin lymphoma | 154 (5) |
| Brain tumors | 137 (5) |
| Treatment, n (%) | |
| Surgery only | 287 (10) |
| Chemotherapy only | 287 (10) |
| Radiation only | 2 (<1) |
| Chemotherapy + radiation | 487 (17) |
| Surgery + chemotherapy | 573 (20) |
| Surgery + radiation | 238 (8) |
| Surgery + chemotherapy + radiation | 942 (33) |
| Stem cell transplant | 32 (1) |
Female survivors of childhood cancer older than 18 years who continued to menstruate for at least 5 years after their cancer diagnosis and were exposed to less than 30 Gy of cranial irradiation.
Characteristics of patients in the CCSS cohort included in this study *
| Characteristic . | Value . |
|---|---|
| Total No. | 2819 |
| Median age at diagnosis (range), y | 7 (0–20) |
| Median age at study (range), y | 29 (18–50) |
| Diagnosis, n (%) | |
| Leukemia | 1025 (36) |
| Hodgkin lymphoma | 404 (14) |
| Bone tumors | 324 (11) |
| Kidney tumors | 297 (11) |
| Sarcomas | 271 (10) |
| Neuroblastoma | 207 (7) |
| Non-Hodgkin lymphoma | 154 (5) |
| Brain tumors | 137 (5) |
| Treatment, n (%) | |
| Surgery only | 287 (10) |
| Chemotherapy only | 287 (10) |
| Radiation only | 2 (<1) |
| Chemotherapy + radiation | 487 (17) |
| Surgery + chemotherapy | 573 (20) |
| Surgery + radiation | 238 (8) |
| Surgery + chemotherapy + radiation | 942 (33) |
| Stem cell transplant | 32 (1) |
| Characteristic . | Value . |
|---|---|
| Total No. | 2819 |
| Median age at diagnosis (range), y | 7 (0–20) |
| Median age at study (range), y | 29 (18–50) |
| Diagnosis, n (%) | |
| Leukemia | 1025 (36) |
| Hodgkin lymphoma | 404 (14) |
| Bone tumors | 324 (11) |
| Kidney tumors | 297 (11) |
| Sarcomas | 271 (10) |
| Neuroblastoma | 207 (7) |
| Non-Hodgkin lymphoma | 154 (5) |
| Brain tumors | 137 (5) |
| Treatment, n (%) | |
| Surgery only | 287 (10) |
| Chemotherapy only | 287 (10) |
| Radiation only | 2 (<1) |
| Chemotherapy + radiation | 487 (17) |
| Surgery + chemotherapy | 573 (20) |
| Surgery + radiation | 238 (8) |
| Surgery + chemotherapy + radiation | 942 (33) |
| Stem cell transplant | 32 (1) |
Female survivors of childhood cancer older than 18 years who continued to menstruate for at least 5 years after their cancer diagnosis and were exposed to less than 30 Gy of cranial irradiation.
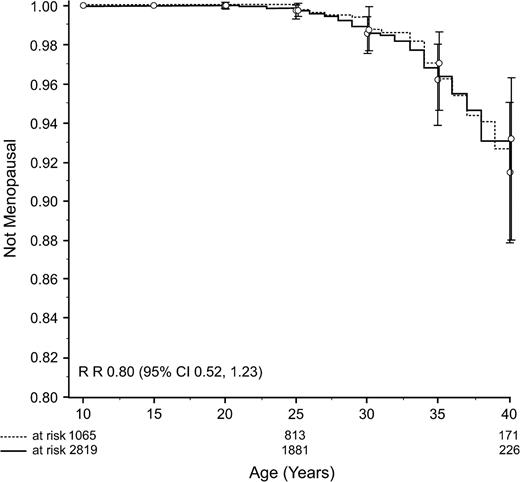
For 61 (48%) of 126 survivors and 31 (94%) of 33 siblings with premature menopause, the menopause had been surgically induced. The incidence of surgically induced premature menopause was similar among survivors and sibling control subjects ( Fig. 2 ). The rate ratio of a surgical menopause was 0.80 (95% CI = 0.52 to 1.23; P = .31) for survivors compared with sibling control subjects.
Cumulative incidence curves of surgically induced premature menopause in survivors ( solid line ) compared with siblings ( broken line ). Vertical bars indicate 95% confidence intervals.
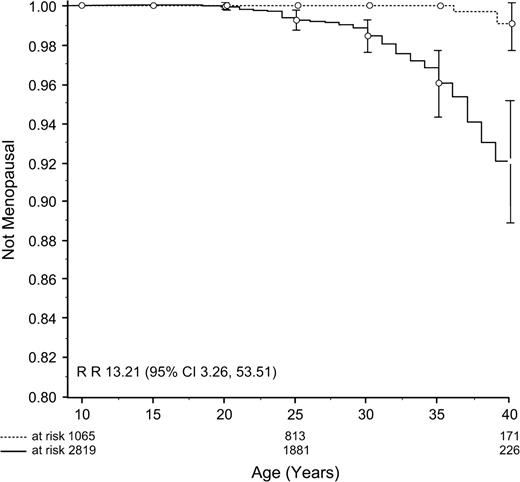
In contrast to the finding for surgical menopause, the incidence of nonsurgical premature menopause was higher among survivors than control siblings (8% versus 0.8% as determined by cumulative incidence curves; Fig. 3 ). For survivors, the rate ratio of nonsurgical premature menopause was 13.21 (95% CI = 3.26 to 53.51; P <.001) compared with control siblings. Survivors with nonsurgical premature menopause were older at diagnosis and at follow-up than survivors who did not develop nonsurgical premature menopause; they were also less likely to have been diagnosed with leukemia, were more likely to have been diagnosed with Hodgkin lymphoma, and were more likely to have been exposed to alkylating agents and abdominopelvic irradiation ( Table 2 ).
Cumulative incidence curves of nonsurgical premature menopause in survivors ( solid line ) compared with siblings ( broken line ). Vertical bars indicate 95% confidence intervals.
Characteristics of survivors with and without nonsurgical premature menopause (NSPM)
| Variable . | NSPM (n = 65) . | No NSPM (n = 2754) . | P* . |
|---|---|---|---|
| Mean age at diagnosis, y (range) | 12.9 (0–20) | 8.2 (0–20) | <.001 |
| Mean age at menarche, y (range) | 13.1 (9–20) | 12.4 (6–23) | .02 |
| Mean age at study, y (range) | 36.8 (21–48) | 29.3 (18–50) | <.001 |
| Diagnosis, n (%) | <.001 | ||
| Leukemia | 9 (14) | 1016 (37) | |
| Hodgkin lymphoma | 38 (56) | 366 (13) | |
| Non-Hodgkin lymphoma | 2 (3) | 152 (6) | |
| Bone tumors | 1 (2) | 323 (12) | |
| Kidney tumors | 7 (10) | 290 (11) | |
| Sarcomas | 6 (9) | 265 (10) | |
| Neuroblastoma | 2 (3) | 205 (7) | |
| Brain tumors | 0 (0) | 137 (5) | |
| Treatment, n (%) | |||
| Alkylating agents | 49 (75) | 1310 (48) | <.001 |
| Alkylating agent score † | |||
| 0 | 16 (29) | 1440 (55) | |
| 1 | 7 (13) | 532 (20) | |
| 2 | 9 (16) | 364 (14) | |
| 3 | 23 (42) | 270 (10) | |
| Abdominopelvic RT ‡ | 29 (44) | 483 (18) | <.001 |
| Radiation to ovaries, cGy | |||
| No RT | 9 (15) | 1072 (43) | |
| 1–99 | 32 (53) | 1108 (44) | |
| 100–999 | 11 (18) | 247 (10) | |
| ≥1000 | 8 (13) | 66 (3) | |
| Stem cell transplant | 2 (3) | 30 (1) | .1 |
| Variable . | NSPM (n = 65) . | No NSPM (n = 2754) . | P* . |
|---|---|---|---|
| Mean age at diagnosis, y (range) | 12.9 (0–20) | 8.2 (0–20) | <.001 |
| Mean age at menarche, y (range) | 13.1 (9–20) | 12.4 (6–23) | .02 |
| Mean age at study, y (range) | 36.8 (21–48) | 29.3 (18–50) | <.001 |
| Diagnosis, n (%) | <.001 | ||
| Leukemia | 9 (14) | 1016 (37) | |
| Hodgkin lymphoma | 38 (56) | 366 (13) | |
| Non-Hodgkin lymphoma | 2 (3) | 152 (6) | |
| Bone tumors | 1 (2) | 323 (12) | |
| Kidney tumors | 7 (10) | 290 (11) | |
| Sarcomas | 6 (9) | 265 (10) | |
| Neuroblastoma | 2 (3) | 205 (7) | |
| Brain tumors | 0 (0) | 137 (5) | |
| Treatment, n (%) | |||
| Alkylating agents | 49 (75) | 1310 (48) | <.001 |
| Alkylating agent score † | |||
| 0 | 16 (29) | 1440 (55) | |
| 1 | 7 (13) | 532 (20) | |
| 2 | 9 (16) | 364 (14) | |
| 3 | 23 (42) | 270 (10) | |
| Abdominopelvic RT ‡ | 29 (44) | 483 (18) | <.001 |
| Radiation to ovaries, cGy | |||
| No RT | 9 (15) | 1072 (43) | |
| 1–99 | 32 (53) | 1108 (44) | |
| 100–999 | 11 (18) | 247 (10) | |
| ≥1000 | 8 (13) | 66 (3) | |
| Stem cell transplant | 2 (3) | 30 (1) | .1 |
Two-sided t test and chi-square test.
Alkylating agent score and radiation dose to ovaries were calculated only when the treatment records were complete and of sufficient quality to permit an accurate assessment of exposure.
RT = radiation therapy.
Characteristics of survivors with and without nonsurgical premature menopause (NSPM)
| Variable . | NSPM (n = 65) . | No NSPM (n = 2754) . | P* . |
|---|---|---|---|
| Mean age at diagnosis, y (range) | 12.9 (0–20) | 8.2 (0–20) | <.001 |
| Mean age at menarche, y (range) | 13.1 (9–20) | 12.4 (6–23) | .02 |
| Mean age at study, y (range) | 36.8 (21–48) | 29.3 (18–50) | <.001 |
| Diagnosis, n (%) | <.001 | ||
| Leukemia | 9 (14) | 1016 (37) | |
| Hodgkin lymphoma | 38 (56) | 366 (13) | |
| Non-Hodgkin lymphoma | 2 (3) | 152 (6) | |
| Bone tumors | 1 (2) | 323 (12) | |
| Kidney tumors | 7 (10) | 290 (11) | |
| Sarcomas | 6 (9) | 265 (10) | |
| Neuroblastoma | 2 (3) | 205 (7) | |
| Brain tumors | 0 (0) | 137 (5) | |
| Treatment, n (%) | |||
| Alkylating agents | 49 (75) | 1310 (48) | <.001 |
| Alkylating agent score † | |||
| 0 | 16 (29) | 1440 (55) | |
| 1 | 7 (13) | 532 (20) | |
| 2 | 9 (16) | 364 (14) | |
| 3 | 23 (42) | 270 (10) | |
| Abdominopelvic RT ‡ | 29 (44) | 483 (18) | <.001 |
| Radiation to ovaries, cGy | |||
| No RT | 9 (15) | 1072 (43) | |
| 1–99 | 32 (53) | 1108 (44) | |
| 100–999 | 11 (18) | 247 (10) | |
| ≥1000 | 8 (13) | 66 (3) | |
| Stem cell transplant | 2 (3) | 30 (1) | .1 |
| Variable . | NSPM (n = 65) . | No NSPM (n = 2754) . | P* . |
|---|---|---|---|
| Mean age at diagnosis, y (range) | 12.9 (0–20) | 8.2 (0–20) | <.001 |
| Mean age at menarche, y (range) | 13.1 (9–20) | 12.4 (6–23) | .02 |
| Mean age at study, y (range) | 36.8 (21–48) | 29.3 (18–50) | <.001 |
| Diagnosis, n (%) | <.001 | ||
| Leukemia | 9 (14) | 1016 (37) | |
| Hodgkin lymphoma | 38 (56) | 366 (13) | |
| Non-Hodgkin lymphoma | 2 (3) | 152 (6) | |
| Bone tumors | 1 (2) | 323 (12) | |
| Kidney tumors | 7 (10) | 290 (11) | |
| Sarcomas | 6 (9) | 265 (10) | |
| Neuroblastoma | 2 (3) | 205 (7) | |
| Brain tumors | 0 (0) | 137 (5) | |
| Treatment, n (%) | |||
| Alkylating agents | 49 (75) | 1310 (48) | <.001 |
| Alkylating agent score † | |||
| 0 | 16 (29) | 1440 (55) | |
| 1 | 7 (13) | 532 (20) | |
| 2 | 9 (16) | 364 (14) | |
| 3 | 23 (42) | 270 (10) | |
| Abdominopelvic RT ‡ | 29 (44) | 483 (18) | <.001 |
| Radiation to ovaries, cGy | |||
| No RT | 9 (15) | 1072 (43) | |
| 1–99 | 32 (53) | 1108 (44) | |
| 100–999 | 11 (18) | 247 (10) | |
| ≥1000 | 8 (13) | 66 (3) | |
| Stem cell transplant | 2 (3) | 30 (1) | .1 |
Two-sided t test and chi-square test.
Alkylating agent score and radiation dose to ovaries were calculated only when the treatment records were complete and of sufficient quality to permit an accurate assessment of exposure.
RT = radiation therapy.
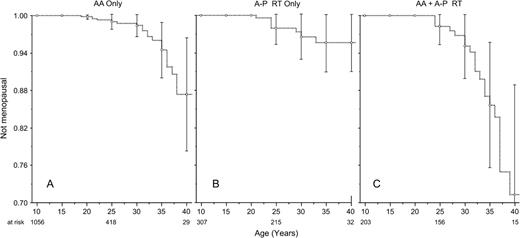
A multiple Poisson regression model indicated the following risk factors for nonsurgical premature menopause: attained age, radiation dose to the ovary (minimum and maximum), alkylating agent score, and a diagnosis of Hodgkin lymphoma ( Table 3 ). There was an interaction between a diagnosis of Hodgkin lymphoma and radiation dose to the ovary. Among those who received no radiation to the ovaries, survivors of Hodgkin lymphoma had a rate of nonsurgical premature menopause that was 9.18-fold higher than that of survivors with other types of childhood cancer. Although exposure to higher doses of ovarian irradiation was associated with an increased risk of nonsurgical premature menopause, the risk was more pronounced for survivors of cancers other than Hodgkin lymphoma ( Table 3 ). For survivors treated with alkylating agents plus abdominopelvic radiation, the cumulative incidence of nonsurgical premature menopause approached 30% ( Fig. 4 ).
Cumulative incidence curves of nonsurgical premature menopause in survivors according to treatment exposures. A ) Survivors who were treated with alkylating agents (AA) but not with abdominopelvic radiation. B ) Survivors who were treated with abdominopelvic (A-P) radiation (RT) but not alkylating agents. C ) Survivors who were treated with alkylating agents (AA) and abdominopelvic (A-P) radiation (RT). Vertical bars indicate 95% confidence intervals.
Multiple Poisson regression model for risk of nonsurgical premature menopause among survivors of Hodgkin lymphoma (HL) and other chilhood cancers *
| Variable . | RR (95% CI) . | P . |
|---|---|---|
| Attained age | 1.15 (1.09 to 1.21) | <.001 |
| Other, † minimum ovarian RT, cGy | ||
| No RT | 1.00 (Referent) | .03 |
| 1–99 | 4.30 (1.20 to 15.47) | .04 |
| 100–999 | 5.70 (1.12 to 28.99) | <.001 |
| ≥1000 | 109.59 (28.15 to 426.70) | |
| HL, minimum ovarian RT, cGy | ||
| No RT | 9.18 (1.52 to 55.24) | .02 |
| 1–99 | 12.26 (3.41 to 44.14) | <.001 |
| 100–999 | 11.41 (2.75 to 47.26) | <.001 |
| ≥1000 | 6.74 (0.63 to 71.74) | .11 |
| Alkylating agent score | ||
| 0 | 1.00 (Referent) | |
| 1–2 | 2.30 (1.08 to 4.90) | .03 |
| 3 | 5.78 (2.90 to 11.55) | <.001 |
| Variable . | RR (95% CI) . | P . |
|---|---|---|
| Attained age | 1.15 (1.09 to 1.21) | <.001 |
| Other, † minimum ovarian RT, cGy | ||
| No RT | 1.00 (Referent) | .03 |
| 1–99 | 4.30 (1.20 to 15.47) | .04 |
| 100–999 | 5.70 (1.12 to 28.99) | <.001 |
| ≥1000 | 109.59 (28.15 to 426.70) | |
| HL, minimum ovarian RT, cGy | ||
| No RT | 9.18 (1.52 to 55.24) | .02 |
| 1–99 | 12.26 (3.41 to 44.14) | <.001 |
| 100–999 | 11.41 (2.75 to 47.26) | <.001 |
| ≥1000 | 6.74 (0.63 to 71.74) | .11 |
| Alkylating agent score | ||
| 0 | 1.00 (Referent) | |
| 1–2 | 2.30 (1.08 to 4.90) | .03 |
| 3 | 5.78 (2.90 to 11.55) | <.001 |
P values calculated with multiple Poisson regression likelihood ratio test (two-sided). RR = rate ratio; CI = confidence interval; RT = radiation therapy.
Other = diagnoses other than Hodgkin lymphoma.
Multiple Poisson regression model for risk of nonsurgical premature menopause among survivors of Hodgkin lymphoma (HL) and other chilhood cancers *
| Variable . | RR (95% CI) . | P . |
|---|---|---|
| Attained age | 1.15 (1.09 to 1.21) | <.001 |
| Other, † minimum ovarian RT, cGy | ||
| No RT | 1.00 (Referent) | .03 |
| 1–99 | 4.30 (1.20 to 15.47) | .04 |
| 100–999 | 5.70 (1.12 to 28.99) | <.001 |
| ≥1000 | 109.59 (28.15 to 426.70) | |
| HL, minimum ovarian RT, cGy | ||
| No RT | 9.18 (1.52 to 55.24) | .02 |
| 1–99 | 12.26 (3.41 to 44.14) | <.001 |
| 100–999 | 11.41 (2.75 to 47.26) | <.001 |
| ≥1000 | 6.74 (0.63 to 71.74) | .11 |
| Alkylating agent score | ||
| 0 | 1.00 (Referent) | |
| 1–2 | 2.30 (1.08 to 4.90) | .03 |
| 3 | 5.78 (2.90 to 11.55) | <.001 |
| Variable . | RR (95% CI) . | P . |
|---|---|---|
| Attained age | 1.15 (1.09 to 1.21) | <.001 |
| Other, † minimum ovarian RT, cGy | ||
| No RT | 1.00 (Referent) | .03 |
| 1–99 | 4.30 (1.20 to 15.47) | .04 |
| 100–999 | 5.70 (1.12 to 28.99) | <.001 |
| ≥1000 | 109.59 (28.15 to 426.70) | |
| HL, minimum ovarian RT, cGy | ||
| No RT | 9.18 (1.52 to 55.24) | .02 |
| 1–99 | 12.26 (3.41 to 44.14) | <.001 |
| 100–999 | 11.41 (2.75 to 47.26) | <.001 |
| ≥1000 | 6.74 (0.63 to 71.74) | .11 |
| Alkylating agent score | ||
| 0 | 1.00 (Referent) | |
| 1–2 | 2.30 (1.08 to 4.90) | .03 |
| 3 | 5.78 (2.90 to 11.55) | <.001 |
P values calculated with multiple Poisson regression likelihood ratio test (two-sided). RR = rate ratio; CI = confidence interval; RT = radiation therapy.
Other = diagnoses other than Hodgkin lymphoma.
Among all nonmenopausal women, 543 (20%) survivors and 262 (24%) siblings were currently taking oral contraceptives. Because menopausal status is difficult to assess in individuals receiving oral contraceptives, we reanalyzed the data after excluding women in this category. The results after exclusion were nearly identical to those of the entire cohort. The cumulative incidence of nonsurgical premature menopause remained at approximately 8% and the rate ratio of a survivor developing nonsurgical premature menopause was 12.82 (95% CI = 3.17 to 51.87; P <.001) compared with siblings. Moreover, the multiple Poisson regression model was unchanged.
D ISCUSSION
The results of this study indicate that for childhood cancer survivors who continued to have spontaneous menses more than 5 years after their cancer diagnosis, the risk of developing nonsurgical premature menopause was 13-fold higher than that of siblings, with a cumulative incidence of 8% by age 40 years. The risk factors we identified for nonsurgical premature menopause, i.e., attained age, a diagnosis of Hodgkin lymphoma, and exposure to increasing doses of both alkylating agents and radiation to the ovaries, are in broad agreement with the findings from previous studies ( 19 , 20 ) . This study is the first, to our knowledge, to incorporate detailed information on therapeutic exposures, including estimates of radiation doses to the ovaries.
Our data indicate that, of all treatment exposures, radiation to the ovary is associated with the greatest risk of nonsurgical premature menopause. Although the risk of nonsurgical premature menopause was most elevated among survivors exposed to the highest doses of ovarian irradiation (≥1000 cGy), exposure to doses as low as 1–99 cGy were associated with an increased risk of nonsurgical premature menopause compared with that in survivors who received no radiation. This finding is consistent with a large body of literature indicating that the ovaries are susceptible to damage from external beam radiation, in a dose-dependent manner ( 3 , 4 , 31 – 33 ) . In this study, however, the association between ovarian irradiation and risk of nonsurgical premature menopause was less apparent in survivors of Hodgkin lymphoma than in survivors of other cancer types. The most likely explanation for this finding is that the number of Hodgkin disease survivors treated at each dose level of ovarian radiation was small (e.g., 100–999 cGy, n = 6; ≥1000 cGy, n = 1), limiting our ability to assess a dose effect.
Treatment with alkylating agents was also associated with an increased risk of nonsurgical premature menopause. In a previous study, Chiarelli et al. ( 20 ) noted a statistically significantly increased risk for premature menopause only for survivors treated with the greatest quantities of alkylating agents. In this study, we observed that the risk for nonsurgical premature menopause was increased at all levels of exposure. Furthermore, the risk increased with exposure to increasing quantities of alkylating agents. For survivors treated with both alkylating agents and abdominopelvic radiation, the cumulative risk of nonsurgical premature menopause approached 30%.
We found an increased risk of nonsurgical premature menopause in survivors with a diagnosis of Hodgkin lymphoma compared with survivors with other cancer diagnoses. This risk persisted after controlling for age at diagnosis, attained age, and exposure to ovarian irradiation and alkylating agents. Even though our assessment of radiation and alkylating agent exposure was detailed and comprehensive, we cannot rule out the possibility that the increased risk of nonsurgical premature menopause noted in survivors of Hodgkin lymphoma is the result of residual confounding. In future studies it will be important to evaluate if Hodgkin lymphoma confers an independent risk of nonsurgical premature menopause.
Although we found that the risk of nonsurgical premature menopause was associated with attained age, we found no association with age at diagnosis after adjusting for attained age. Thus, our findings differ from those reported from the Five-Center Study, in which older age at diagnosis was associated with an increased risk of premature menopause ( 19 ) . Our results should be viewed in light of the large size of the CCSS cohort and the fact that our study included a substantially larger number of nonsurgical premature menopause outcomes among survivors diagnosed at younger ages. This fact, along with the fact that eligibility into the CCSS cohort was not restricted on the basis of attained age, likely provided us with a better opportunity than in previous studies to evaluate the association between age at diagnosis and risk of nonsurgical premature menopause.
In nearly 50% of the childhood cancer survivors with premature menopause, the menopause was surgically induced, in agreement with the data of Chiarelli et al. ( 20 ) . The cumulative incidence of surgically induced premature menopause was nearly identical between survivors and sibling control subjects. Assuming that, in most cases of surgical menopause in our study, the surgery performed included a hysterectomy, the 8% cumulative incidence of surgical menopause by age 40 years observed in our study is consistent with the approximately 20% lifetime prevalence of self-reported hysterectomy that was observed in a population-based sample ( 34 ) .
When interpreting the results of this study, certain limitations should be taken into account. We relied on self-report to determine menopausal status, without confirmation from medical records or hormonal assays. Thus, some misclassification could have occurred, particularly for women with surgically induced menopause. Both the relatively young age of our cohort and the fact that a sizable percentage of participants who were classified as “not menopausal” were taking oral contraceptives likely resulted in an underestimate of the incidence of nonsurgical premature menopause among study participants. Also, some patients received rapidly changing doses of radiation therapy near their ovaries, where a few millimeters in either direction could result in a sizable change in the dose estimation. Nevertheless, this difference is unlikely to have resulted in a systematic over- or underestimation of ovarian radiation dose. Finally, data for this study are derived from treatments that were used from 1970 to 1986. Given that therapies may have changed over time, results of this study may not be strictly applicable to individuals who have been treated on more recent protocols.
This study, using the unique resource of the CCSS, provides new information regarding the incidence and risk factors for nonsurgical premature menopause. Because of the size of the study population and the detailed characterization of treatment-specific exposures, we can provide more precise estimates of risk than previous studies.
In conclusion, we have demonstrated that survivors of childhood cancer are at increased risk of developing a nonsurgical premature menopause compared with women who have never had cancer. The results of this study well facilitate counseling current survivors about their risk of experiencing an early menopause and will assist researchers in designing new therapeutic protocols that aim to reduce late ovarian toxicity.
Supported by grants U24-CA55727 and R01CA79024 (CS) from the National Institutes of Health, which played no role in the study design, analysis or interpretation of the data, writing of the manuscript, or the decision to submit the manuscript for publication.
This research used the CCSS, a resource supported by the National Cancer Institute to promote and facilitate research on long-term survivors of cancer diagnosed in childhood and adolescence. Investigators may apply to use the CCSS by proposing an analysis of existing data or proposing new initiatives that would use the cohort. Interested investigators are encouraged to visit the CCSS Web site at http://www.cancer.umn.edu/ccss to learn more about this unique resource.
CCSS institutions and investigators include the following (*, Institutional Principal Investigator; †, Former Institutional Principal Investigator; ‡, Member CCSS Steering Committee):
University of California–San Francisco: Robert Goldsby, MD*, Arthur Ablin, MD†; University of Alabama, Birmingham: Roger Berkow, MD*; International Epidemiology Institute, Rockville, MD: John Boice, ScD‡; University of Washington, Seattle: Norman Breslow, PhD‡; University of Texas Southwestern Medical Center at Dallas: Gail Tomlinson, MD*, George R. Buchanan, MD†; Cincinnati Children's Hospital Medical Center, OH: Stella Davies, MD, PhD‡; Dana-Farber Cancer Institute, Boston, MA: Lisa Diller, MD*, Holcombe Grier, MD†, Frederick Li, MD‡; Texas Children's Center, Houston: Zoann Dreyer, MD*; Children's Hospital and Medical Center, Seattle, WA: Debra Friedman, MD, MPH*, Thomas Pendergrass, MD†; Roswell Park Cancer Institute, Buffalo, NY: Daniel M. Green, MD*‡; Hospital for Sick Children, Toronto, ON: Mark Greenberg, MB, ChB*; St. Louis Children's Hospital, St. Louis, MO: Robert Hayashi, MD*, Teresa Vietti, MD†; St. Jude Children's Research Hospital, Memphis, TN: Leslie L. Robison, PhD*‡, Melissa Hudson, MD*‡; University of Michigan, Ann Arbor: Raymond Hutchinson, MD*; Stanford University School of Medicine, Stanford, CA: Neyssa Marina, MD*, Michael P. Link, MD†, Sarah S. Donaldson, MD‡; Emory University, Atlanta, GA: Lillian Meacham, MD*; Children's Hospital of Philadelphia, PA: Anna Meadows, MD*‡, Bobbie Bayton‡; Children's Hospital, Oklahoma City, OK: John Mulvihill, MD‡; Children's Hospital, Denver, CO: Brian Greffe*, Lorrie Odom, MD†; Children's Hospitals and Clinics of Minnesota, MN: Joanna Perkins, MD*, Maura O'Leary, MD†; Columbus Children's Hospital, Columbus, OH: Amanda Termuhlen, MD*, Frederick Ruymann, MD†, Stephen Qualman, MD‡; Children's National Medical Center, Washington, DC: Gregory Reaman, MD*, Roger Packer, MD‡; Children's Hospital of Pittsburgh, PA: A. Kim Ritchey, MD*, Julie Blatt, MD†; University of Minnesota, Minneapolis: Ann Mertens, PhD*‡, Joseph Neglia, MD, MPH‡, Mark Nesbit, MD‡; Children's Hospital Los Angeles, CA: Kathy Ruccione, RN, MPH*; Memorial Sloan-Kettering Cancer Center, New York, NY: Charles Sklar, MD*‡, Kevin Oeffinger, MD‡; National Cancer Institute, Bethesda, MD: Barry Anderson, MD‡, Peter Inskip, ScD‡; Mayo Clinic, Rochester, MN: Vilmarie Rodriguez, MD*, W. Anthony Smithson, MD, Gerald Gilchrist, MD†; University of Texas M. D. Anderson Cancer Center, Houston: Louise Strong, MD*‡, Marilyn Stovall, PhD‡; Riley Hospital for Children, Indianapolis, IN: Terry A. Vik, MD*, Robert Weetman, MD†; Fred Hutchinson Cancer Research Center, Seattle, WA: Wendy Leisenring, ScD*‡, John Potter, MD, PhD†‡; University of Alberta, Edmonton: Yutaka Yasui, PhD†‡; University of California–Los Angeles: Lonnie Zeltzer, MD*‡.
References
Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. Ries LA, Smith MA, Gurney JG, Linet M, Tamra T, Young JL et al., editors. NIH Pub. No. 99-4649.
Sklar C. Reproductive physiology and treatment-related loss of sex hormone production.
Sklar C. Maintenance of ovarian function and risk of premature menopause related to cancer treatment.
Sarafoglou K, Boulad F, Gillio A, Sklar C. Gonadal function after bone marrow transplantation for acute leukemia during childhood.
Critchley HO, Thomson AB, Wallace WH. Ovarian and uterine function and reproductive potential. In: Wallace WH, Green DM, editors. Late effects of childhood cancer. London (UK): Arnold;
Shalet SM, Beardwell CG, Morris-Jones PH, Pearson D, Orrell DH. Ovarian failure following abdominal irradiation in childhood.
Thibaud E, Rodriguez-Macias K, Trivin C, Esperou H, Michon J, Brauner R. Ovarian function after bone marrow transplantation during childhood.
Meirow D. Reproduction post-chemotherapy in young cancer patients.
Chemaitilly W, Mertens AC, Mitby P, Whitton J, Stovall M, Yasui Y, et al. Acute ovarian failure in the Childhood Cancer Survivor Study.
Himelstein-Braw R, Peters H, Faber M. Influence of irradiation and chemotherapy on the ovaries of children with abdominal tumors.
Nicosia SV, Matus-Ridley M, Meadows AT. Gonadal effects of cancer therapy in girls.
Larsen EC, Muller J, Schmiegelow K, Rechnitzer C, Andersen AN. Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer.
Faddy MJ, Gosden RG. A model conforming the decline in follicle numbers to the age of menopause in women.
Pouilles JM, Tremollieres F, Bonneu M, Ribot C. Influence of early age at menopause on vertebral bone mass.
Vega EM, Egea MA, Mautalen CA. Influence of menopausal age on the severity of osteoporosis in women with vertebral fractures.
de Kleijn MJJ, van der Schouw YT, Verbeek AL, Peeters PH, Banga J, van der Graaf Y. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women.
Schover L. Sexuality and body image in younger women treated for breast cancer.
Byrne J, Fears TR, Gail MH, Pee D, Connelly RR, Austin DF, et al. Early menopause in long-term survivors of cancer during adolescence.
Chiarelli AM, Marrett LD, Darlington G. Early menopause and infertility in females after treatment for childhood cancer diagnosed in 1964–1988 in Ontario, Canada.
Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project.
Kaufert PA, Gilbert P, Tate R. Defining menopausal status: the impact of longitudinal data.
Ceccarelli C, Bencivelli W, Morciano D, Pinchera A, Pacini F. 131I therapy for differentiated thyroid cancer leads to an earlier onset of menopause: results of a retrospective study.
Stovall M, Donaldson SS, Weathers RE, Robison LL, Mertens AC, Winther JF, et al. Genetic effects of radiotherapy for childhood cancer: gonadal dose reconstruction.
Tucker MA, D'Angio GJ, Boice JD Jr, Strong LC, Li FP, Stovall M, et al. Bone sarcomas linked to radiotherapy and chemotherapy in children.
Neglia JP, Friedman DL, Yasui Y, Mertens AC, Hammond S, Stovall M, et al. Second malignant neoplasms in five-year survivors of childhood cancer: Childhood Cancer Survivor Study.
Breslow NE, Day NE. The Design and Analysis of Cohort Studies. [2].
Zeger SL, Liang KY, Abert PS. Models for longitudinal data: a generalized estimating equation approach.
Kaplan EL, Meier P. Nonparametric observation from incomplete observations.
Akaike H. A new look at the statistical identification model.
Stillman RJ, Schinfeld JS, Schiff I, Gelber RD, Greenberger J, Larson M, et al. Ovarian failure in long-term survivors of childhood malignancy.
Wallace WH, Thomson AB, Kelsey TW. The radiosensitivity of the human oocyte.
Wallace WH, Thomson AB, Saran F, Kelsey TW. Predicting age at ovarian failure after radiation to a field that includes the ovaries.