-
PDF
- Split View
-
Views
-
Cite
Cite
Ian J. Lewis, Marianne A. Nooij, Jeremy Whelan, Matthew R. Sydes, Robert Grimer, Pancras C. W. Hogendoorn, Muhammad A. Memon, Simon Weeden, Barbara M. Uscinska, Martine van Glabbeke, Anne Kirkpatrick, Esther I. Hauben, Alan W. Craft, Antonie H. M. Taminiau, On behalf of MRC BO06 and EORTC 80931 collaborators and European Osteosarcoma Intergroup, Improvement in Histologic Response But Not Survival in Osteosarcoma Patients Treated With Intensified Chemotherapy: A Randomized Phase III Trial of the European Osteosarcoma Intergroup, JNCI: Journal of the National Cancer Institute, Volume 99, Issue 2, 17 January 2007, Pages 112–128, https://doi.org/10.1093/jnci/djk015
Close - Share Icon Share
Abstract
Previous randomized controlled trials that used the two-drug chemotherapy regimen of cisplatin and doxorubicin as the conventional arm showed no evidence of benefit from an increase in the number of agents or the length of treatment. It was then proposed that survival could be improved by increasing the planned dose intensity of cisplatin and doxorubicin.
Previously untreated patients with nonmetastatic, high-grade, central osteosarcoma of an extremity were randomly assigned to Regimen-C (conventional treatment with six 3-week cycles of cisplatin [100 mg/m 2 by 24-hour infusion] and doxorubicin [25 mg/m 2 /day by 4-hour infusion for 3 days]) or to Regimen-DI (intensified treatment with identical total doses of cisplatin and doxorubicin, planned as six 2-week cycles supported by granulocyte colony stimulating factor (G-CSF). Surgery was scheduled for week 6 in both arms. Primary and secondary outcome measures were overall and progression-free survival, respectively. Intention-to-treat analyses were performed using standard survival analysis methods. Landmark analyses were performed in patients with known surgical details and centrally reviewed histologic response. All statistical tests were two-sided.
Between May 1993 and September 2002, treatment was randomly allocated to 497 eligible patients. Six cycles of chemotherapy were completed by 78% of patients in Regimen-C and 80% of patients in Regimen-DI. The delivered preoperative median dose intensity of cisplatin was 86% in Regimen-C and 111% in Regimen-DI (as the percentage of that planned for the conventional regimen). Postoperative median dose intensity of cisplatin was 82% in Regimen-C and 110% in Regimen-DI (the corresponding figures for doxorubicin dose intensity were similar). Regimen-DI was associated with lower risks of severe leucopenia and neutropenia and higher risks of thrombocytopenia and mucositis. Good histologic response (>90% tumor necrosis) was observed in 36% of Regimen-C patients and 50% of Regimen-DI patients ( P = .003, χ 2 test). There was no evidence of a difference in overall survival (hazard ratio [HR] = 0.94, 95% CI = 0.71 to 1.24; P = .64) or progression-free survival (HR = 0.98, 95% CI = 0.77 to 1.24; P = .83). Landmark analyses showed similar results.
Planned intensification of chemotherapy with cisplatin and doxorubicin increased received dose intensity and resulted in a statistically significant increase in favorable histologic response rate, but not in increased progression-free or overall survival. Our results call into question the use of histologic response as a surrogate outcome measure in trials of this disease.
Chemotherapy has improved outcomes for patients with osteosarcoma such that 50%–70% of them are alive 5 years after diagnosis. However, in recent trials, treatments in which the number of chemotherapeutic agents or the duration of chemotherapy was increased did not improve patient survival compared to conventional chemotherapy.
This is a randomized clinical trial comparing two treatment regimens with overall and progression-free survival as the clinical endpoints.
This trial investigated whether increasing the planned dose intensity of the chemotherapeutic agents cisplatin and doxorubicin would improve outcomes compared to conventional treatment with these drugs. The investigators found that increased dose intensity, while improving histologic response, did not prolong patient survival.
Testing of additional therapeutic strategies will be required to improve survival of patients with osteosarcoma, and histologic response may not be a valid surrogate outcome in osteosarcoma trials.
Due to the rarity of osteosarcoma, this trial took a long time to recruit and mature, and it involved many countries with different standard approaches to chemotherapy and surgery.
During the last three decades, 5-year survival of patients with localized high-grade osteosarcoma has risen from less than 20% to 50%–70%, and parallel advances in conserving surgical techniques have led to limb salvage rates of more than 75% ( 1 ). These improved outcomes followed the introduction of effective adjuvant and neoadjuvant chemotherapy ( 2 – 11 ). At present, the four principal active drugs for osteosarcoma are cisplatin ( 12 , 13 ), doxorubicin ( 14 ), high-dose methotrexate ( 15 , 16 ), and ifosfamide ( 17 , 18 ), but the most effective combination of these agents is unknown ( 4 – 11 ).
The European Osteosarcoma Intergroup (EOI) was formed in 1982 to conduct randomized controlled trials for patients with osteosarcoma. The first EOI randomized controlled trial (Medical Research Council [MRC] BO02, European Organization for Research and Treatment of Cancer [EORTC] 80831) ( 6 ) compared six cycles of a two-drug regimen (cisplatin and doxorubicin) ( 19 ) with four cycles of a three-drug regimen (cisplatin, doxorubicin, and high-dose methotrexate). In 198 patients with primary nonmetastatic limb osteosarcoma, the two-drug regimen was associated with a statistically significant increase in progression-free survival at 5 years (57% versus 41%, hazard ratio [HR] = 0.63 (95% confidence interval [CI] = 0.42 to 0.94). The second EOI randomized controlled trial (MRC BO03, EORTC 80861) ( 7 ) compared an 18-week, two-drug regimen consisting of cisplatin and doxorubicin with a 47-week multidrug regimen consisting of high-dose methotrexate, doxorubicin, cisplatin, bleomycin, cyclophosphamide, and actinomycin-D that was based on the Rosen T-10 protocol ( 18 ). Between 1986 and 1993, 391 eligible patients with primary nonmetastatic limb osteosarcoma were randomly assigned to these two treatments. No difference was observed in progression-free survival (HR = 1.01, 95% CI = 0.77 to 1.33) or overall survival, with 5-year progression-free survival and overall survival of 44% and 55%, respectively, in both arms.
In experimental animal models for the treatment of chemosensitive tumors, most cytotoxic drugs exhibit a sigmoidal dose–response curve ( 20 ) with lag, linear, and plateau phases. Increasing total dose or dose intensity such that drug concentration increases to higher values within the linear phase of the dose–response curve should minimize drug resistance and maximize effective cell kill ( 21 ). In the clinical setting, the nature of dose–response curves are unknown, and, in particular, the slopes of the linear phase and the plateau doses have not been determined.
Clinical studies in various adult and childhood malignancies indicated that dose intensity may be important in determining survival: retrospective studies in breast cancer ( 22 ), Hodgkin lymphoma ( 23 ), non-Hodgkin lymphoma ( 24 , 25 ), and neuroblastoma ( 26 ) showed a positive association between planned dose intensity and survival in conventional dosing schedules. Evidence as to whether increasing planned dose intensity above conventional levels by dose compression improves outcome has been evolving in a number of malignancies ( 27 – 30 ). More recently, this concept of increasing dose intensity has been referred to as “dose density.” The hypothesized advantage of dose-dense chemotherapy is that compressing conventional schedules of drug administration can achieve greater efficacy by minimizing regrowth of tumor cells between treatment cycles ( 31 ).
Several retrospective studies have identified dose intensity as a potentially important determinant of outcome in patients treated for osteosarcoma. In a review of 16 studies, Smith et al. ( 32 ) found a strong relationship between planned doxorubicin dose intensity and histologic tumor necrosis. Other retrospective studies concluded that doxorubicin dose ( 33 ) or planned high-dose methotrexate intensity ( 34 , 35 ) influenced outcome. The EOI investigated the impact of received dose intensity on the conventional two-drug cisplatin and doxorubicin regimen given every 3 weeks ( 36 ). This retrospective analysis of trial data failed to demonstrate any benefit for those patients who received this regimen most intensively. However, both overall survival and progression-free survival were lowest for patients receiving the lowest dose intensity, and progression-free survival was lower for those who received between one and five treatment cycles compared to those who received all six cycles.
Previous EOI trials showed no additional benefit from increasing the number of agents or length of treatment, and therefore, the two-drug cisplatin and doxorubicin remained our standard. It was hypothesized that a further increase in the planned dose intensity of cisplatin and doxorubicin would improve outcome. A pilot study demonstrated the feasibility of increasing the dose intensity of the two-drug regimen by 50%, by using granulocyte colony-stimulating factor (G-CSF) to shorten the treatment cycle from 3 to 2 weeks ( 37 ). This demonstration has formed the basis of the trial (MRC BO06/EORTC 80931) reported here. To our knowledge, this is the first randomized trial of the effectiveness of increased dose intensity in the treatment of high-grade osteosarcoma.
Patients and Methods
Trial Design
This was an open-label randomized controlled trial with the primary outcome measure of overall survival (time to death from any cause) and a secondary outcome measure of progression-free survival, i.e., time to nontumor death or any relapse or recurrence including distant metastases (but not including local disease progression before primary surgery). For the trial, 165 events were planned to detect an improvement of 15% in 5-year survival (55%–70%, HR = 0.6) with 90% power and a two-sided statistical significance level of 5%. Randomizations were performed separately and centrally at the two trials offices (Medical Research Council Clinical Trials Unit [MRC CTU] and EORTC Data Center). Minimization was performed with two stratification factors: center and age (0–16 or ≥17 years).
Patients
Patients aged 40 years or less with a histologically confirmed diagnosis of high-grade osteosarcoma in an extremity long bone ( 38 ) were eligible for the trial ( 39 , 40 ). An EOI pathologist reviewed each diagnosis and assigned histologic subtype. Patients with paraosteal, periosteal, Paget-related, or radiation-induced osteosarcoma were ineligible. Also excluded were patients with prior malignancy, any chemotherapy before trial entry, reduced glomerular filtration rate (<60 mL/min/1.73 m 2 ), cardiac dysfunction, or raised bilirubin. In addition, to be eligible, patients needed to commence chemotherapy within 28 days after biopsy, with normal leukocyte (≥3.5 × 10 9 /L) and platelet (≥100 × 10 9 /L) counts. Informed consent was obtained according to local requirements.
Initial staging investigations included plain radiography of tumor and chest, computerized tomography (CT) or magnetic resonance image of affected limb, CT scan of thorax, and isotope bone scan. Patients with demonstrable metastases were ineligible, but equivocal radiology results without confirmed metastases did not render patients ineligible.
Patients had full assessment at diagnosis of hematologic and biochemical parameters. This assessement included determination of hemoglobin levels, total and differential white blood cell counts, platelet counts, and examination of blood film. Plasma levels of creatinine, urea, sodium, potassium, chloride, calcium, magnesium, and phosphate were determined. Liver function tests included measurement of bilirubin and alkaline phosphatase and transaminase activity. Renal function was assessed by either 51 chromium-EDTA or 51 chromium-DTPA isotopic renal clearance. Audiometry was performed according to local practice. Cardiac function was assessed by echocardiogram or an equivalent test.
Quality Assurance
The trial was run from the MRC CTU, London, United Kingdom (formerly MRC Cancer Trials Office, Cambridge, United Kingdom) and the EORTC Data Center, Brussels, Belgium. Data were collated for analysis at MRC CTU. The trial was overseen by the Trial Management Group and reviewed on three occasions by an independent Data Monitoring Committee. No formal stopping rules were specified. On the occasion of each review, the Data Monitoring Committee recommended that the trial continue. This trial was conducted in compliance with the Declaration of Helsinki, and the protocol was approved by the appropriate Research Ethics Committees.
Chemotherapy
As planned, the conventional two-drug regimen (Regimen-C) consisted of six 3-week cycles, each commencing with administration of doxorubicin (25 mg/m 2 ) by 4-hour infusion (repeated on days 2 and 3) and cisplatin (100 mg/m 2 ) by 24-hour infusion, with surgery planned between cycles 2 and 3. Regimen-DI consisted of six cycles of treatment with the same doses of cisplatin and doxorubicin as in the conventional regimen, but with cycles planned to commence every 2 weeks. Patients treated with this regimen were supported by G-CSF (5 μg/kg daily) by subcutaneous injection on days 4–13 of each cycle). Surgery was planned between cycles 3 and 4. For both regimens, cisplatin infusion was preceded by 4 hours of predehydration followed by a 24-hour posthydration schedule, which included a forced mannitol diuresis. It was recommended that cisplatin be given as a 24-hour infusion diluted in isotonic saline with added potassium chloride and mannitol. In both arms, the surgery window was 14 days in duration. G-CSF was lenograstim (Amgen, United Kingdom) or filgrastim (Chugai, United Kingdom) according to local arrangements. The complete schedules for both regimens are shown in Fig. 1 .
Schedule of drug treatment and surgery and planned dosage in the trial. A ) Schedule of drug treatment cycles and surgery for the conventional regimen (Regimen-C) and the dose-intensive regimen (Regimen-DI) that were compared in the trial. B ) Schedule of drug doses within each cycle. In both regimens, C = administration of cisplatin as a 100 mg/m 2 24-hour infusion, D = administration of doxorubicin as a 25 mg/m 2 4-hour intravenous infusion, and G = granulocyte colony-stimulating factor (GCSF) given as a 5 μg/kg injection. Cisplatin infusion was preceded by 4 hours of predehydration followed by a 24-hour posthydration schedule, which included a forced mannitol diuresis. C ) Planned total doses of each drug according to regimen in preoperative, postoperative, and full period of treatment.
Toxicity Monitoring and Dose Modification.
Height (cm), weight (kg), surface area (m 2 ), glomerular function, and levels of creatinine, sodium, potassium, calcium, magnesium, chloride, phosphates, alkaline phosphatase, bilirubin, and transaminases were determined before each cycle according to local practice. Blood counts were obtained before each cycle and at the expected nadir of the count (day 10 in Regimen-C, day 8 in Regimen-DI). Doses could be delayed or reduced according to specific criteria as follows: Dose reductions of 20% were advised for patients with febrile neutropenia requiring antibiotics or platelet transfusion. Delay was advised where neutrophil counts were less than 1 × 10 9 /L and/or platelets were less than 100 × 10 9 /L, amended in 1997 to platelets 75 × 10 9 /L. Toxicity was graded using the World Health Organization (WHO) toxicity coding scheme ( 41 ). Delay or dose reduction was advised for renal toxicity or mucositis (WHO grade 3–4 in both cases). Discontinuation of cisplatin was advised if the glomerular filtration rate fell below 60 mL/min/1.73 m 2 for more than 1 week or if grade 3–4 neurotoxicity or symptomatic ototoxicity was observed. Cardiac function was assessed by echocardiogram or equivalent before treatment and before cycles 3–6. The Children's Cancer Study Group anthracycline administration guideline ( 42 ) was adopted during the trial to provide more consistency to the management of doxorubicin-induced cardiotoxicity.
Calculation of Dose Intensity.
Surgery
Patients were clinical and radiologically assessed for response in the week before surgery. Local progression was defined as increase in tumor size or worsening pain and systemic progression as the development of distant metastases. Patients with local progression alone were advised to have surgery and continue on protocol; the appearance of metastases was deemed a progression-free survival event, and such patients were treated off protocol.
Surgery was planned for day 42 in both arms or as soon thereafter as hematologic recovery allowed. Decisions about the type of planned surgery, i.e., limb sparing or amputation, were made by clinical teams. Surgical margins were defined as radical, wide, marginal, or intralesional. Further details about surgery will be published separately.
Histologic Response Assessment
Resected tumor specimens were assessed for histologic response to preoperative chemotherapy ( 43 ). Using the definitions employed in the two previous EOI trials, tumor necrosis more than 90% was defined as “good” histopathologic response; 10% or more viable tumor after chemotherapy was defined “poor”. As tumors with a substantial chondroblastic component are intrinsically less responsive to chemotherapy, the extent of chondroblastic differentiation was assessed using a standard method of microscopic examination to stratify cases. Tumors in which there was more than 30% volume of chondroblastic differentiation relative to tumor volume were defined as chondroblastic. Specimens were reviewed centrally by EOI pathologists wherever possible.
End of Treatment and Off-treatment Monitoring
Full radiologic and toxicity assessment was advised following completion of treatment. Investigators were advised to perform clinical follow-up with chest radiography monthly for the first 6 months after treatment, every 2 months until the end of year 1, every 3 months during year 2, every 4 months during year 3, and every 6 months until year 5. After 5 years, follow-up was annual. Cardiac function was assessed at 1, 3, and 5 years by echocardiography or equivalent local practice.
Statistical Analyses
Analyses were performed on a modified intention-to-treat basis: all patients were included in the analysis except for patients determined to be histologically ineligible during central pathology review, which was performed without reference to treatment allocation. These patients were excluded because it was independently confirmed that they were ineligible before randomization and should not have been entered. Patients who were found after random assignment to have violated other eligibility criteria were included in the analysis because such assessment was not blind to treatment allocation. Baseline characteristics of the patients and their tumors were described but not formally tested for differences across arms.
All tests were two-sided. No formal adjustments were made for multiple testing. Confidence intervals were calculated at the 95% level. Histologic response to preoperative chemotherapy was quantified using the chi-square test for association and toxicities using relative risks (RRs). Time-to-event data were summarized using hazard ratio and Kaplan–Meier plots and compared with log-rank tests. All comparisons including relative risks and hazard ratios were relative to Regimen-C; therefore, a hazard ratio of less than 1.0 indicated an advantage of Regimen-DI. For time-to-event calculations, time was from randomization unless specified. Adjusted hazard ratios were obtained using Cox regression models; the assumption of proportionality was checked both visually and by using tools in the Stata software. Tests for heterogeneity of effect across groups were tested with chi-square tests for interaction. Statistical analyses were performed using Stata 9 software (Stata Corporation, College Station, TX).
Exploratory and Landmark Analyses.
The purpose of the landmark analyses was to allow for comparison of treatment arms accounting for histologic response, which was not known until after surgery, the primary treatment, had been performed. For patients included in the landmark analyses, both surgical details and histologic response had to be ascertained and time was taken as starting from 60 days after randomization at which time surgery should have been performed and histologic response known. Histologic response was unobtainable for some patients. Therefore, exploratory survival models were constructed using the landmark approach to look at how histologic response (good, poor, unobtainable, or no surgery) affected outcome. Additionally, a series of univariate and multivariable Cox regression models were constructed to examine the effect of overall and preoperative dose and dose intensity on overall survival. Finally, sensitivity analyses were performed on overall survival and progression-free survival for the whole population of all 504 randomly assigned patients including those patients excluded for reasons of prerandomization ineligibility.
Results
Accrual, Patient Characteristics, and Follow-up
Between May 1, 1993, and September 30, 2002, 504 patients with presumed nonmetastatic operable osteosarcoma were enrolled—292 through the MRC CTU (United Kingdom, Argentina, and Chile) and 212 through the EORTC (including Netherlands, Saudi Arabia, Belgium, and Denmark) (full details are presented in the Appendix ). A Consolidated Standards of Reporting Trials flow diagram of the trial is presented in Fig. 2 . Pathologic review identified seven patients as ineligible due to inappropriate histologic diagnoses, and these were excluded from further analyses. Therefore, 497 patients (245 assigned to Regimen-C and 252 assigned to Regimen-DI) were analyzed, including 36 patients who were not centrally reviewed. Seven patients were found to not fulfill eligibility criteria after random assignment (three due to the presence of metastases, three because their osteosarcoma was at an incorrect site, and one due to renal insufficiency) but were included in the analyses. The baseline characteristics of the 490 eligible patients are shown in Table 1 . More than 60% were 16 years old or younger, and 60% were male. Distal femur was the most common site of osteosarcoma. Common-type osteosarcoma was the most frequent histologic subtype, followed by chondroblastic osteosarcoma. Other subtypes occurred infrequently. The treatment groups were balanced in terms of tumor site, location within the bone, and a combination of site and location (data not shown).
Trial flow diagram (Consolidated Standards of Reporting Clincial Trials [CONSORT] diagram).
Baseline characteristics *
| . | Allocated treatment . | . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| . | Regimen-C . | . | Regimen-DI . | . | Overall . | . | |||
| Baseline characteristics . | N . | % . | N . | % . | N . | % . | |||
| Eligibility † | |||||||||
| Eliglible | 241 | 96 | 249 | 98 | 490 | 97 | |||
| Ineligible—pathology | 4 | 2 | 1 | 0 | 5 | 1 | |||
| Ineligible—other | 4 | 2 | 3 | 1 | 7 | 1 | |||
| Ineligible—pathology and other | 1 | 0 | 1 | 0 | 2 | 0 | |||
| Sex ‡ | |||||||||
| Male | 144 | 60 | 149 | 59 | 293 | 59 | |||
| Female | 98 | 40 | 103 | 41 | 201 | 41 | |||
| Age (y) | |||||||||
| ≤16 | 158 | 64 | 161 | 64 | 319 | 64 | |||
| >16 | 87 | 36 | 91 | 36 | 178 | 36 | |||
| Median (quartiles) | 15 (12–18) | 15 (13–18) | 15 (12–18) | ||||||
| Minimum/maximum | 4/38 | 5/41 | 4/41 | ||||||
| Site | |||||||||
| Femur | 143 | 59 | 153 | 61 | 296 | 60 | |||
| Tibia | 60 | 25 | 56 | 22 | 116 | 24 | |||
| Fibula | 13 | 5 | 12 | 5 | 25 | 5 | |||
| Humerus | 22 | 9 | 26 | 10 | 48 | 10 | |||
| Radius | 3 | 1 | 2 | 1 | 5 | 1 | |||
| Ulna | 0 | 0 | 1 | 0 | 1 | 0 | |||
| Missing | 4 | – | 2 | – | 6 | – | |||
| Location within bone | |||||||||
| Proximal | 93 | 39 | 97 | 39 | 190 | 39 | |||
| Midshaft | 8 | 3 | 4 | 2 | 12 | 2 | |||
| Distal | 139 | 58 | 149 | 60 | 288 | 59 | |||
| Missing | 5 | – | 2 | – | 7 | – | |||
| Pathology | |||||||||
| Common type | 131 | 60 | 152 | 68 | 283 | 64 | |||
| Chondroblastic | 25 | 12 | 26 | 12 | 51 | 12 | |||
| Fibroblastic | 9 | 4 | 6 | 3 | 15 | 3 | |||
| Osteoclast rich | 2 | 1 | 7 | 3 | 9 | 2 | |||
| Anaplastic | 9 | 4 | 12 | 5 | 21 | 5 | |||
| Small cell osteo | 3 | 1 | 2 | 1 | 5 | 1 | |||
| Telangiectatic | 22 | 10 | 7 | 3 | 29 | 7 | |||
| Other type | 16 | 7 | 12 | 5 | 28 | 6 | |||
| Missing § | 28 | – | 28 | – | 56 | – | |||
| Total included | 245 | 100 | 252 | 100 | 497 | 100 | |||
| Ineligible | 5 | – | 2 | – | 7 | – | |||
| Total randomly assigned | 250 | 254 | 504 | ||||||
| . | Allocated treatment . | . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| . | Regimen-C . | . | Regimen-DI . | . | Overall . | . | |||
| Baseline characteristics . | N . | % . | N . | % . | N . | % . | |||
| Eligibility † | |||||||||
| Eliglible | 241 | 96 | 249 | 98 | 490 | 97 | |||
| Ineligible—pathology | 4 | 2 | 1 | 0 | 5 | 1 | |||
| Ineligible—other | 4 | 2 | 3 | 1 | 7 | 1 | |||
| Ineligible—pathology and other | 1 | 0 | 1 | 0 | 2 | 0 | |||
| Sex ‡ | |||||||||
| Male | 144 | 60 | 149 | 59 | 293 | 59 | |||
| Female | 98 | 40 | 103 | 41 | 201 | 41 | |||
| Age (y) | |||||||||
| ≤16 | 158 | 64 | 161 | 64 | 319 | 64 | |||
| >16 | 87 | 36 | 91 | 36 | 178 | 36 | |||
| Median (quartiles) | 15 (12–18) | 15 (13–18) | 15 (12–18) | ||||||
| Minimum/maximum | 4/38 | 5/41 | 4/41 | ||||||
| Site | |||||||||
| Femur | 143 | 59 | 153 | 61 | 296 | 60 | |||
| Tibia | 60 | 25 | 56 | 22 | 116 | 24 | |||
| Fibula | 13 | 5 | 12 | 5 | 25 | 5 | |||
| Humerus | 22 | 9 | 26 | 10 | 48 | 10 | |||
| Radius | 3 | 1 | 2 | 1 | 5 | 1 | |||
| Ulna | 0 | 0 | 1 | 0 | 1 | 0 | |||
| Missing | 4 | – | 2 | – | 6 | – | |||
| Location within bone | |||||||||
| Proximal | 93 | 39 | 97 | 39 | 190 | 39 | |||
| Midshaft | 8 | 3 | 4 | 2 | 12 | 2 | |||
| Distal | 139 | 58 | 149 | 60 | 288 | 59 | |||
| Missing | 5 | – | 2 | – | 7 | – | |||
| Pathology | |||||||||
| Common type | 131 | 60 | 152 | 68 | 283 | 64 | |||
| Chondroblastic | 25 | 12 | 26 | 12 | 51 | 12 | |||
| Fibroblastic | 9 | 4 | 6 | 3 | 15 | 3 | |||
| Osteoclast rich | 2 | 1 | 7 | 3 | 9 | 2 | |||
| Anaplastic | 9 | 4 | 12 | 5 | 21 | 5 | |||
| Small cell osteo | 3 | 1 | 2 | 1 | 5 | 1 | |||
| Telangiectatic | 22 | 10 | 7 | 3 | 29 | 7 | |||
| Other type | 16 | 7 | 12 | 5 | 28 | 6 | |||
| Missing § | 28 | – | 28 | – | 56 | – | |||
| Total included | 245 | 100 | 252 | 100 | 497 | 100 | |||
| Ineligible | 5 | – | 2 | – | 7 | – | |||
| Total randomly assigned | 250 | 254 | 504 | ||||||
Regimen-C = conventional regimen; Regimen-DI = dose-intensive regimen. Percentages may not add to 100% because of rounding.
Randomly assigned patients were excluded only if determined not to meet the pathology criteria on central pathology review.
Sex was missing for three patients on Regimen-C.
Histology was determined on central pathology review using the initial biopsy sample or the resected specimen. No samples were available for 43 patients. One or both of these were available for 461 (91%) of randomly assigned patients. In 10 of these patients, the material could not be assessed, and class was not available for 10 further patients. Patients were included in the analyses unless they were shown to be pathologically ineligible. Therefore, 497 patients are included.
Baseline characteristics *
| . | Allocated treatment . | . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| . | Regimen-C . | . | Regimen-DI . | . | Overall . | . | |||
| Baseline characteristics . | N . | % . | N . | % . | N . | % . | |||
| Eligibility † | |||||||||
| Eliglible | 241 | 96 | 249 | 98 | 490 | 97 | |||
| Ineligible—pathology | 4 | 2 | 1 | 0 | 5 | 1 | |||
| Ineligible—other | 4 | 2 | 3 | 1 | 7 | 1 | |||
| Ineligible—pathology and other | 1 | 0 | 1 | 0 | 2 | 0 | |||
| Sex ‡ | |||||||||
| Male | 144 | 60 | 149 | 59 | 293 | 59 | |||
| Female | 98 | 40 | 103 | 41 | 201 | 41 | |||
| Age (y) | |||||||||
| ≤16 | 158 | 64 | 161 | 64 | 319 | 64 | |||
| >16 | 87 | 36 | 91 | 36 | 178 | 36 | |||
| Median (quartiles) | 15 (12–18) | 15 (13–18) | 15 (12–18) | ||||||
| Minimum/maximum | 4/38 | 5/41 | 4/41 | ||||||
| Site | |||||||||
| Femur | 143 | 59 | 153 | 61 | 296 | 60 | |||
| Tibia | 60 | 25 | 56 | 22 | 116 | 24 | |||
| Fibula | 13 | 5 | 12 | 5 | 25 | 5 | |||
| Humerus | 22 | 9 | 26 | 10 | 48 | 10 | |||
| Radius | 3 | 1 | 2 | 1 | 5 | 1 | |||
| Ulna | 0 | 0 | 1 | 0 | 1 | 0 | |||
| Missing | 4 | – | 2 | – | 6 | – | |||
| Location within bone | |||||||||
| Proximal | 93 | 39 | 97 | 39 | 190 | 39 | |||
| Midshaft | 8 | 3 | 4 | 2 | 12 | 2 | |||
| Distal | 139 | 58 | 149 | 60 | 288 | 59 | |||
| Missing | 5 | – | 2 | – | 7 | – | |||
| Pathology | |||||||||
| Common type | 131 | 60 | 152 | 68 | 283 | 64 | |||
| Chondroblastic | 25 | 12 | 26 | 12 | 51 | 12 | |||
| Fibroblastic | 9 | 4 | 6 | 3 | 15 | 3 | |||
| Osteoclast rich | 2 | 1 | 7 | 3 | 9 | 2 | |||
| Anaplastic | 9 | 4 | 12 | 5 | 21 | 5 | |||
| Small cell osteo | 3 | 1 | 2 | 1 | 5 | 1 | |||
| Telangiectatic | 22 | 10 | 7 | 3 | 29 | 7 | |||
| Other type | 16 | 7 | 12 | 5 | 28 | 6 | |||
| Missing § | 28 | – | 28 | – | 56 | – | |||
| Total included | 245 | 100 | 252 | 100 | 497 | 100 | |||
| Ineligible | 5 | – | 2 | – | 7 | – | |||
| Total randomly assigned | 250 | 254 | 504 | ||||||
| . | Allocated treatment . | . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| . | Regimen-C . | . | Regimen-DI . | . | Overall . | . | |||
| Baseline characteristics . | N . | % . | N . | % . | N . | % . | |||
| Eligibility † | |||||||||
| Eliglible | 241 | 96 | 249 | 98 | 490 | 97 | |||
| Ineligible—pathology | 4 | 2 | 1 | 0 | 5 | 1 | |||
| Ineligible—other | 4 | 2 | 3 | 1 | 7 | 1 | |||
| Ineligible—pathology and other | 1 | 0 | 1 | 0 | 2 | 0 | |||
| Sex ‡ | |||||||||
| Male | 144 | 60 | 149 | 59 | 293 | 59 | |||
| Female | 98 | 40 | 103 | 41 | 201 | 41 | |||
| Age (y) | |||||||||
| ≤16 | 158 | 64 | 161 | 64 | 319 | 64 | |||
| >16 | 87 | 36 | 91 | 36 | 178 | 36 | |||
| Median (quartiles) | 15 (12–18) | 15 (13–18) | 15 (12–18) | ||||||
| Minimum/maximum | 4/38 | 5/41 | 4/41 | ||||||
| Site | |||||||||
| Femur | 143 | 59 | 153 | 61 | 296 | 60 | |||
| Tibia | 60 | 25 | 56 | 22 | 116 | 24 | |||
| Fibula | 13 | 5 | 12 | 5 | 25 | 5 | |||
| Humerus | 22 | 9 | 26 | 10 | 48 | 10 | |||
| Radius | 3 | 1 | 2 | 1 | 5 | 1 | |||
| Ulna | 0 | 0 | 1 | 0 | 1 | 0 | |||
| Missing | 4 | – | 2 | – | 6 | – | |||
| Location within bone | |||||||||
| Proximal | 93 | 39 | 97 | 39 | 190 | 39 | |||
| Midshaft | 8 | 3 | 4 | 2 | 12 | 2 | |||
| Distal | 139 | 58 | 149 | 60 | 288 | 59 | |||
| Missing | 5 | – | 2 | – | 7 | – | |||
| Pathology | |||||||||
| Common type | 131 | 60 | 152 | 68 | 283 | 64 | |||
| Chondroblastic | 25 | 12 | 26 | 12 | 51 | 12 | |||
| Fibroblastic | 9 | 4 | 6 | 3 | 15 | 3 | |||
| Osteoclast rich | 2 | 1 | 7 | 3 | 9 | 2 | |||
| Anaplastic | 9 | 4 | 12 | 5 | 21 | 5 | |||
| Small cell osteo | 3 | 1 | 2 | 1 | 5 | 1 | |||
| Telangiectatic | 22 | 10 | 7 | 3 | 29 | 7 | |||
| Other type | 16 | 7 | 12 | 5 | 28 | 6 | |||
| Missing § | 28 | – | 28 | – | 56 | – | |||
| Total included | 245 | 100 | 252 | 100 | 497 | 100 | |||
| Ineligible | 5 | – | 2 | – | 7 | – | |||
| Total randomly assigned | 250 | 254 | 504 | ||||||
Regimen-C = conventional regimen; Regimen-DI = dose-intensive regimen. Percentages may not add to 100% because of rounding.
Randomly assigned patients were excluded only if determined not to meet the pathology criteria on central pathology review.
Sex was missing for three patients on Regimen-C.
Histology was determined on central pathology review using the initial biopsy sample or the resected specimen. No samples were available for 43 patients. One or both of these were available for 461 (91%) of randomly assigned patients. In 10 of these patients, the material could not be assessed, and class was not available for 10 further patients. Patients were included in the analyses unless they were shown to be pathologically ineligible. Therefore, 497 patients are included.
With reverse Kaplan–Meier censoring ( 44 ), median follow-up time was 62 months (range 0–126 months). Of the patients last reported alive, 91%, 68%, and 44% had follow-up of ≥1, 3, and 5 years, respectively. The corresponding percentages were similar in both treatment groups. Deaths and progression-free survival events were reported in 193 (39%) and 272 (55%) patients, respectively.
Chemotherapy
Among eligible patients, 486 (98%) started trial chemotherapy, most within 1 day of random assignment. Figure 1 shows preoperative and postoperative chemotherapy cycles of Regimen-C (control regimen) and regiment-DI (dose-intensive regimen) and the planned schedule of doses (of cisplatin, doxorubicin, and G-CSF) within a cycle for the two regimens. The planned number of preoperative cycles was administered to 170 (69%) and 195 (77%) patients allocated to Regimen-C and Regimen-DI, respectively, and 192 (78%) and 201 (80%) patients received six cycles of Regimen-C and Regimen-DI, respectively ( Table 2 ). The majority (80%) of patients stopped chemotherapy on completion of the protocol treatment, although 24 (10%) and 25 (10%), in Regimen-C and Regimen-DI, respectively, stopped prematurely due to excessive toxicity or patient choice. Three deaths occurred during the protocol treatment period, all among patients allocated to Regimen-DI. One patient who died received a very large, nonprotocol dose of cisplatin during cycle 1. G-CSF was administered to 20 (8%) of patients undergoing Regimen-C and to 244 (99%) of patients undergoing Regimen-DI. Figure 3, A , depicts time to stopping trial chemotherapy and shows how the time of chemotherapy was shorter for patients allocated Regimen-DI.
Kaplan–Meier analysis of time from randomization to trial milestones. The proportion of patients in the conventional regimen (Regimen-C [ solid line ]) and the dose-intensive regimen (Regimen-DI [ dashed line ]) not yet having completed chemotherapy (A) and not yet having completed surgery if surgery was eventually performed (B) are shown. Surgery was expected at 42 days.
Chemotherapy and surgery data *
| . | Allocated treatment . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Chemotherapy and surgery . | Regimen-C . | . | Regimen-DI . | . | |||
| . | N . | % . | N . | % . | |||
| Given intended number of cycles | |||||||
| Preoperatively | 170 | 69 | 195 | 77 | |||
| Postoperatively | 137 | 54 | 174 | 71 | |||
| Overall | 192 | 78 | 201 | 80 | |||
| Reason for terminating chemotherapy | |||||||
| Treatment completed | 189 | 79 | 201 | 82 | |||
| Disease progression | 11 | 5 | 8 | 3 | |||
| Excessive toxicity | 12 | 5 | 17 | 7 | |||
| Treatment refusal | 12 | 5 | 8 | 3 | |||
| Other reason | 16 | 7 | 10 | 4 | |||
| Not available | 0 | – | 2 | – | |||
| Histologic response † | |||||||
| Good response | 71 | 36 | 103 | 50 | |||
| Poor response | 128 | 64 | 101 | 50 | |||
| Response unknown | 22 | – | 28 | – | |||
| Surgery not reported | 24 | – | 20 | – | |||
| Surgery performed | |||||||
| Limb salvage | 164 | 74 | 170 | 73 | |||
| Amputation | 38 | 17 | 46 | 20 | |||
| Disarticulation | 9 | 4 | 10 | 4 | |||
| Rotation plasty | 10 | 5 | 6 | 3 | |||
| No surgery reported | 24 | – | 20 | – | |||
| Total included | 245 | 100 | 252 | 100 | |||
| . | Allocated treatment . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Chemotherapy and surgery . | Regimen-C . | . | Regimen-DI . | . | |||
| . | N . | % . | N . | % . | |||
| Given intended number of cycles | |||||||
| Preoperatively | 170 | 69 | 195 | 77 | |||
| Postoperatively | 137 | 54 | 174 | 71 | |||
| Overall | 192 | 78 | 201 | 80 | |||
| Reason for terminating chemotherapy | |||||||
| Treatment completed | 189 | 79 | 201 | 82 | |||
| Disease progression | 11 | 5 | 8 | 3 | |||
| Excessive toxicity | 12 | 5 | 17 | 7 | |||
| Treatment refusal | 12 | 5 | 8 | 3 | |||
| Other reason | 16 | 7 | 10 | 4 | |||
| Not available | 0 | – | 2 | – | |||
| Histologic response † | |||||||
| Good response | 71 | 36 | 103 | 50 | |||
| Poor response | 128 | 64 | 101 | 50 | |||
| Response unknown | 22 | – | 28 | – | |||
| Surgery not reported | 24 | – | 20 | – | |||
| Surgery performed | |||||||
| Limb salvage | 164 | 74 | 170 | 73 | |||
| Amputation | 38 | 17 | 46 | 20 | |||
| Disarticulation | 9 | 4 | 10 | 4 | |||
| Rotation plasty | 10 | 5 | 6 | 3 | |||
| No surgery reported | 24 | – | 20 | – | |||
| Total included | 245 | 100 | 252 | 100 | |||
Regimen-C = conventional regimen; Regimen-DI = dose-intensive regimen.
Good response is ≤10% viable tumor; poor response is >10% viable tumor.
Chemotherapy and surgery data *
| . | Allocated treatment . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Chemotherapy and surgery . | Regimen-C . | . | Regimen-DI . | . | |||
| . | N . | % . | N . | % . | |||
| Given intended number of cycles | |||||||
| Preoperatively | 170 | 69 | 195 | 77 | |||
| Postoperatively | 137 | 54 | 174 | 71 | |||
| Overall | 192 | 78 | 201 | 80 | |||
| Reason for terminating chemotherapy | |||||||
| Treatment completed | 189 | 79 | 201 | 82 | |||
| Disease progression | 11 | 5 | 8 | 3 | |||
| Excessive toxicity | 12 | 5 | 17 | 7 | |||
| Treatment refusal | 12 | 5 | 8 | 3 | |||
| Other reason | 16 | 7 | 10 | 4 | |||
| Not available | 0 | – | 2 | – | |||
| Histologic response † | |||||||
| Good response | 71 | 36 | 103 | 50 | |||
| Poor response | 128 | 64 | 101 | 50 | |||
| Response unknown | 22 | – | 28 | – | |||
| Surgery not reported | 24 | – | 20 | – | |||
| Surgery performed | |||||||
| Limb salvage | 164 | 74 | 170 | 73 | |||
| Amputation | 38 | 17 | 46 | 20 | |||
| Disarticulation | 9 | 4 | 10 | 4 | |||
| Rotation plasty | 10 | 5 | 6 | 3 | |||
| No surgery reported | 24 | – | 20 | – | |||
| Total included | 245 | 100 | 252 | 100 | |||
| . | Allocated treatment . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Chemotherapy and surgery . | Regimen-C . | . | Regimen-DI . | . | |||
| . | N . | % . | N . | % . | |||
| Given intended number of cycles | |||||||
| Preoperatively | 170 | 69 | 195 | 77 | |||
| Postoperatively | 137 | 54 | 174 | 71 | |||
| Overall | 192 | 78 | 201 | 80 | |||
| Reason for terminating chemotherapy | |||||||
| Treatment completed | 189 | 79 | 201 | 82 | |||
| Disease progression | 11 | 5 | 8 | 3 | |||
| Excessive toxicity | 12 | 5 | 17 | 7 | |||
| Treatment refusal | 12 | 5 | 8 | 3 | |||
| Other reason | 16 | 7 | 10 | 4 | |||
| Not available | 0 | – | 2 | – | |||
| Histologic response † | |||||||
| Good response | 71 | 36 | 103 | 50 | |||
| Poor response | 128 | 64 | 101 | 50 | |||
| Response unknown | 22 | – | 28 | – | |||
| Surgery not reported | 24 | – | 20 | – | |||
| Surgery performed | |||||||
| Limb salvage | 164 | 74 | 170 | 73 | |||
| Amputation | 38 | 17 | 46 | 20 | |||
| Disarticulation | 9 | 4 | 10 | 4 | |||
| Rotation plasty | 10 | 5 | 6 | 3 | |||
| No surgery reported | 24 | – | 20 | – | |||
| Total included | 245 | 100 | 252 | 100 | |||
Regimen-C = conventional regimen; Regimen-DI = dose-intensive regimen.
Good response is ≤10% viable tumor; poor response is >10% viable tumor.
Nearly all (468 or 97%) the patients who had chemotherapy experienced severe (grade 3–4) toxicity ( Table 3 ). Regimen-DI was associated with lower risk of severe leucopenia (RR = 0.85, 95% CI = 0.77 to 0.95) and neutropenia (RR = 0.83, 95% CI = 0.76 to 0.89) and higher risk of thrombocytopenia (RR = 1.33, 95% CI = 1.17 to 1.52) and mucositis (RR = 1.30, 95% CI = 0.99 to 1.70). A small increase in ototoxicity was reported for patients on Regimen-DI. Table 4 shows the number of, and reasons for, dose reductions of 20% or greater of either drug and delays longer than 3 days in the 2639 reported cycles. There were more patients with at least one delay on Regimen-DI than on Regimen-C (190 patients on Regimen-C versus 227 patients on Regimen-DI,
Grade 3 or 4 toxicity during protocol chemotherapy *
| . | Regimen-C . | . | Regimen-DI . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|
| Type . | N . | % . | N . | % . | RR (95% CI) † . | χ 2 , P value . | ||
| WBC ‡ | 193 | 81 | 170 | 69 | 0.85 (0.77 to 0.95) | 9.27, <.001 | ||
| Platelets ‡ | 137 | 58 | 189 | 77 | 1.33 (1.17 to 1.52) | 20.42, <.001 | ||
| Neutrophil ‡ | 220 | 92 | 188 | 76 | 0.83 (0.76 to 0.89) | 23.44, <.001 | ||
| Nausea | 113 | 48 | 131 | 53 | 1.12 (0.94 to 1.33) | 1.50, .22 | ||
| Mucositis | 64 | 27 | 86 | 35 | 1.30 (0.99 to 1.70) | 3.69, .06 | ||
| Infection | 65 | 27 | 61 | 25 | 0.91 (0.67 to 1.23) | 0.40, .53 | ||
| Neurologic | 0 | 0 | 2 | 1 | – | 1.94, .16 | ||
| Ototoxicity | 1 | 0 | 7 | 3 | 6.8 (0.84 to 54.84) | 4.40, .04 | ||
| Cardiac | 6 | 3 | 2 | 1 | 0.32 (0.07 to 1.58) | 2.17, .14 | ||
| Any toxicities | 232 | 97 | 236 | 96 | 0.98 (0.95 to 1.02) | 0.90, .34 | ||
| . | Regimen-C . | . | Regimen-DI . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|
| Type . | N . | % . | N . | % . | RR (95% CI) † . | χ 2 , P value . | ||
| WBC ‡ | 193 | 81 | 170 | 69 | 0.85 (0.77 to 0.95) | 9.27, <.001 | ||
| Platelets ‡ | 137 | 58 | 189 | 77 | 1.33 (1.17 to 1.52) | 20.42, <.001 | ||
| Neutrophil ‡ | 220 | 92 | 188 | 76 | 0.83 (0.76 to 0.89) | 23.44, <.001 | ||
| Nausea | 113 | 48 | 131 | 53 | 1.12 (0.94 to 1.33) | 1.50, .22 | ||
| Mucositis | 64 | 27 | 86 | 35 | 1.30 (0.99 to 1.70) | 3.69, .06 | ||
| Infection | 65 | 27 | 61 | 25 | 0.91 (0.67 to 1.23) | 0.40, .53 | ||
| Neurologic | 0 | 0 | 2 | 1 | – | 1.94, .16 | ||
| Ototoxicity | 1 | 0 | 7 | 3 | 6.8 (0.84 to 54.84) | 4.40, .04 | ||
| Cardiac | 6 | 3 | 2 | 1 | 0.32 (0.07 to 1.58) | 2.17, .14 | ||
| Any toxicities | 232 | 97 | 236 | 96 | 0.98 (0.95 to 1.02) | 0.90, .34 | ||
Regimen-C = conventional regimen; Regimen-DI = dose-intensive regimen; WBC = white blood cell. Missing data (Regimen-C, Regimen-DI): WBC 2, 0; platelets 2, 0; neutrophil 2, 0; nausea 3, 0; mucositis 3, 1; infection 3, 1; neurologic 3, 1; ototoxicity 3, 2; cardiac 3, 1; other toxicities 5, 0; all toxicities 2, 0.
RR = relative risk; CI = confidence interval.
WBC, platelet, and neutrophil toxicity based on nadir counts before, during, and after each cycle.
Grade 3 or 4 toxicity during protocol chemotherapy *
| . | Regimen-C . | . | Regimen-DI . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|
| Type . | N . | % . | N . | % . | RR (95% CI) † . | χ 2 , P value . | ||
| WBC ‡ | 193 | 81 | 170 | 69 | 0.85 (0.77 to 0.95) | 9.27, <.001 | ||
| Platelets ‡ | 137 | 58 | 189 | 77 | 1.33 (1.17 to 1.52) | 20.42, <.001 | ||
| Neutrophil ‡ | 220 | 92 | 188 | 76 | 0.83 (0.76 to 0.89) | 23.44, <.001 | ||
| Nausea | 113 | 48 | 131 | 53 | 1.12 (0.94 to 1.33) | 1.50, .22 | ||
| Mucositis | 64 | 27 | 86 | 35 | 1.30 (0.99 to 1.70) | 3.69, .06 | ||
| Infection | 65 | 27 | 61 | 25 | 0.91 (0.67 to 1.23) | 0.40, .53 | ||
| Neurologic | 0 | 0 | 2 | 1 | – | 1.94, .16 | ||
| Ototoxicity | 1 | 0 | 7 | 3 | 6.8 (0.84 to 54.84) | 4.40, .04 | ||
| Cardiac | 6 | 3 | 2 | 1 | 0.32 (0.07 to 1.58) | 2.17, .14 | ||
| Any toxicities | 232 | 97 | 236 | 96 | 0.98 (0.95 to 1.02) | 0.90, .34 | ||
| . | Regimen-C . | . | Regimen-DI . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|
| Type . | N . | % . | N . | % . | RR (95% CI) † . | χ 2 , P value . | ||
| WBC ‡ | 193 | 81 | 170 | 69 | 0.85 (0.77 to 0.95) | 9.27, <.001 | ||
| Platelets ‡ | 137 | 58 | 189 | 77 | 1.33 (1.17 to 1.52) | 20.42, <.001 | ||
| Neutrophil ‡ | 220 | 92 | 188 | 76 | 0.83 (0.76 to 0.89) | 23.44, <.001 | ||
| Nausea | 113 | 48 | 131 | 53 | 1.12 (0.94 to 1.33) | 1.50, .22 | ||
| Mucositis | 64 | 27 | 86 | 35 | 1.30 (0.99 to 1.70) | 3.69, .06 | ||
| Infection | 65 | 27 | 61 | 25 | 0.91 (0.67 to 1.23) | 0.40, .53 | ||
| Neurologic | 0 | 0 | 2 | 1 | – | 1.94, .16 | ||
| Ototoxicity | 1 | 0 | 7 | 3 | 6.8 (0.84 to 54.84) | 4.40, .04 | ||
| Cardiac | 6 | 3 | 2 | 1 | 0.32 (0.07 to 1.58) | 2.17, .14 | ||
| Any toxicities | 232 | 97 | 236 | 96 | 0.98 (0.95 to 1.02) | 0.90, .34 | ||
Regimen-C = conventional regimen; Regimen-DI = dose-intensive regimen; WBC = white blood cell. Missing data (Regimen-C, Regimen-DI): WBC 2, 0; platelets 2, 0; neutrophil 2, 0; nausea 3, 0; mucositis 3, 1; infection 3, 1; neurologic 3, 1; ototoxicity 3, 2; cardiac 3, 1; other toxicities 5, 0; all toxicities 2, 0.
RR = relative risk; CI = confidence interval.
WBC, platelet, and neutrophil toxicity based on nadir counts before, during, and after each cycle.
Reported reasons for calculated reductions and delays during treatment *
| . | Delays of ≥3 days, No. (%) . | . | Cisplatin reduction ≥20%, No. (%) . | . | Doxorubicin reduction ≥20%, No. (%) . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Deviation type . | Regimen-C . | Regimen-DI . | Regimen-C . | Regimen-DI . | Regimen-C . | Regimen-DI . | |||
| Patients with deviations † | |||||||||
| At least one cycle affected | 190 (80) | 227 (92) | 69 (29) | 70 (28) | 64 (27) | 70 (28) | |||
| No cycles affected | 48 (20) | 19 (8) | 169 (71) | 176 (72) | 174 (73) | 176 (72) | |||
| Total | 238 | 246 | 238 | 246 | 238 | 246 | |||
| Cycles with deviations ‡ | |||||||||
| Yes, reason known | 200 (16) | 343 (25) | 127 (10) | 130 (10) | 113 (9) | 132 (10) | |||
| Yes, reason not known | 220 (17) | 342 (25) | 35 (3) | 16 (1) | 32 (2) | 15 (1) | |||
| No | 862 (67) | 672 (50) | 1120 (87) | 1211 (89) | 1137 (89) | 1210 (89) | |||
| Total | 1282 | 1357 | 1282 | 1357 | 1282 | 1357 | |||
| Reasons for deviations § | |||||||||
| Administrative | 15 (8) | 25 (7) | 3 (2) | 6 (5) | 1 (1) | 6 (5) | |||
| Hematologic | 104 (52) | 205 (60) | 63 (50) | 58 (45) | 62 (55) | 68 (52) | |||
| Renal | 4 (2) | 4 (1) | 6 (5) | 10 (8) | 1 (1) | 0 (0) | |||
| Ototoxicity | 1 (1) | 4 (1) | 6 (5) | 8 (6) | 0 (0) | 0 (0) | |||
| Infection | 32 (16) | 30 (9) | 15 (12) | 7 (5) | 14 (12) | 9 (7) | |||
| Other | 36 (18) | 66 (19) | 19 (15) | 14 (11) | 23 (20) | 31 (23) | |||
| Any combination | 8 (4) | 9 (3) | 15 (12) | 27 (21) | 12 (11) | 18 (14) | |||
| Total | 200 | 343 | 127 | 130 | 113 | 132 | |||
| . | Delays of ≥3 days, No. (%) . | . | Cisplatin reduction ≥20%, No. (%) . | . | Doxorubicin reduction ≥20%, No. (%) . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Deviation type . | Regimen-C . | Regimen-DI . | Regimen-C . | Regimen-DI . | Regimen-C . | Regimen-DI . | |||
| Patients with deviations † | |||||||||
| At least one cycle affected | 190 (80) | 227 (92) | 69 (29) | 70 (28) | 64 (27) | 70 (28) | |||
| No cycles affected | 48 (20) | 19 (8) | 169 (71) | 176 (72) | 174 (73) | 176 (72) | |||
| Total | 238 | 246 | 238 | 246 | 238 | 246 | |||
| Cycles with deviations ‡ | |||||||||
| Yes, reason known | 200 (16) | 343 (25) | 127 (10) | 130 (10) | 113 (9) | 132 (10) | |||
| Yes, reason not known | 220 (17) | 342 (25) | 35 (3) | 16 (1) | 32 (2) | 15 (1) | |||
| No | 862 (67) | 672 (50) | 1120 (87) | 1211 (89) | 1137 (89) | 1210 (89) | |||
| Total | 1282 | 1357 | 1282 | 1357 | 1282 | 1357 | |||
| Reasons for deviations § | |||||||||
| Administrative | 15 (8) | 25 (7) | 3 (2) | 6 (5) | 1 (1) | 6 (5) | |||
| Hematologic | 104 (52) | 205 (60) | 63 (50) | 58 (45) | 62 (55) | 68 (52) | |||
| Renal | 4 (2) | 4 (1) | 6 (5) | 10 (8) | 1 (1) | 0 (0) | |||
| Ototoxicity | 1 (1) | 4 (1) | 6 (5) | 8 (6) | 0 (0) | 0 (0) | |||
| Infection | 32 (16) | 30 (9) | 15 (12) | 7 (5) | 14 (12) | 9 (7) | |||
| Other | 36 (18) | 66 (19) | 19 (15) | 14 (11) | 23 (20) | 31 (23) | |||
| Any combination | 8 (4) | 9 (3) | 15 (12) | 27 (21) | 12 (11) | 18 (14) | |||
| Total | 200 | 343 | 127 | 130 | 113 | 132 | |||
The reasons for deviations are only known where they were reported explicitly; no reasons have been deduced where a reduction or delay was calculated but not reported explicitly. Regimen-C = conventional regimen; Regimen-DI = dose-intensive regimen.
% as percentage of all patients starting chemotherapy.
% as percentage of all cycles administered.
% as percentage of cycles with known reason delay or reduction.
Reported reasons for calculated reductions and delays during treatment *
| . | Delays of ≥3 days, No. (%) . | . | Cisplatin reduction ≥20%, No. (%) . | . | Doxorubicin reduction ≥20%, No. (%) . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Deviation type . | Regimen-C . | Regimen-DI . | Regimen-C . | Regimen-DI . | Regimen-C . | Regimen-DI . | |||
| Patients with deviations † | |||||||||
| At least one cycle affected | 190 (80) | 227 (92) | 69 (29) | 70 (28) | 64 (27) | 70 (28) | |||
| No cycles affected | 48 (20) | 19 (8) | 169 (71) | 176 (72) | 174 (73) | 176 (72) | |||
| Total | 238 | 246 | 238 | 246 | 238 | 246 | |||
| Cycles with deviations ‡ | |||||||||
| Yes, reason known | 200 (16) | 343 (25) | 127 (10) | 130 (10) | 113 (9) | 132 (10) | |||
| Yes, reason not known | 220 (17) | 342 (25) | 35 (3) | 16 (1) | 32 (2) | 15 (1) | |||
| No | 862 (67) | 672 (50) | 1120 (87) | 1211 (89) | 1137 (89) | 1210 (89) | |||
| Total | 1282 | 1357 | 1282 | 1357 | 1282 | 1357 | |||
| Reasons for deviations § | |||||||||
| Administrative | 15 (8) | 25 (7) | 3 (2) | 6 (5) | 1 (1) | 6 (5) | |||
| Hematologic | 104 (52) | 205 (60) | 63 (50) | 58 (45) | 62 (55) | 68 (52) | |||
| Renal | 4 (2) | 4 (1) | 6 (5) | 10 (8) | 1 (1) | 0 (0) | |||
| Ototoxicity | 1 (1) | 4 (1) | 6 (5) | 8 (6) | 0 (0) | 0 (0) | |||
| Infection | 32 (16) | 30 (9) | 15 (12) | 7 (5) | 14 (12) | 9 (7) | |||
| Other | 36 (18) | 66 (19) | 19 (15) | 14 (11) | 23 (20) | 31 (23) | |||
| Any combination | 8 (4) | 9 (3) | 15 (12) | 27 (21) | 12 (11) | 18 (14) | |||
| Total | 200 | 343 | 127 | 130 | 113 | 132 | |||
| . | Delays of ≥3 days, No. (%) . | . | Cisplatin reduction ≥20%, No. (%) . | . | Doxorubicin reduction ≥20%, No. (%) . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Deviation type . | Regimen-C . | Regimen-DI . | Regimen-C . | Regimen-DI . | Regimen-C . | Regimen-DI . | |||
| Patients with deviations † | |||||||||
| At least one cycle affected | 190 (80) | 227 (92) | 69 (29) | 70 (28) | 64 (27) | 70 (28) | |||
| No cycles affected | 48 (20) | 19 (8) | 169 (71) | 176 (72) | 174 (73) | 176 (72) | |||
| Total | 238 | 246 | 238 | 246 | 238 | 246 | |||
| Cycles with deviations ‡ | |||||||||
| Yes, reason known | 200 (16) | 343 (25) | 127 (10) | 130 (10) | 113 (9) | 132 (10) | |||
| Yes, reason not known | 220 (17) | 342 (25) | 35 (3) | 16 (1) | 32 (2) | 15 (1) | |||
| No | 862 (67) | 672 (50) | 1120 (87) | 1211 (89) | 1137 (89) | 1210 (89) | |||
| Total | 1282 | 1357 | 1282 | 1357 | 1282 | 1357 | |||
| Reasons for deviations § | |||||||||
| Administrative | 15 (8) | 25 (7) | 3 (2) | 6 (5) | 1 (1) | 6 (5) | |||
| Hematologic | 104 (52) | 205 (60) | 63 (50) | 58 (45) | 62 (55) | 68 (52) | |||
| Renal | 4 (2) | 4 (1) | 6 (5) | 10 (8) | 1 (1) | 0 (0) | |||
| Ototoxicity | 1 (1) | 4 (1) | 6 (5) | 8 (6) | 0 (0) | 0 (0) | |||
| Infection | 32 (16) | 30 (9) | 15 (12) | 7 (5) | 14 (12) | 9 (7) | |||
| Other | 36 (18) | 66 (19) | 19 (15) | 14 (11) | 23 (20) | 31 (23) | |||
| Any combination | 8 (4) | 9 (3) | 15 (12) | 27 (21) | 12 (11) | 18 (14) | |||
| Total | 200 | 343 | 127 | 130 | 113 | 132 | |||
The reasons for deviations are only known where they were reported explicitly; no reasons have been deduced where a reduction or delay was calculated but not reported explicitly. Regimen-C = conventional regimen; Regimen-DI = dose-intensive regimen.
% as percentage of all patients starting chemotherapy.
% as percentage of all cycles administered.
% as percentage of cycles with known reason delay or reduction.
Received Dose and Dose Intensity Analysis
Preoperative, postoperative, and overall received dose and relative dose intensity of cisplatin and doxorubicin for a given regimen were calculated ( Fig. 4 ). We plotted received dose intensity against received total dose in individual patients for each drug and allocated treatment. Overall, the scatter plots show that Regimen-DI led to more higher doses of drug and greater dose intensity preoperatively, lower doses of drug but great dose intensity postoperatively, and so similar doses of drug but greater dose intensity overall. The clear scattering of values in each of the plots shows the deviation from the summary figures for each treatment and each phase of treatment.
Total dose and dose intensity for patients treated with chemotherapy for osteosarcoma relative to expectations of the conventional regimen. Scatter plots of standardized dose intensity versus standardized total dose of cisplatin (CDDP [ A ]) or doxorubicin (Dox [ B ]) for the preoperative ( top panels ), postoperative ( middle panels ), and the full course of treatment ( lower panels ) achieved for patients on the conventional regimen (Regimen-C [ open circles ]) or the dose-intensive regimen (Regimen-DI [ solid circles ]). Dose and dose intensity values are given as a percent of the expectations of the control regimen. In each graph, the top right quadrant defined by solid lines marks out patients whose dose and dose intensity exceeded 80%. The dashed lines indicate planned dose and dose intensity.
Dose.
The median standardized received cisplatin doses for Regimen-C and Regimen-DI were 565/600 (94%) and 593/600 (99%), respectively, and the median standardized received doxorubicin doses were 428/450 (95%) and 440/450 (98%), respectively ( Table 5 ). Thus, overall, higher total doses of cisplatin and doxorubicin were delivered to patients allocated to the intensified regimen. The explanation may be that, while in both arms the median preoperative received dose for each drug was as planned, there was some evidence of greater postoperative dose reductions in Regimen-C compared to Regimen-DI.
Summary of total dose and dose intensity by allocated regimen *
| . | Regimen-C . | . | . | Regimen-DI . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Observed . | . | . | Observed . | . | ||||
| Timing . | Planned . | Median . | Quartiles . | Planned . | Median . | Quartiles . | ||||
| Preoperative period | ||||||||||
| Duration (days) | 42 | 52 | 45–64 | 42 | 56 | 49–64 | ||||
| Cisplatin dose | 200 | 200 | 199–218 | 300 | 300 | 289–301 | ||||
| Cisplatin DI | ||||||||||
| Regimen-C plan | 100 † | 86 | 76–93 | 150 † | 111 | 95–123 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 74 | 64–82 | ||||
| Doxorubicin dose | 150 | 150 | 149–184 | 225 | 225 | 217–226 | ||||
| Doxorubicin DI | ||||||||||
| Regimen-C plan | 100 † | 86 | 77–94 | 150 † | 111 | 96–124 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 74 | 64–83 | ||||
| Surgical period | ||||||||||
| Preoperative chemotherapy to surgery § (days) | 21 | 26 | 23–30 | 14 | 23 | 19–27 | ||||
| Surgery to postoperative chemotherapy ‖ (days) | 14 | 18 | 14–22 | 14 | 17 | 14–21 | ||||
| Postoperative period | ||||||||||
| Duration (days) | 84 | 87 | 73–98 | 42 | 55 | 48–59 | ||||
| Cisplatin dose | 400 | 335 | 288–400 | 300 | 296 | 219–300 | ||||
| Cisplatin DI | ||||||||||
| Regimen-C plan | 100 † | 82 | 72–93 | 150 † | 110 | 93–121 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 73 | 62–81 | ||||
| Doxorubicin dose | 300 | 253 | 217–299 | 225 | 221 | 165–225 | ||||
| Doxorubicin DI | ||||||||||
| Regimen-C plan | 100 † | 83 | 73–95 | 150 † | 111 | 93–121 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 74 | 62–81 | ||||
| Overall | ||||||||||
| Duration including surgery (days) | 140 | 155 | 144–168 | 98 | 126 | 112–138 | ||||
| Duration excluding surgery (days) | 126 | 139 | 129–150 | 84 | 109 | 97–120 | ||||
| Cisplatin dose | 600 | 565 | 465–600 | 600 | 593 | 493–600 | ||||
| Cisplatin DI ¶ | ||||||||||
| Regimen-C plan | 100 † | 83 | 75–92 | 150 † | 108 | 95–122 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 72 | 63–82 | ||||
| Doxorubicin dose | 450 | 428 | 354–449 | 450 | 440 | 373–450 | ||||
| Doxorubicin DI ¶ | ||||||||||
| Regimen-C plan | 100 * | 83 | 75–92 | 150 † | 109 | 94–122 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 73 | 62–81 | ||||
| G-CSF dose | – | 0 | 0–0 | – | 9202 | (7900–10 598) | ||||
| . | Regimen-C . | . | . | Regimen-DI . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Observed . | . | . | Observed . | . | ||||
| Timing . | Planned . | Median . | Quartiles . | Planned . | Median . | Quartiles . | ||||
| Preoperative period | ||||||||||
| Duration (days) | 42 | 52 | 45–64 | 42 | 56 | 49–64 | ||||
| Cisplatin dose | 200 | 200 | 199–218 | 300 | 300 | 289–301 | ||||
| Cisplatin DI | ||||||||||
| Regimen-C plan | 100 † | 86 | 76–93 | 150 † | 111 | 95–123 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 74 | 64–82 | ||||
| Doxorubicin dose | 150 | 150 | 149–184 | 225 | 225 | 217–226 | ||||
| Doxorubicin DI | ||||||||||
| Regimen-C plan | 100 † | 86 | 77–94 | 150 † | 111 | 96–124 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 74 | 64–83 | ||||
| Surgical period | ||||||||||
| Preoperative chemotherapy to surgery § (days) | 21 | 26 | 23–30 | 14 | 23 | 19–27 | ||||
| Surgery to postoperative chemotherapy ‖ (days) | 14 | 18 | 14–22 | 14 | 17 | 14–21 | ||||
| Postoperative period | ||||||||||
| Duration (days) | 84 | 87 | 73–98 | 42 | 55 | 48–59 | ||||
| Cisplatin dose | 400 | 335 | 288–400 | 300 | 296 | 219–300 | ||||
| Cisplatin DI | ||||||||||
| Regimen-C plan | 100 † | 82 | 72–93 | 150 † | 110 | 93–121 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 73 | 62–81 | ||||
| Doxorubicin dose | 300 | 253 | 217–299 | 225 | 221 | 165–225 | ||||
| Doxorubicin DI | ||||||||||
| Regimen-C plan | 100 † | 83 | 73–95 | 150 † | 111 | 93–121 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 74 | 62–81 | ||||
| Overall | ||||||||||
| Duration including surgery (days) | 140 | 155 | 144–168 | 98 | 126 | 112–138 | ||||
| Duration excluding surgery (days) | 126 | 139 | 129–150 | 84 | 109 | 97–120 | ||||
| Cisplatin dose | 600 | 565 | 465–600 | 600 | 593 | 493–600 | ||||
| Cisplatin DI ¶ | ||||||||||
| Regimen-C plan | 100 † | 83 | 75–92 | 150 † | 108 | 95–122 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 72 | 63–82 | ||||
| Doxorubicin dose | 450 | 428 | 354–449 | 450 | 440 | 373–450 | ||||
| Doxorubicin DI ¶ | ||||||||||
| Regimen-C plan | 100 * | 83 | 75–92 | 150 † | 109 | 94–122 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 73 | 62–81 | ||||
| G-CSF dose | – | 0 | 0–0 | – | 9202 | (7900–10 598) | ||||
Regimen-C = conventional regimen; Regimen-DI = dose-intensive regimen; G-CSF = granulocyte colony-stimulating factor.
Compared to expectations of Regimen-C.
Compared to expectations of Regimen-DI.
Time from first day of last preoperative chemotherapy cycle to day of surgery.
Time from day of surgery to first day of postoperative chemotherapy.
Overall dose intensity was calculated using the overall duration including the surgical period; the surgical period was not included in the preoperative or postoperative DI calculations.
Summary of total dose and dose intensity by allocated regimen *
| . | Regimen-C . | . | . | Regimen-DI . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Observed . | . | . | Observed . | . | ||||
| Timing . | Planned . | Median . | Quartiles . | Planned . | Median . | Quartiles . | ||||
| Preoperative period | ||||||||||
| Duration (days) | 42 | 52 | 45–64 | 42 | 56 | 49–64 | ||||
| Cisplatin dose | 200 | 200 | 199–218 | 300 | 300 | 289–301 | ||||
| Cisplatin DI | ||||||||||
| Regimen-C plan | 100 † | 86 | 76–93 | 150 † | 111 | 95–123 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 74 | 64–82 | ||||
| Doxorubicin dose | 150 | 150 | 149–184 | 225 | 225 | 217–226 | ||||
| Doxorubicin DI | ||||||||||
| Regimen-C plan | 100 † | 86 | 77–94 | 150 † | 111 | 96–124 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 74 | 64–83 | ||||
| Surgical period | ||||||||||
| Preoperative chemotherapy to surgery § (days) | 21 | 26 | 23–30 | 14 | 23 | 19–27 | ||||
| Surgery to postoperative chemotherapy ‖ (days) | 14 | 18 | 14–22 | 14 | 17 | 14–21 | ||||
| Postoperative period | ||||||||||
| Duration (days) | 84 | 87 | 73–98 | 42 | 55 | 48–59 | ||||
| Cisplatin dose | 400 | 335 | 288–400 | 300 | 296 | 219–300 | ||||
| Cisplatin DI | ||||||||||
| Regimen-C plan | 100 † | 82 | 72–93 | 150 † | 110 | 93–121 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 73 | 62–81 | ||||
| Doxorubicin dose | 300 | 253 | 217–299 | 225 | 221 | 165–225 | ||||
| Doxorubicin DI | ||||||||||
| Regimen-C plan | 100 † | 83 | 73–95 | 150 † | 111 | 93–121 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 74 | 62–81 | ||||
| Overall | ||||||||||
| Duration including surgery (days) | 140 | 155 | 144–168 | 98 | 126 | 112–138 | ||||
| Duration excluding surgery (days) | 126 | 139 | 129–150 | 84 | 109 | 97–120 | ||||
| Cisplatin dose | 600 | 565 | 465–600 | 600 | 593 | 493–600 | ||||
| Cisplatin DI ¶ | ||||||||||
| Regimen-C plan | 100 † | 83 | 75–92 | 150 † | 108 | 95–122 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 72 | 63–82 | ||||
| Doxorubicin dose | 450 | 428 | 354–449 | 450 | 440 | 373–450 | ||||
| Doxorubicin DI ¶ | ||||||||||
| Regimen-C plan | 100 * | 83 | 75–92 | 150 † | 109 | 94–122 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 73 | 62–81 | ||||
| G-CSF dose | – | 0 | 0–0 | – | 9202 | (7900–10 598) | ||||
| . | Regimen-C . | . | . | Regimen-DI . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Observed . | . | . | Observed . | . | ||||
| Timing . | Planned . | Median . | Quartiles . | Planned . | Median . | Quartiles . | ||||
| Preoperative period | ||||||||||
| Duration (days) | 42 | 52 | 45–64 | 42 | 56 | 49–64 | ||||
| Cisplatin dose | 200 | 200 | 199–218 | 300 | 300 | 289–301 | ||||
| Cisplatin DI | ||||||||||
| Regimen-C plan | 100 † | 86 | 76–93 | 150 † | 111 | 95–123 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 74 | 64–82 | ||||
| Doxorubicin dose | 150 | 150 | 149–184 | 225 | 225 | 217–226 | ||||
| Doxorubicin DI | ||||||||||
| Regimen-C plan | 100 † | 86 | 77–94 | 150 † | 111 | 96–124 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 74 | 64–83 | ||||
| Surgical period | ||||||||||
| Preoperative chemotherapy to surgery § (days) | 21 | 26 | 23–30 | 14 | 23 | 19–27 | ||||
| Surgery to postoperative chemotherapy ‖ (days) | 14 | 18 | 14–22 | 14 | 17 | 14–21 | ||||
| Postoperative period | ||||||||||
| Duration (days) | 84 | 87 | 73–98 | 42 | 55 | 48–59 | ||||
| Cisplatin dose | 400 | 335 | 288–400 | 300 | 296 | 219–300 | ||||
| Cisplatin DI | ||||||||||
| Regimen-C plan | 100 † | 82 | 72–93 | 150 † | 110 | 93–121 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 73 | 62–81 | ||||
| Doxorubicin dose | 300 | 253 | 217–299 | 225 | 221 | 165–225 | ||||
| Doxorubicin DI | ||||||||||
| Regimen-C plan | 100 † | 83 | 73–95 | 150 † | 111 | 93–121 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 74 | 62–81 | ||||
| Overall | ||||||||||
| Duration including surgery (days) | 140 | 155 | 144–168 | 98 | 126 | 112–138 | ||||
| Duration excluding surgery (days) | 126 | 139 | 129–150 | 84 | 109 | 97–120 | ||||
| Cisplatin dose | 600 | 565 | 465–600 | 600 | 593 | 493–600 | ||||
| Cisplatin DI ¶ | ||||||||||
| Regimen-C plan | 100 † | 83 | 75–92 | 150 † | 108 | 95–122 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 72 | 63–82 | ||||
| Doxorubicin dose | 450 | 428 | 354–449 | 450 | 440 | 373–450 | ||||
| Doxorubicin DI ¶ | ||||||||||
| Regimen-C plan | 100 * | 83 | 75–92 | 150 † | 109 | 94–122 | ||||
| Regimen-DI plan | – | – | – | 100 ‡ | 73 | 62–81 | ||||
| G-CSF dose | – | 0 | 0–0 | – | 9202 | (7900–10 598) | ||||
Regimen-C = conventional regimen; Regimen-DI = dose-intensive regimen; G-CSF = granulocyte colony-stimulating factor.
Compared to expectations of Regimen-C.
Compared to expectations of Regimen-DI.
Time from first day of last preoperative chemotherapy cycle to day of surgery.
Time from day of surgery to first day of postoperative chemotherapy.
Overall dose intensity was calculated using the overall duration including the surgical period; the surgical period was not included in the preoperative or postoperative DI calculations.
Time.
The median time from the start of chemotherapy to surgery was 52 and 56 days for Regimen-C and Regimen-DI, respectively. Including the surgical period, patients allocated to Regimen-C had a median time to completion of 155 days compared to 140 days planned, a relative increase of 10%. Regimen-DI had a median time to completion of 126 days compared to 98 days, an increase of 29%. Excluding the surgical period, patients allocated to Regimen-C had a median time to completion of 139 days compared to 126 days planned, a 10% increase, and Regimen-DI had an actual median time to completion of 109 days compared to 84 days planned, a 30% increase. Overall, the patients in Regimen-DI also experienced more delays than did patients in Regimen-C.
Dose Intensity.
For purposes of comparison, dose intensities were calculated relative to the planned dose intensity for Regimen-C (100%) The median preoperative dose intensity achieved on Regimen-C was 86% for both cisplatin and doxorubicin and it was 111% on Regimen-DI for both drugs ( Table 5 ). Thus, there was a 29% relative increase in actual preoperative dose intensity for Regimen-DI compared to the actual preoperative dose intensity of Regimen-C. Postoperatively, the median cisplatin dose intensities on Regimen-C and Regimen-DI were 82% and 110%, respectively, a relative increase of 34% for Regimen-DI; the median dose intensities for doxorubicin were 83% and 111%, respectively, a relative 33% increase for Regimen-DI. In most cases observed dose intensity was below that planned. Excluding the surgery window, overall dose intensity achieved for cisplatin was 83% in Regimen-C and 108% in Regimen-DI, a relative increase of 29%, and for doxorubicin it was 83% and 109%, respectively, a relative increase of 31%. Thus, in each case, the received chemotherapy for Regimen-DI was more dose intensive than for Regimen-C. With Regimen-DI, overall dose intensity was 73% for doxorubicin and 72% for cisplatin of that expected for Regimen-DI protocol. Only seven Regimen-DI patients achieved a dose intensity of 150% and only 10 Regimen-C patients achieved a dose intensity of 100%. The observed reductions in dose intensity were due to reductions in treatment dose and delays in administering treatment.
Surgery
Surgical data were available for 453 (90%) patients. The median time to surgery in Regimen-C was 52 days (95% CI = 49 to 54), and in Regimen-DI it was 56 days (95% CI = 55 to 57), HR = 0.89, P = .20 ( Fig. 3, B ). Limb salvage was the most common operation and was performed in 164 (74%) and 170 (73%) patients in Regimen-C and regimen-D1, respectively. Of patients who had surgery, 84% underwent the planned operation. There was no evidence of a difference in immediate or late postoperative complication rates according to treatment regimen.
Histologic Response
Histologic diagnosis was confirmed in 461 (93%) patients, and histologic subtype was assessable in 441 (89%) patients of the 497 patients eligible ( Table 1 ). Resected tumor specimens were reviewed in 403 (89%) of the 453 trial patients with surgical details. Good response was observed in 71 (36%) of the assessable Regimen-C patients and 103 (50%) Regimen-DI patients (RR = 1.30; 95% CI = 1.09 to 1.54;
The proportions of Regimen-C and Regimen-DI patients with a good response after two preoperative cycles were 26% (95% CI = 20% to 34%) and 14% (95% CI = 3% to 36%). The proportions of patients with a good response after three preoperative cycles were 40% (95% CI = 26% to 56%) and 45% (95% CI = 38% to 52%) on Regimen-C and Regimen-DI, respectively. Among the 11 Regimen-C and the 20 Regimen-DI patients who received four preoperative cycles, the proportion with a good response was 64% (95% CI = 31% to 89%) and 65% (95% CI = 41% to 85%). Thus, the proportion of patients with observed good histologic response increased with the number of preoperative cycles.
Clinical Outcome Measures
Progression-free survival. Kaplan–Meier estimates of progression-free survival according to the received treatment were derived ( Fig. 5, A ). The estimated 5-year progression-free survival rates were 39% (95% CI = 33% to 46%) for Regimen-C and 41% (95% CI = 34% to 47%) for Regimen-DI (HR = 0.98, 95% CI = 0.77 to 1.24). There was no evidence of a statistically significant difference in progression-free survival between treatment regimens based on the log-rank test (χ 2 = 0.05, P = .83).
Progression-free survival according to allocated treatment. Progression-free survival (PFS) for patients treated with conventional regimen (Regimen-C [ solid line ]) and dose-intensive regimen (Regimen-DI [ dashed line ]) was calculated from the time of randomization (hazard ratio [HR] = 0.98, 95% confidence interval [CI] = 0.77 to 1.24) ( A ) or from 60 days after randomization (HR = 0.82, 95% CI = 0.63 to 1.08) ( B ) when histologic response was known. C ) Progression-free survival for patients allocated to Regimen-C ( left panel ) or Regimen-DI ( right panel ) according to histologic response (good [ solid line ] or poor [ dashed line ]). Hatch marks denote censoring events. Numbers at risk are shown below each graph.
In landmark analyses timed from 60 days after randomization and including 395 patients, the hazard ratio for progression-free survival was 0.82 (95% CI = 0.63 to 1.08; log-rank χ 2 = 2.06, P = .15) in favor of Regimen-DI ( Fig. 5, B ). Stratifying for histologic response (good or poor), the landmark analysis hazard ratio for progression-free survival was 0.88 (95% CI = 0.67 to 1.16; log-rank χ 2 = 0.84, P = .36) in favor of Regimen-DI. Using the landmark time and patient group in a Cox model including allocated treatment, histologic response, and an interaction term for allocated treatment and histologic response, allocated treatment was not a statistically significant determinant of outcome (HR = 1.23, 95% CI = 0.75 to 2.02) whereas histologic response was prognostic (HR = 2.64, 95% CI = 1.69 to 4.12). There was no good evidence of interaction between these factors ( P = .10). Reported progression at any time on Regimen-C and Regimen-DI, respectively, were as follows: local progression 22 (9%) versus 11 (4%), local recurrence 44 (18%) versus 23 (9%), and distant metastases 107 (44%) versus 113 (45%). Regardless of allocated treatment, patients with good histologic response had improved progression-free survival in landmark analyses (compared with patients with poor response, HR = 0.50, 95% CI = 0.38 to 0.66, χ 2 = 23.30, P <.001), with 3-year and 5-year progression-free survival rates of 60% and 56%, respectively, in good-response patients and 38% and 33%, respectively, in poor response patients.
Overall Survival.
At the time of analysis (February 2005), 193 deaths had been reported (98 in Regimen-C and 95 in Regimen-DI). The reported causes of deaths were similar according to allocated treatment with the deaths of 93 patients (95%) on Regimen-C and 88 patients (93%) on Regimen-DI attributed to osteosarcoma. The other 12 deaths were attributed to treatment, although treatment-related deaths occurred during the protocol treatment period for only three patients, all on Regimen-DI. The hazard ratio for overall survival was 0.94 (95% CI = 0.71 to 1.24) with no evidence of a difference between treatment regimens based on Kaplan–Meier estimates ( Fig. 6, A ; log-rank χ 2 = 0.22, P = .64). The 5-year overall survival rates were 55% (95% CI = 48% to 62%) for Regimen-C and 58% (95% CI = 51% to 65%) for Regimen-DI.
Overall survival according to allocated treatment. Survival for patients treated with conventional regimen (Regimen-C [ solid line ]) and dose-intensive regimen (Regimen-DI [ dashed line ]) was calculated from the time of randomization (hazard ratio (HR) = 0.94, 95% confidence interval [CI] = 0.71 to 1.24) ( A ) or from 60 days after randomization (HR = 0.83, 95% CI = 0.60 to 1.15) ( B ) when histologic response was known. C ) Overall survival for patients allocated to Regimen-C ( left panel ) or Regimen-DI ( right panel ) according to histologic response (good [ solid line ] or poor [ dashed line ]). Hatch marks denote censoring events. Numbers at risk are shown below each graph.
In landmark analyses, 145 of the 395 patients eligible for these analyses had died (HR = 0.83, 95% CI = 0.60 to 1.15; log-rank χ 2 = 1.24, P = .26) in favor of Regimen-DI ( Fig. 6, B ). Kaplan–Meier estimates for progression-free survival on both regimens in which patients are stratified by histologic response were derived ( Fig. 6, C ). Stratifying for good and poor histologic response, the landmark analysis hazard ratio for survival was 0.91 (95% CI = 0.66 to 1.26; log-rank χ 2 = 0.31, P = .58) in favor of Regimen-DI. In a Cox model that included allocated treatment, histologic response, and an interaction term for these variables, treatment was not statistically important (HR = 0.89, 95% CI = 0.49 to 1.62) and histologic response was important (HR = 2.01, 95% CI = 1.20 to 3.38), and there was no evidence of interaction between these factors ( P = .92). Regardless of allocated treatment, patients with good histologic response had improved survival over patients with poor histologic response (HR = 0.50, 95% CI = 0.36 to 0.70, χ 2 = 15.52, P = .0001); the 3-year and 5-year survival rates were 78% and 71%, respectively, in good-response patients and 63% and 49%, respectively, in patients with poor response.
In exploratory landmark analyses using the 487 patients who were alive 60 days after randomization, by which time surgery should have been performed, those patients who had surgery but for whom histologic response was not available had poorer survival than both good-response patients (HR = 2.61, 95% CI = 1.53 to 2.75) and poor-response patients (HR = 1.29, 95% CI = 0.76 to 2.20). Patients for whom no surgery was reported had even worse survival compared with good responders (HR = 4.05, 95% CI = 1.97 to 8.31) ( Fig. 7, A ). A similar trend was noted for progression-free survival ( Fig. 7, B ).
Overall and progression-free survival (PFS) according to histologic response and whether surgery was performed. Overall survival ( A ) and progression-free survival ( B ) were calculated from 60 days after randomization for patients on the conventional regimen (Regimen-C, left panels ) or the dose-intensive regimen (Regimen-DI, right panels ) according to histologic response (good [ solid black lines ], poor [ solid gray lines ], or unknown [NK; dashed black lines ] and in cases where surgery was not performed [No surg; gray dashed lines ]). Numbers at risk are shown below each graph.
A series of univariate and multivariate Cox regression models based on chemotherapy and outcome data from 485 patients who received chemotherapy (one patient with a large overdose was excluded) showed no good evidence that received cisplatin dose, cisplatin dose intensity, doxorubicin dose, or doxorubicin dose intensity (whether preoperative or overall) affected overall survival. There was a suggestion that increased preoperative and overall cisplatin dose may be associated with improved survival: each 10% increase in preoperative standardized cisplatin dose was associated with an improvement in survival (HR = 0.87, 95% CI = 0.76 to 0.99) as was each 10% increase in overall standardized cisplatin dose (HR = 0.88, 95% CI = 0.77 to 1.00).
Sensitivity analyses were performed to assess the impact of the modified intention-to-treat approach by examining the results of all 504 randomly assigned patients. The hazard ratios for progression-free survival and overall survival in this total population were the same as for the modified intention-to-treat population (n = 497) that was analyzed.
Discussion
Here, we have presented results for the third EOI randomized controlled trial in osteosarcoma. This trial was designed to prospectively test the hypothesis that increasing the planned dose intensity of the EOI cisplatin and doxorubicin regimen by a factor of 1.5 would improve outcome for patients with nonmetastatic limb osteosarcoma. The trial involved 48 centers in 11 countries. In this complex setting, we have demonstrated that patients randomly assigned to the dose-intensive arm, Regimen-DI, received treatment at increased dose intensity compared to patients in the conventional arm, Regimen-C. Despite the relatively large number of patients, 5-year median follow-up, and more events reported than were planned, the trial was only powered to detect a relatively large 15% absolute difference (HR = 0.6) between arms. Consequently, the trial had limited power to detect a smaller but still clinically relevant benefit of Regimen-DI. With this data an absolute difference in overall survival of 10% cannot be excluded.
Perhaps not surprisingly, patients receiving G-CSF in Regimen-DI had substantially less grade 3–4 neutropenia but statistically significantly increased thrombocytopenia compared with patients in Regimen-C. Hematologic toxicity was the main reason for delays and dose reductions. There were concerns that a dose-dense schedule might lead to increases in other acute toxicities, but there was no good evidence from this trial to support this possibility, except for increases in mucositis and ototoxicity that were of borderline statistical significance. There were also particular concerns before the trial that doxorubicin every 2 weeks might provoke increased early cardiotoxicity, but there was no consistent evidence of this (data not shown).
Overall in this trial, patients receiving Regimen-DI received their total treatment at 31% greater intensity relative to those receiving Regimen-C—less than the planned 50% increase. As expected from clinical experience, the actual intensity of both arms was less than planned. In fact, the observed intensity for Regimen-DI was close to, but more than, the planned intensity for Regimen-C. Although patients in both arms experienced delays and dose reductions, patients allocated to Regimen-C were more likely to have their chemotherapy doses reduced because of toxicity. They were also more likely than those on Regimen-DI to receive treatment on time. Patients allocated Regimen-DI had close to full-dose treatment, but with substantially increased delays. There was no evidence that dose intensity varied systematically according to disease or patient characteristics.
Histologic response to chemotherapy has repeatedly been demonstrated to be a major prognostic indicator in osteosarcoma and is sometimes used as a surrogate marker of outcome (3235). This study confirmed that patients with good response (>90% tumor necrosis) had outcomes that were improved to a statistically significant extent compared to those with poor response (≥10% viable cells), with reported 5-year survival of rates 71% and 51%, respectively. This trial also demonstrated a statistically significant increase in the rate of good histologic response for Regimen-DI over that of Regimen-C (50% versus 36%). However, this advantage in histologic response did not translate into a demonstrable survival benefit. This suggests that histologic response would not be a good surrogate measure despite previous indications, and this observation demands further exploration.
One possible explanation for the fact that histologic response was not associated with overall survival is that the association was obscured by the results of patients whose histologic response was not assessed. In this trial, patients with unknown histologic response had worse outcomes than would have been predicted for a randomly selected representative group of trial patients ( Fig. 7 ). However, the landmark analyses accounted for this by including only patients for whom both surgery and histologic response data were available.
Most Regimen-DI patients received three cycles of preoperative chemotherapy, one cycle more than that received by most Regimen-C patients, and they received a 50% greater preoperative chemotherapy dose. This raises the possibility that histologic response might be dependent on dose per se, rather than on dose intensity. There is support for this hypothesis because exploratory analyses showed similar increases in histologic response rates in both arms according to the number of preoperative cycles. Although the proportion of histologic responders increased with increased standardized preoperative dose, the proportion of responders did not show a clear increase with increasing standardized dose intensity. These observed trends are not based on a randomized comparison and therefore should be treated with caution.
The favorable histologic response rate (50%) for Regimen-DI in this trial is higher than that seen with cisplatin and doxorubicin in previous trials (response rates 30%–35%) ( 6 , 7 ), even though preoperative doses were the same and planned time to surgery was shorter (6 versus 9 weeks). Thus, either increasing dose intensity has led to increased tumor necrosis or the technical assessment of histologic response has changed over time so that increased numbers of tumors are deemed to have responded. The latter possibility seems unlikely given that the methodology was the same as that used in previous EOI trials (with several of the same reference pathologists analyzing the data) ( 43 ) and the fact that changes in assessment should have affected both trial arms. An alternative explanation for the observed increase in favorable histologic response in Regimen-DI is that more intensive chemotherapy delivery alters sensitivity and specificity of the histologic response assessment so that despite the appearance of good histologic response, greater numbers of cells survive. Progression-free survival for patients with good histologic response was slightly less in Regimen-DI than in Regimen-C and for poor responders slightly better ( Fig. 5, C ), suggesting that the interpretation of good response may be different for patients receiving the dose-intensive schedule. Certainly, these results challenge the emphasis placed on histologic response as the key treatment-related prognostic factor ( 20 , 45 ) and suggest that the predictive value of histologic response may be protocol specific. Although inappropriate as a surrogate outcome measure, pathological response to preoperative chemotherapy is able to select for patients with good prognosis.
With the completion of this trial, the EOI has carried out three randomized controlled trials since 1983, all incorporating a control arm planned to deliver six cycles of cisplatin and doxorubicin every 3 weeks with surgery after two or three cycles. None of the research arms (three drug, multidrug, or dose intensified) have demonstrated any statistically significant benefit over this conventional arm. In this trial, the 5-year progression-free survival and overall survival for nonmetastatic high-grade limb osteosarcoma in the conventional arm were 39% and 55%, respectively. These percentages do not compare favorably with outcomes reported by other large cooperative groups. Cooperative Osteosarkomstudiengruppe (COSS) reported 10-year progression-free survival of 49% and overall survival of 60% in 1702 patients entered into all their studies. The COSS trial encompassed several randomized controlled trials and included patients with axial primaries and metastases ( 46 ). The Children's Oncology Group (COG), after conducting the largest multi-institutional randomized controlled trial in osteosarcoma and using the three-drug regimen of cisplatin, doxorubicin, and high-dose methotrexate, reported 3-year progression-free survival and overall survivals of 71% and 80%, respectively ( 11 ). Results of broadly similar magnitude have been reported by other multi-institutional groups although not in randomized controlled trials ( 45 , 47 ). Comparisons across trials and between groups are complex and unreliable, but it is difficult not to conclude there might be identifiable factors accounting for the results from the EOI trials. One difference is that other groups have, in general, used longer regimens with more drugs.
This EOI trial recruited 504 patients over a period of 9 years and is the second largest osteosarcoma randomized controlled trial. Although this is a relatively large randomized controlled trial in the field of osteosarcoma, it clearly has its limitations because of the trial size. EOI has recently joined with other large trials groups (COG, COSS, and Scandinavian Sarcoma Group) to form EURAMOS: the EURopean and AMerican OsteoSarcoma group. This is an international intergroup aiming to develop and conduct randomized controlled trials pertaining to osteosarcoma and associated studies. EURAMOS plans to recruit more than 400 patients per year, and the first EURAMOS randomized controlled trial, EURAMOS 1, opened to recruitment in 2005 ( 48 ). EURAMOS 1 is using pathologic response to stratify patients by risk and then perform separate randomizations for good-response and poor-response patients; the standard arm in each group uses a three-drug regimen of cisplatin, doxorubicin, and high-dose methotrexate; good responders are randomly assigned to continue standard treatment and follow this with maintenance therapy with pegylated interferon; poor responders are randomly assigned to supplement the cisplatin, doxorubicin, and high-dose methotrexate with ifosfamide and etoposide.
Our trial had a number of limitations and compromises. We had to make a pragmatic decision about the timing of surgery, and there were positives and negatives to each of the approaches: either choice could have affected the surrogate outcome of histologic response to preoperative treatment. The negative is that patients did not have a fixed dose of chemotherapy before surgery, but we felt this was outweighed by the positives of ensuring that surgery, the primary therapy for these patients, was at a fixed time on each arm and that histologic response was assessed at the same point in time. The trial took a long time to recruit and mature. Largely, this is because of the rarity of the condition. This has led to the decision to participate with other cooperative groups in future trials. The multicountry nature of the trial added some difficulties, especially where countries vary in their standard approaches to chemotherapy and surgery. In this context, central pathology review was not complete in all cases and data collection was difficult to organize. With the main participating countries, these were not problematic, but data collection and histologic response were more difficult to organize in certain countries which had communication difficulties.
In summary, this was one of the largest trials in osteosarcoma, and it tested the use of dose-intensified perioperative chemotherapy. Both regimens planned the same total chemotherapy doses and both planned surgery at day 42. Most patients experienced some form of grade 3 or 4 toxicity. Regimen-DI resulted in more tumors having good histologic response to preoperative chemotherapy, but this did not translate into a demonstrable patient benefit in overall survival or progression-free survival over Regimen-C. The emphasis placed on histologic response as the key treatment-related predictive factor is thereby challenged ( 20 ). Clinical outcomes measures for this patient group need to be further improved.
Appendix
The EOI was formed in 1982 to facilitate sufficiently sized randomized controlled trials. It has had representation from National Cancer Research Institute Sarcoma Clinical Studies Group, U.K. Children's Cancer Study Group, Société Internationale d'Oncologie Paediatrique (SIOP), and EORTC Soft Tissue and Bone Sarcoma Group.
Writing Committee: I. Lewis, M. R. Sydes, M. Nooij, R. Grimer, P. C. W. Hogendoorn, J. Whelan, B. Uscinska, A. H. M. Taminiau; Chief Investigators: I. Lewis, M. Nooij, W. Steward; Trial/Data Managers: B. Uscinska, A. Kirkpatrick; Statisticians: D. Machin (to 1998), M. van Glabbeke, M. K. B. Parmar (from 1998), S. Weeden (to 2004), M. R. Sydes (from 2004); Analysis: M. R. Sydes; EOI Chairs: J. W. van der Eijken (1990–1995), A. W. Craft (1995–1999), A. Taminiau (from 1999).
MRC Participating Centers (country: number of patients—hospital, oncologists)
U.K. (232): The London Bone Tumor Service (104; J. Whelan, R. L. Souhami, A. M. Kilby, M. Michelagnoli); Leeds St James's University Hospital (16; I. Lewis, M. Leahy); Newcastle Royal Victoria Infirmary (14; A. W. Craft, J. P. Hale); Manchester Christie Hospital (10; H. R. Gattamaneni, B. Brennan, D. Crowther, O. B. Eden); Llandough Hospital (8; M. B. Phillips, M. English, M. E. Jenney); Glasgow Royal Hospital for Sick Children (7; E. Simpson); Southampton General Hospital (7; J. Kohler, M. Radford); Liverpool Alder Hey Hospital (6; H. McDowell, J. Martin, B. L. Pizer); Birmingham Children's Hospital (6; B. J. Morland); Edinburgh Royal Hospital for Sick Children (6; H. Wallace); Sutton Royal Marsden Hospital (6; C. R. Pinkerton, K. Pritchard-Jones); Bath Royal United Hospital (4; E. D. Gilby); Cambridge Addenbrooke's Hospital (4; V. A. Broadbent, H. Earl, D. Williams); Leicester Royal Infirmary (4; D. Heney, R. S. Shannon, W. P. Steward); Manchester Royal Children's Hospital (4; B. Brennan); Nottingham City Hospital (4; P. J. Woll, M. P. J. Sokal); Oxford John Radcliffe Hospital (4; C. Mitchell, K. Wheeler); Birmingham Royal Orthopaedic Hospital (3; D. Spooner, D. Peake); Newcastle Northern Centre for Cancer Treatment (3; M. Verrill, J. T. Roberts); Cardiff Velindre Hospital (2; P. F. Salaman); Glasgow Beatson Oncology Centre (2; A. Barrett); Sheffield Children's Hospital (2; M. P. Gerrard); Aberdeen Royal Children's Hospital (1; D. J. King); London Great Ormond Street Hospital (1; J. Pritchard); London Royal Hospital (1; A. G. Shankar); Nottingham University Hospital (1; D. A. Walker); Oxford Churchill Hospital (1; A. C. Jones); Poole General Hospital (1; G. P. Clein); Argentina ( 39 ): Buenos Aires, Hospital De Pediatria G. P. Garrahan (39; S. Casak, P. Zubizarreta, M. Scopinaro); Chile ( 19 ): Santiago, Luis Calvo Mackenna Hospital (19; J. Quintana); Canada ( 2 ): London, Ontario London Regional Cancer Centre (2; V. Bramwell).
EORTC Participating Centers (country: number of patients—hospital, oncologists)
The Netherlands (78): Leiden University Hospital (40; M. Nooij); UMC Nijmegen (20; J. Bokkerink); Amsterdam, Emma Kinderziekenhuis (12; H. van den Berg); Amsterdam, Onze Lieve Vrouw Gasthuis (5; K. Rozendaal); Utrecht UMC Academisch Ziekenhuis (1; A. de Graeff); Saudi Arabia (52): Riyadh, King Faisal Specialist Hospital and Research Centre (52; M. Memon); Belgium ( 38 ): Leuven, U. Z. Gasthuisberg (14; A. T. Van Oosterom, A. Uyttebreock); Brussels, Cliniques Universitaires St Luc (11; B. Brichard, A. Zenebergh); Universitair Ziekenhuis Antwerpen (8; J. B. Vermorken); Brussels Institut Jules Bordet (5; A. Awada, T. H. Gil); Denmark ( 25 ): Aarhus Kommunehospital (23; O. S. Nielsen); Copenhagen, Rigshospitalet (2; C. Rechnitzer); South Africa ( 6 ): Pretoria Academic Hospital University (6; L. de Jager); Greece ( 5 ): Athens, Aghia Sophia Children's Hospital (5; S. Polychronopoulou); Portugal ( 5 ): Instituto Portugues de Oncologia de Francisco Gentil—Centro De Lisboa (5; A. Fernandes); France ( 2 ): Rennes, Centre Eugene Marquis (2; P. Kerbrat); Slovenia ( 1 ): The Institute of Oncology (1; B. Zakotnic).
MRC Surgeons (who performed seven or more trial operations)
United Kingdom: S. R. Cannon, R. J. Grimer, T. W. R. Briggs, J. Cobb, S. R. Carter, S. A. Murray, J. Pooley; Argentina: J. L. Massa; Chile: N. Chamas.
EORTC Surgeons (who performed seven or more trial operations)
The Netherlands: A. H. M. Taminiau, R. P. H. Veth, J. Van der Eijken; SaudiArabia: D. Younge; Belgium: I. Sanson, C. H. Delloye, J. Somville; Denmark: R. Weth.
MRC Reference Pathologists
J. Pringle, C. Mangham, A. Malcolm
EORTC Reference Pathologists
E. Hauben, H. Noel, J. Bras, P. C. W. Hogendoorn
Trial Steering Committee
H. Kitchener (chair), T. Maughan, M. Seymour
Independent Data Monitoring Committee
O. Dalesio, A. Gregor, H. Jurgens (chair)
The trial was supported and coordinated by the U.K. MRC and the EORTC. The funding agencies had no role in the design of this trial, data collection, analysis and interpretation of the results, or the writing of the manuscript.
Discounted growth factor support was supplied from Amgen and Chugain on an individual basis with hospitals.
The trial received initial input from the Cancer Therapy Committee.
Preliminary analyses were presented in part at ASCO 2003, SIOP 2003, ECCO 2003, and BCRM 2003.
We thank Robert Souhami, Mahesh Parmar, and the U.K. Children's Cancer Study Group Manuscript Review Committee for their helpful comments.
Funding to pay the Open Access publication charges for this article was provided by Medical Research Council, UK.
References
Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, Belasco JB, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity.
Eilber F, Giuliano A, Eckardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial.
Winkler K, Beron G, Kotz R, Salzer-Kuntschik M, Beck J, Beck W, et al. Neoadjuvant chemotherapy for osteogenic sarcoma: results of a Cooperative German/Austrian study.
Krailo M, Ertel I, Makley J, Fryer CJ, Baum E, Weetman R, et al. A randomized study comparing high-dose methotrexate with moderate-dose methotrexate as components of adjuvant chemotherapy in childhood nonmetastatic osteosarcoma: a report from the Childrens Cancer Study Group.
Bramwell VH, Burgers M, Sneath R, Souhami R, van Oosterom AT, Voute PA, et al. A comparison of two short intensive adjuvant chemotherapy regimens in operable osteosarcoma of limbs in children and young adults: the first study of the European Osteosarcoma Intergroup.
Souhami RL, Craft AW, Van der Eijken JW, Nooij M, Spooner D, Bramwell VH, et al. Randomised trial of two regimens of chemotherapy in operable osteosarcoma: a study of the European Osteosarcoma Intergroup.
Provisor AJ, Ettinger LJ, Nachman JB, Krailo MD, Makley JT, Yunis EJ, et al. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children's Cancer Group.
Fuchs N, Bielack SS, Epler D, Bieling P, Delling G, Korholz D, et al. Long-term results of the co-operative German-Austrian-Swiss osteosarcoma study group's protocol COSS-86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs.
Bacci G, Briccoli A, Ferrari S, Longhi A, Mercuri M, Capanna R, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremity: long-term results of the Rizzoli's 4th protocol.
Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate.
Ochs JJ, Freeman AI, Douglass HO Jr, Higby DS, Mindell ER, Sinks LF. cis-Dichlorodiammineplatinum (II) in advanced osteogenic sarcoma.
Baum ES, Gaynon P, Greenberg L, Krivit W, Hammond D. Phase II study of cis-dichlorodiammineplatinum(II) in childhood osteosarcoma: Children's Cancer Study Group Report.
Cortes EP, Holland JF, Wang JJ, Sinks LF. Doxorubicin in disseminated osteosarcoma.
Jaffe N, Paed D, Farber S, Traggis D, Geiser C, Kim BS, et al. Favorable response of metastatic osteogenic sarcoma to pulse high-dose methotrexate with citrovorum rescue and radiation therapy.
Rosen G. Preoperative (neoadjuvant) chemotherapy for osteogenic sarcoma: a ten year experience.
Harris MB, Cantor AB, Goorin AM, Shochat SJ, Ayala AG, Ferguson WS, et al. Treatment of osteosarcoma with ifosfamide: comparison of response in pediatric patients with recurrent disease versus patients previously untreated: a Pediatric Oncology Group study.
Harris MB, Gieser P, Goorin AM, Ayala A, Shochat SJ, Ferguson WS, et al. Treatment of metastatic osteosarcoma at diagnosis: a Pediatric Oncology Group Study.
Ettinger LJ, Douglass HO Jr, Higby DJ, Mindell ER, Nime F, Ghoorah J, et al. Adjuvant adriamycin and cis-diamminedichloroplatinum (cis-platinum) in primary osteosarcoma.
DeVita VT. Principles of cancer management: chemotherapy. In: DeVita VT, Hellman S, Rosenberg SA, editors. Principles and practice of oncology. 5th ed. Philadelphia (PA): Lipincott-Raven;
Coldman AJ, Goldie JH. Impact of dose-intense chemotherapy on the development of permanent drug resistance.
Hryniuk W, Levine MN. Analysis of dose intensity for adjuvant chemotherapy trials in stage II breast cancer.
van Rijswijk RE, Haanen C, Dekker AW, de Meijer AJ, Verbeek J. Dose intensity of MOPP chemotherapy and survival in Hodgkin's disease.
Meyer RM, Hryniuk WM, Goodyear MD. The role of dose intensity in determining outcome in intermediate-grade non-Hodgkin's lymphoma.
Pettengell R, Crowther D. Haemopoietic growth factor and dose intensity in high-grade and intermediate-grade lymphoma.
Cheung NV, Heller G. Chemotherapy dose intensity correlates strongly with response, median survival, and median progression-free survival in metastatic neuroblastoma.
Savarese DM, Hsieh C, Stewart FM. Clinical impact of chemotherapy dose escalation in patients with hematologic malignancies and solid tumors.
Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741.
Cocconi G, Bella M, Lottici R, Leonardi F, Ceci G, Passalacqua R, et al. Mature results of a prospective randomized trial comparing a three-weekly with an accelerated weekly schedule of cisplatin in advanced ovarian carcinoma.
Hudis CA, Schmitz N. Dose-dense chemotherapy in breast cancer and lymphoma.
Norton L. Theoretical concepts and the emerging role of taxanes in adjuvant therapy.
Smith MA, Ungerleider RS, Horowitz ME, Simon R. Influence of doxorubicin dose intensity on response and outcome for patients with osteogenic sarcoma and Ewing's sarcoma.
Bacci G, Picci P, Ferrari S, Casadei R, Brach DP, Tienghi A, et al. Influence of adriamycin dose in the outcome of patients with osteosarcoma treated with multidrug neoadjuvant chemotherapy: results of two sequential studies.
Delepine N, Delepine G, Bacci G, Rosen G, Desbois JC. Influence of methotrexate dose intensity on outcome of patients with high grade osteogenic osteosarcoma. Analysis of the literature.
Bacci G, Ferrari S, Picci P, Zolezzi C, Gherlinzoni F, Iantorno D, et al. Methotrexate serum concentration and histological response to multiagent primary chemotherapy for osteosarcoma of the limbs.
Lewis IJ, Weeden S, Machin D, Stark D, Craft AW. Received dose and dose-intensity of chemotherapy and outcome in nonmetastatic extremity osteosarcoma. European Osteosarcoma Intergroup.
Ornadel D, Souhami RL, Whelan J, Nooy M, Ruiz de Elvira C, Pringle J, et al. Doxorubicin and cisplatin with granulocyte colony-stimulating factor as adjuvant chemotherapy for osteosarcoma: phase II trial of the European Osteosarcoma Intergroup.
Raymond A, Ayala A, Knuutila S. Conventional osteosarcoma. In: Fletcher C, Unni K, Mertens F, editors. World Health Organisation Classification of Tumours. Pathology and genetics of tumours of soft tissue and bone. Lyon (France): IARC Press;
Matsuno T, Okada K, Knuutila S. Telangiectatic osteosarcoma. In: Fletcher C, Unni K, Mertens F, editors. World Health Organisation Classification of Tumours. Pathology and genetics of tumours of soft tissue and bone. Lyon (France): IARC Press;
Kalil R, Bridge J. Small cell osteosarcoma. In: Fletcher C, Unni K, Mertens F, editors. World Health Organisation Classification of Tumours. Pathology and genetics of tumours of soft tissue and bone. Lyon (France): IARC Press;
Handbook for reporting results of cancer treatment. Geneva (Switzerland): World Health Organization;
Steinherz LJ, Graham T, Hurwitz R, Sondheimer HM, Schwartz RG, Shaffer EM, et al. Guidelines for cardiac monitoring of children during and after anthracycline therapy: report of the Cardiology Committee of the Childrens Cancer Study Group.
Hauben EI, Weeden S, Pringle J, Van Marck EA, Hogendoorn PC. Does the histological subtype of high-grade central osteosarcoma influence the response to treatment with chemotherapy and does it affect overall survival? A study on 570 patients of two consecutive trials of the European Osteosarcoma Intergroup.
Parmar MKB, Machin D. Survival analysis: a practical approach. 1st ed. Chichester (U.K.): John Wiley & Sons;
Smeland S, Muller C, Alvegard TA, Wiklund T, Wiebe T, Bjork O, et al. Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders.
Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols.
Ferrari S, Smeland S, Mercuri M, Bertoni F, Longhi A, Ruggieri P, et al. Neoadjuvant chemotherapy with high-dose lfosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups.
EURAMOS. Available at: http://www.euramos.org . [Last accessed: December


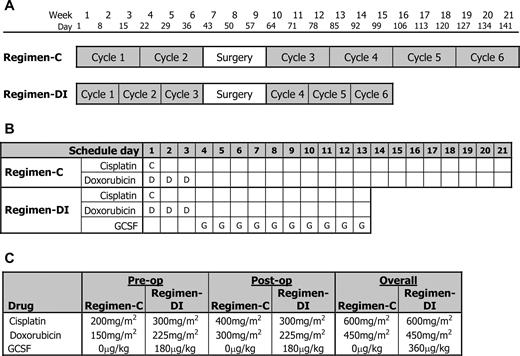
![Trial flow diagram (Consolidated Standards of Reporting Clincial Trials [CONSORT] diagram).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jnci/99/2/10.1093_jnci_djk015/2/m_jncidjk015f02_ht.jpeg?Expires=1716387770&Signature=moiyQp67ylC8r3b-CvgcX98iPrrRoLwg7pCMbQyHQUWeF~wwPsxOYKEZzKVzNOYKAc-uqdUaw0LCVVFa3p6HUzZlhj2SSacP8kCzUw47Gzb-bFjqYPwpj81xCVHvzWuiRWHVdqMn8iCB-JRx~57MIa4QGzjZxT6tABDX~-gta5ZS9Bk6Au3qo2LjXKWZQUh~zUSYa~1fRqFcW~~2X5r6aXR-B-7alxtr8bBxWBD5xZZxQRxvOqHd3u0ZLe9o-FVskP8U6GEWU4YETXNhBg4EzLxhUPf2UQtl-9m9oyHnZG1Rpsc55VHb8VqVd5JfBCBn3Vb-nfqD~mMIgWFAcEJrYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Kaplan–Meier analysis of time from randomization to trial milestones. The proportion of patients in the conventional regimen (Regimen-C [ solid line ]) and the dose-intensive regimen (Regimen-DI [ dashed line ]) not yet having completed chemotherapy (A) and not yet having completed surgery if surgery was eventually performed (B) are shown. Surgery was expected at 42 days.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jnci/99/2/10.1093_jnci_djk015/2/m_jncidjk015f03_lw.jpeg?Expires=1716387770&Signature=3~~3-YhzdPhZ7Cztgd8XyVqgSFNOQJ2Icqr3-iQJA3ouiw9c8ox4zNLjZGSHxJ7xtvevhB9pZ7mblgWIEoL7an32ZOkkTDKzC423hQH70OkcTmL8KcwPmTV8Acg~~Br4CCf8jZBukbqPGTMSvv2UoRiY9ru7G6BVq0Sid9L1tV7IzRi0iMEtcXX9RDAZgQnGShpyk83R2z-gKUon0dzD6~2dSDYK6B-L9t2PkIdImxM5imglJ3I4HIfefpbQjymZzm1jsfTRNvLY88Z5tqffSVXm1acxoqWpewqwa29SJKKhzInRoMh5cfz6VKOLyrmRThiFgL4KLWUEg9fbhrIqPw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Total dose and dose intensity for patients treated with chemotherapy for osteosarcoma relative to expectations of the conventional regimen. Scatter plots of standardized dose intensity versus standardized total dose of cisplatin (CDDP [ A ]) or doxorubicin (Dox [ B ]) for the preoperative ( top panels ), postoperative ( middle panels ), and the full course of treatment ( lower panels ) achieved for patients on the conventional regimen (Regimen-C [ open circles ]) or the dose-intensive regimen (Regimen-DI [ solid circles ]). Dose and dose intensity values are given as a percent of the expectations of the control regimen. In each graph, the top right quadrant defined by solid lines marks out patients whose dose and dose intensity exceeded 80%. The dashed lines indicate planned dose and dose intensity.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jnci/99/2/10.1093_jnci_djk015/2/m_jncidjk015f04_lw.jpeg?Expires=1716387770&Signature=xcVLljHvawJdCwCmzbXsh34uqeCoZEIwVkiZMCxBDd0J7vX0w-8GcG5w9GmmwFCWNtbQvq37uBQAo607SvZ~cvSTcY2EC32CG8UMFsjdlwhkadMWSTRfVoYxmpIZGFvlNboM7e9D~ws5zQSoZ-220hZ3joYBZzWEPW19P30V5amLY4JLiGPGQPfH1g1Cs4NXZ7w774yTMH6LEPyuLoJ1JsynjS3zGr-QNkCXZZiNQMBYV2NO-CzqFBS5an5gV8XfvMolEhcK15LapvtN9a2wp2cgQ5bh8G1mvGMmYhwhvMyRWdkCvE~QaSRWVrM82mgbpydJlAMqUv4xpEQz435E7A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Progression-free survival according to allocated treatment. Progression-free survival (PFS) for patients treated with conventional regimen (Regimen-C [ solid line ]) and dose-intensive regimen (Regimen-DI [ dashed line ]) was calculated from the time of randomization (hazard ratio [HR] = 0.98, 95% confidence interval [CI] = 0.77 to 1.24) ( A ) or from 60 days after randomization (HR = 0.82, 95% CI = 0.63 to 1.08) ( B ) when histologic response was known. C ) Progression-free survival for patients allocated to Regimen-C ( left panel ) or Regimen-DI ( right panel ) according to histologic response (good [ solid line ] or poor [ dashed line ]). Hatch marks denote censoring events. Numbers at risk are shown below each graph.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jnci/99/2/10.1093_jnci_djk015/2/m_jncidjk015f05_ht.jpeg?Expires=1716387770&Signature=bnaBMU5g9Qnnl5HlEjlriAXfULCe957bYoRmovBv30Bt98Da6YdcTqDvnURFYRCx8Kl2M6K~iepB46MqN-uw4FGerDeRBU3fBgYc9t-CUTIdhYclWwq6wHdsmVclBQqIJBHhwHU-3kJ8Zolaty0EY1s2Jmu4qWxatrevSb01Zl3JcER9R9aCA5ONDqeRwHAuOIYB9y8kEv-EmdrSxcZXqMiUDQtc~TQblq54YwO6chdJyKo~M0y6XK82f1NvDLSaSuS9KzUKdDakrbQbgojSsM4B8X5dZzkz9iGDDXPpugyxVW9XBf5Ug5~AP-koL6nY0VZLO7YjfaPBtJ6ato6Ecg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Overall survival according to allocated treatment. Survival for patients treated with conventional regimen (Regimen-C [ solid line ]) and dose-intensive regimen (Regimen-DI [ dashed line ]) was calculated from the time of randomization (hazard ratio (HR) = 0.94, 95% confidence interval [CI] = 0.71 to 1.24) ( A ) or from 60 days after randomization (HR = 0.83, 95% CI = 0.60 to 1.15) ( B ) when histologic response was known. C ) Overall survival for patients allocated to Regimen-C ( left panel ) or Regimen-DI ( right panel ) according to histologic response (good [ solid line ] or poor [ dashed line ]). Hatch marks denote censoring events. Numbers at risk are shown below each graph.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jnci/99/2/10.1093_jnci_djk015/2/m_jncidjk015f06_ht.jpeg?Expires=1716387770&Signature=K69-eDED9VvGCX13Ffie2nF1CXiVYQpUPbaYU6DKHPLvkDA2eGORGFGbPnuGYUdoxoGXyo9Tplx4SDQIoUF7vizp8Bb~oGAVFhj4Yc-BOSWeJfK4vcXYeWJr44IpD50b~a7omJaLgQHuOraUeI3Z2551vaTktu~RAkVQXduKnEk8xRZlyJaw90fEBhdm5m~MrBxo7WuVN-lMi7v~wBzuNPHIHPq0Ixa96QZzhhn4qAYc9WtMP0D-8BTCj7ybrjVor8p5AUDGHUSff1DDFLIqTHEIcFQcZAtRpbX63PpGOlKRYsZ-AJiWeaO0fXglPiuD8I4PZ~YeQOZlbUKUuew8Fg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Overall and progression-free survival (PFS) according to histologic response and whether surgery was performed. Overall survival ( A ) and progression-free survival ( B ) were calculated from 60 days after randomization for patients on the conventional regimen (Regimen-C, left panels ) or the dose-intensive regimen (Regimen-DI, right panels ) according to histologic response (good [ solid black lines ], poor [ solid gray lines ], or unknown [NK; dashed black lines ] and in cases where surgery was not performed [No surg; gray dashed lines ]). Numbers at risk are shown below each graph.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jnci/99/2/10.1093_jnci_djk015/2/m_jncidjk015f07_ht.jpeg?Expires=1716387770&Signature=PLLl5bStqyCsqMxhzsJbnRbL1roHNffW8Ctjhz2mvDtSqKpIcdAYFrkW-2XRTqPUrqBpcwth7kH6q6RfgoAle73Nwl6dJpKj0uu99y129j-Lb0~WqXQMz02KP6agP3aLpE~z6NRpGxFe~AdciNys7X2y1EXD0~TLUQ5Is33xvgOaQfK7jqUsgoLc3-f3DdlgtupSq4f-AM76pAlpq5oMJ-KHloamBVkS0XA4zOSfMmwDLwTHTStXWazrv5-A1Lw52eSL1UGtXYe1IP10pwWwRhWm3YCMxh5q2MC5-olNeMtg5gbTJCCpK7oQppI-yeuYZaxmbP4iDVFmjR8kkLrYFQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
