-
PDF
- Split View
-
Views
-
Cite
Cite
Mitch Dowsett, Ian E. Smith, Stephen R. Ebbs, J. Michael Dixon, Anthony Skene, Roger A'Hern, Janine Salter, Simone Detre, Margaret Hills, Geraldine Walsh, On behalf of the IMPACT Trialists Group, Prognostic Value of Ki67 Expression After Short-Term Presurgical Endocrine Therapy for Primary Breast Cancer, JNCI: Journal of the National Cancer Institute, Volume 99, Issue 2, 17 January 2007, Pages 167–170, https://doi.org/10.1093/jnci/djk020
Close - Share Icon Share
Abstract
Tumor expression of the proliferation antigen Ki67 is widely used to assess the prognosis of cancer patients. A change in the expression of Ki67 after short-term exposure of patients to therapeutic agents is frequently used as a pharmacodynamic marker of efficacy, particularly among breast cancer patients before undergoing surgery. To determine the clinical significance of the level of tumor cell proliferation during endocrine therapy for breast cancer, we measured the expression of Ki67 in tumor biopsy samples taken before and after 2 weeks of presurgical treatment with anastrozole or tamoxifen or the combination of anastrozole plus tamoxifen in 158 patients with hormone receptor–positive primary disease. In a multivariable analysis, we found that higher Ki67 expression after 2 weeks of endocrine therapy was statistically significantly associated with lower recurrence-free survival ( P = .004) whereas higher Ki67 expression at baseline was not. Larger baseline tumor size and lower estrogen receptor level after 2 weeks of treatment were also statistically significantly associated with poorer recurrence-free survival ( P <.001 and P = .04, respectively). Our data indicate that measurements of tumor Ki67 level after short-term endocrine treatment may improve the prediction of recurrence-free survival by integrating the prognostic value of Ki67 level at baseline with changes in Ki67 level that are associated with treatment benefit.
Tumor expression of the cell proliferation antigen Ki67 is widely used to select the best treatment for and to predict the prognosis of breast cancer patients. However, it is not known if tumor Ki67 expression after short-term endocrine therapy can predict recurrence-free survival of individual patients or if it is a more accurate predictor of recurrence-free survival than measurements of tumor Ki67 before treatment.
Ki67 levels in breast tumor biopsy samples taken before and after 2 weeks of presurgical endocrine treatment were evaluated as predictors of recurrence-free survival in postmenopausal primary breast cancer patients enrolled in the IMPACT trial.
Higher tumor Ki67 expression predicted worse recurrence-free survival better after 2 weeks of adjuvant therapy than before adjuvant therapy.
Measurements of tumor Ki67 expression after short-term endocrine treatment may improve the prediction of recurrence-free survival for breast cancer patients.
The conclusions were based on a small number of patients. Larger studies are needed to confirm the results.
Adjuvant medical therapy for patients with primary breast cancer has led to a substantial reduction in mortality from this disease ( 1 ). However, adjuvant therapy trials to test the many new agents that are in development would require thousands of patients, many years of follow-up, and very large resources. In the absence of such trial data, there is an urgent need for biomarkers of efficacy that can reliably predict long-term outcomes. Endocrine treatments for breast cancer appear to act largely by inhibiting tumor cell proliferation. Thus, markers of tumor cell proliferation, such as Ki67, are candidate markers of efficacy that can be evaluated after short-term (e.g., 2 weeks) treatment of primary breast cancer patients, before they undergo surgery.
We have previously reported ( 2 ) that among estrogen receptor (ER)–positive postmenopausal patients with primary breast cancer enrolled in the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) trial, 2 weeks of treatment with the aromatase inhibitor anastrozole suppressed Ki67 expression (measured as a percentage of baseline expression) statistically significantly more than tamoxifen alone or tamoxifen in combination with anastrozole. In an indirect comparison, both the change in tumor Ki67 expression as a percentage of baseline expression and the absolute level of tumor Ki67 expression after 2 weeks of treatment paralleled the greater recurrence-free survival observed among more than 9300 patients treated with anastrozole versus tamoxifen or the combination in an adjuvant trial (the Arimidex, Tamoxifen, Alone or in Combination [ATAC] trial) ( 3 ), suggesting that short-term change in Ki67 expression is a valid predictor of long-term outcome.
On the basis of these findings, we postulated that measurements of residual tumor proliferation after 2 weeks of endocrine therapy as assessed by Ki67 expression might predict the recurrence-free survival of individual patients and that this measurement might be a more accurate predictor of this long-term outcome than measurements of tumor Ki67 before treatment, which would not reflect the effects of treatment. We therefore compared the expression of Ki67 (percentage of immunoreactive tumor cells) after 2 weeks with the expression of Ki67 at baseline as predictors of recurrence-free survival among patients enrolled in the IMPACT trial. This was an unplanned, exploratory analysis.
IMPACT was a double-blind, double-placebo, multicenter trial in which previously untreated postmenopausal women with ER-positive invasive primary breast cancer were randomly assigned to receive anastrozole (1 mg) plus tamoxifen placebo, tamoxifen (20 mg) plus anastrozole placebo, or tamoxifen plus anastrozole once a day for 12 weeks before they underwent surgery ( 2 , 4 ). The same treatment given as neoadjuvant treatment continued as adjuvant treatment. Clinical tumor size was measured using a caliper. Clinical nodal status at baseline was determined by physical examination. Pathologic nodal status was unavailable in this trial at the 2-week analysis point. Core-cut biopsy samples were obtained before therapy (i.e., at baseline) and at 2 weeks after the start of therapy (nonobligatory), and excision biopsy samples were obtained at surgery. Biopsy samples were fixed in neutral-buffered formalin for 24–48 hours and embedded in paraffin wax, and sections of 3–4 μm were taken and stained for Ki67 using a mouse monoclonal antibody (MIB-1 at 1 : 50 dilution; DakoCytomation, Cambridge, U.K.) and for apoptosis using the terminal deoxynucleotidyltransferase-mediated UTP end-labeling (TUNEL) method to incorporate biotinylated deoxyuridine (Roche Diagnostics, Mannheim, Germany) at DNA strand breaks with terminal deoxynucleotidyltransferase (Amersham Biosciences, Piscataway, NJ). Light microscopic visualization of the incorporated biotinylated deoxyuridine was performed after the addition of an avidin–peroxidase conjugate followed by 3′3′diaminobenzidine, as previously described ( 5 ). Ki67- and TUNEL-positive cells were defined by the presence of brown staining nuclei; TUNEL-positive cells were also required to show classical apoptotic morphology (e.g., shrinkage of cytoplasm and condensed misshapen nuclei). The percentages of Ki67- and TUNEL-positive cells were scored among 1000 and 3000 malignant cells, respectively. ER levels were measured immunohistochemically using a mouse monoclonal antibody (clone 6F11; Novocastra, Newcastle upon Tyne, U.K.) at a 1 : 40 dilution and assessed by the H-score, which incorporates both the intensity of staining (scored from 0 to 3) and the percentage of cells stained and ranges from 0 (no ER expression) to 300 (high ER expression).
A total of 330 patients were entered into the IMPACT trial ( 4 ). Baseline biopsy samples were available for 241 eligible patients and 2-week biopsy samples were available for 159 patients ( 2 ). Paired biopsy samples (i.e., baseline and 2-week samples) were available for 158 eligible patients. The primary endpoints of the IMPACT trial—objective response and change in the percentage of Ki67-positive cells between baseline and 2 weeks of treatment—were previously reported in detail, without regard to recurrence-free survival ( 2 , 4 ). The study was approved first by a national multicenter ethics committee and subsequently by individual local research committees of all participating centers. All patients gave written informed consent before study enrollment.
A Cox proportional hazards regression model was used for multivariable analysis of the association between Ki67 expression and recurrence-free survival after transformation of the percentage of Ki67-positive cells to natural logarithms to normalize the data, and hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated for a 1-unit increase in log Ki67. The model was developed using forward stepwise regression and was checked using backward stepwise regression. Both methods gave the same results. Compliance with the proportional hazards assumption was assessed by fitting time-dependent covariates that allowed the hazard ratios to differ with follow-up time. The P values for the covariate size, 2-week ER, and 2-week Ki67 were .67, .95, and .38, respectively. Recurrence-free survival was defined as the time from randomization to first local or distant recurrence; patients who did not have a recurrence were censored on the date of their last follow-up. Kaplan–Meier curves were used to estimate recurrence-free survival according to approximate tertiles of log Ki67, and a log-rank test for trend was used to compare the curves. P less than or equal to .05 was considered statistically significant, and all statistical tests were two-sided.
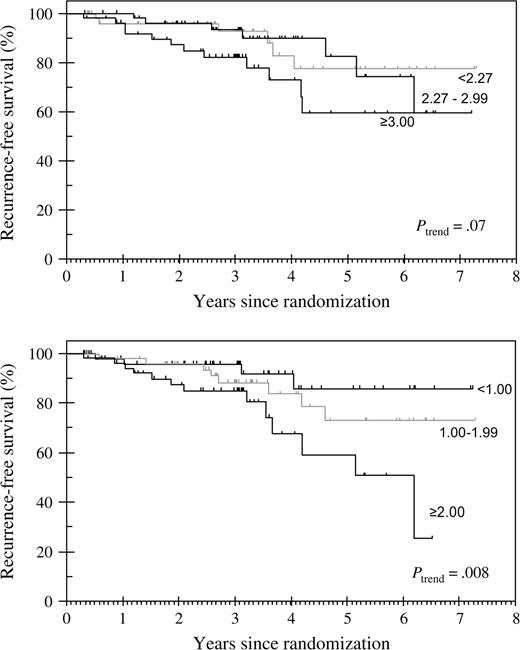
After a median follow-up of 37 months (range = 4–88 months), the median duration of therapy was 30 months. At the 37-month time point, there were 25 relapses: two were local, 18 were distant, and five were both local and distant. Univariate analyses revealed that larger baseline tumor size, higher percentage of Ki67-positive tumor cells at baseline and after 2 weeks of treatment, and lower ER level at baseline and after 2 weeks of treatment were statistically significantly inversely associated with recurrence-free survival ( Table 1 ). Clinical nodal status at baseline and percentage of apoptotic (i.e., TUNEL-positive) cells at baseline and after 2 weeks of treatment were not associated with recurrence-free survival. The type of endocrine treatment received was also not statistically significantly associated with recurrence-free survival: the ATAC trial required many thousands of patients to reveal such associations ( 3 ). The Kaplan–Meier curves for recurrence-free survival ( Fig. 1 ) according to tertiles of tumor Ki67 expression suggested that tumor Ki67 expression after 2 weeks of treatment (log-rank P = .008) was more strongly associated with recurrence-free survival than tumor Ki67 expression at baseline (log-rank P = .07). There was no consistent separation of the curves for the lower two tertiles of baseline Ki67 expression. By contrast, all three curves for tertiles of 2-week Ki67 expression showed substantial and consistent separation after a median duration of treatment of approximately 2.5 years, with 5-year recurrence-free survival rates of 85%, 75%, and 60% for the lowest, middle, and highest tertiles of 2-week Ki67 expression, respectively.
Recurrence-free survival according to tertiles of tumor Ki67 expression at baseline (top panel) and after 2 weeks of treatment (bottom panel) . The divisions refer to the natural logarithm of the percentage of Ki67-positive cells at 2 weeks.
Univariate and multivariable analyses of associations between factors at baseline and after 2 weeks of treatment and recurrence-free survival *
| . | Univariate analysis . | . | Multivariable analysis † . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Factor . | No. of events/No. of patients . | HR (95% CI) . | P . | HR (95% CI) . | P . | |||
| Tumor size at baseline, per 1-cm increase | 25/156 | 1.66 (1.35 to 2.04) | <.001 | 1.69 (1.36 to 2.1) | <.001 | |||
| Nodal status at baseline, positive vs. negative | 25/157 | 1.43 (0.6 to 3.44) | .42 | – | – | |||
| Ki67 expression at baseline, per 2.7-fold increase | 25/157 | 1.85 (1.06 to 3.22) | .03 | – | – | |||
| Ki67 expression after 2 wk of treatment, per 2.7-fold increase | 25/158 | 2.09 (1.41 to 3.08) | <.001 | 1.95 (1.23 to 3.07) | .004 | |||
| ER level at baseline, per 2.7-fold increase | 25/158 | 0.35 (0.2 to 0.62) | <.001 | – | – | |||
| ER level after 2 wk of treatment, per 2.7-fold increase | 25/154 | 0.62 (0.5 to 0.77) | <.001 | 0.78 (0.62 to 0.99) | .04 | |||
| TUNEL level at baseline, per 2.7-fold increase | 25/148 | 1.52 (0.76 to 3.03) | .24 | – | – | |||
| TUNEL level after 2 wk of treatment, per 2.7-fold increase | 23/141 | 1.65 (0.86 to 3.16) | .13 | – | – | |||
| Adjuvant chemotherapy after surgery, yes vs. no | 25/158 | 0.88 (0.35 to 2.21) | .78 | – | – | |||
| . | Univariate analysis . | . | Multivariable analysis † . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Factor . | No. of events/No. of patients . | HR (95% CI) . | P . | HR (95% CI) . | P . | |||
| Tumor size at baseline, per 1-cm increase | 25/156 | 1.66 (1.35 to 2.04) | <.001 | 1.69 (1.36 to 2.1) | <.001 | |||
| Nodal status at baseline, positive vs. negative | 25/157 | 1.43 (0.6 to 3.44) | .42 | – | – | |||
| Ki67 expression at baseline, per 2.7-fold increase | 25/157 | 1.85 (1.06 to 3.22) | .03 | – | – | |||
| Ki67 expression after 2 wk of treatment, per 2.7-fold increase | 25/158 | 2.09 (1.41 to 3.08) | <.001 | 1.95 (1.23 to 3.07) | .004 | |||
| ER level at baseline, per 2.7-fold increase | 25/158 | 0.35 (0.2 to 0.62) | <.001 | – | – | |||
| ER level after 2 wk of treatment, per 2.7-fold increase | 25/154 | 0.62 (0.5 to 0.77) | <.001 | 0.78 (0.62 to 0.99) | .04 | |||
| TUNEL level at baseline, per 2.7-fold increase | 25/148 | 1.52 (0.76 to 3.03) | .24 | – | – | |||
| TUNEL level after 2 wk of treatment, per 2.7-fold increase | 23/141 | 1.65 (0.86 to 3.16) | .13 | – | – | |||
| Adjuvant chemotherapy after surgery, yes vs. no | 25/158 | 0.88 (0.35 to 2.21) | .78 | – | – | |||
Factors found to be statistically significantly associated with recurrence-free survival in the univariate analysis ( P ≤.05) were included in the multivariable analysis. Values of these parameters were transformed using natural logarithms before they were entered into the Cox regression model. A unit change on the transformed scale represents a 2.7-fold change on the untransformed scale. HR = hazard ratio; CI = confidence interval; – = not statistically significant in the univariate or multivariable analyses; ER = estrogen receptor; TUNEL = terminal deoxynucleotidyltransferase-mediated UTP end-labeling.
Number of events/number of patients: 25/152. The numbers of patients differ for the different factors because there were insufficient cells in the tumor sections to make a reliable estimate in a small number of cases and because clinical nodal status was not recorded for one patient.
Univariate and multivariable analyses of associations between factors at baseline and after 2 weeks of treatment and recurrence-free survival *
| . | Univariate analysis . | . | Multivariable analysis † . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Factor . | No. of events/No. of patients . | HR (95% CI) . | P . | HR (95% CI) . | P . | |||
| Tumor size at baseline, per 1-cm increase | 25/156 | 1.66 (1.35 to 2.04) | <.001 | 1.69 (1.36 to 2.1) | <.001 | |||
| Nodal status at baseline, positive vs. negative | 25/157 | 1.43 (0.6 to 3.44) | .42 | – | – | |||
| Ki67 expression at baseline, per 2.7-fold increase | 25/157 | 1.85 (1.06 to 3.22) | .03 | – | – | |||
| Ki67 expression after 2 wk of treatment, per 2.7-fold increase | 25/158 | 2.09 (1.41 to 3.08) | <.001 | 1.95 (1.23 to 3.07) | .004 | |||
| ER level at baseline, per 2.7-fold increase | 25/158 | 0.35 (0.2 to 0.62) | <.001 | – | – | |||
| ER level after 2 wk of treatment, per 2.7-fold increase | 25/154 | 0.62 (0.5 to 0.77) | <.001 | 0.78 (0.62 to 0.99) | .04 | |||
| TUNEL level at baseline, per 2.7-fold increase | 25/148 | 1.52 (0.76 to 3.03) | .24 | – | – | |||
| TUNEL level after 2 wk of treatment, per 2.7-fold increase | 23/141 | 1.65 (0.86 to 3.16) | .13 | – | – | |||
| Adjuvant chemotherapy after surgery, yes vs. no | 25/158 | 0.88 (0.35 to 2.21) | .78 | – | – | |||
| . | Univariate analysis . | . | Multivariable analysis † . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Factor . | No. of events/No. of patients . | HR (95% CI) . | P . | HR (95% CI) . | P . | |||
| Tumor size at baseline, per 1-cm increase | 25/156 | 1.66 (1.35 to 2.04) | <.001 | 1.69 (1.36 to 2.1) | <.001 | |||
| Nodal status at baseline, positive vs. negative | 25/157 | 1.43 (0.6 to 3.44) | .42 | – | – | |||
| Ki67 expression at baseline, per 2.7-fold increase | 25/157 | 1.85 (1.06 to 3.22) | .03 | – | – | |||
| Ki67 expression after 2 wk of treatment, per 2.7-fold increase | 25/158 | 2.09 (1.41 to 3.08) | <.001 | 1.95 (1.23 to 3.07) | .004 | |||
| ER level at baseline, per 2.7-fold increase | 25/158 | 0.35 (0.2 to 0.62) | <.001 | – | – | |||
| ER level after 2 wk of treatment, per 2.7-fold increase | 25/154 | 0.62 (0.5 to 0.77) | <.001 | 0.78 (0.62 to 0.99) | .04 | |||
| TUNEL level at baseline, per 2.7-fold increase | 25/148 | 1.52 (0.76 to 3.03) | .24 | – | – | |||
| TUNEL level after 2 wk of treatment, per 2.7-fold increase | 23/141 | 1.65 (0.86 to 3.16) | .13 | – | – | |||
| Adjuvant chemotherapy after surgery, yes vs. no | 25/158 | 0.88 (0.35 to 2.21) | .78 | – | – | |||
Factors found to be statistically significantly associated with recurrence-free survival in the univariate analysis ( P ≤.05) were included in the multivariable analysis. Values of these parameters were transformed using natural logarithms before they were entered into the Cox regression model. A unit change on the transformed scale represents a 2.7-fold change on the untransformed scale. HR = hazard ratio; CI = confidence interval; – = not statistically significant in the univariate or multivariable analyses; ER = estrogen receptor; TUNEL = terminal deoxynucleotidyltransferase-mediated UTP end-labeling.
Number of events/number of patients: 25/152. The numbers of patients differ for the different factors because there were insufficient cells in the tumor sections to make a reliable estimate in a small number of cases and because clinical nodal status was not recorded for one patient.
Multivariable analysis of variables that were statistically significantly associated with recurrence-free survival in the univariate analysis revealed that baseline tumor size and 2-week Ki67 expression remained strongly associated with recurrence-free survival (baseline tumor size HR = 1.69, 95% CI = 1.36 to 2.1, P <.001; 2-week Ki67 HR = 1.95, 95% CI = 1.23 to 3.07, P = .004) and that 2-week ER level also remained statistically significantly associated with recurrence-free survival, although the magnitude of the latter association had weakened (HR = 0.98, 95% CI = 0.62 to 0.99, P = .04). We reasoned that if the change in the percentage of Ki67-positive tumor cells after treatment was more strongly associated with recurrence-free survival than the absolute percentage of Ki67-positive tumor cells after 2 weeks of treatment, then Ki67 expression at both baseline and after 2 weeks of treatment should be statistically significantly associated with recurrence-free survival in the multivariable analysis, but this was not the case. Although the baseline values for both Ki67 positivity and ER positivity were moderately correlated with the respective 2-week values (Spearman's ρ = .57 [Ki67] and .46 [ER]), when we used the change in Ki67 expression in the multivariable model (instead of the absolute level of Ki67 at 2 weeks), the hazard ratio decreased from 1.95 (95% CI = 1.23 to 3.07) to 1.39 (95% CI = 0.91 to 2.13; P = .13). When both baseline and 2-week Ki67 expression were entered into the multivariable model, the hazard ratio for baseline Ki67 expression was 1.09 (95% CI = 0.60 to 1.99), indicating that this variable was not statistically significantly associated with recurrence-free survival.
This study is small, and thus, our findings must be interpreted cautiously; however, they suggest some potentially important and novel principles with regard to molecular prognostication. First, our data provide support for the notion that the effect of 2 weeks of endocrine treatment on tumor cell Ki67 expression is indicative of the effect of the treatment in terms of long-term patient outcome. Second, the data have important implications for predicting the outcome for individual patients receiving adjuvant endocrine therapy. It is logical that the change in tumor cell Ki67 expression might be related to treatment benefit, and it is well known (and confirmed here) that tumor cell Ki67 expression before treatment is related to prognosis ( 6 , 7 ); by extrapolation we infer that measurements of tumor cell Ki67 expression after 2 weeks of treatment may integrate the intrinsic prognostic value of baseline Ki67 expression measurements and the predictive value of the endocrine treatment–determined changes in Ki67 expression. For these reasons, we expected that tumor cell Ki67 expression after 2 weeks of treatment would predict outcomes on the respective therapies better than tumor cell Ki67 expression at baseline. Indeed our finding that baseline Ki67 expression was not statistically significantly associated with recurrence-free survival in the multivariable model supports the concept that patients who have high baseline Ki67 expression but low 2-week Ki67 expression should have a clinical outcome similar to that of patients who have low baseline Ki67 expression. However, because of the small number of patients in this study, it was not possible to confirm this equivalence. Thus, this concept requires further study before it is widely accepted.
It is possible that the concept of improving the accuracy of prognostic assessment by conducting this assessment on tumor biopsy samples taken after exposure to therapy may apply to other molecular pathologic markers. We have reported in a different series of breast cancer patients that tumor expression of more than 2800 genes changed after 2 weeks of treatment with an aromatase inhibitor (false discovery rate = 0.01), suggesting that biologically responsive tumors undergo radical changes at the transcriptional level in response to short-term therapy ( 8 ). Several studies have reported that RNA profiles from primary breast carcinomas have prognostic value ( 9 , 10 ), and a small number of clinical trials have been designed to test the impact of introducing tumor RNA profiling into clinical practice ( 11 ). Our findings on the improved prognostic value of Ki67 expression after short-term endocrine therapy, when considered in the light of the differences in tumor gene expression we observed after 2 weeks of treatment with an aromatase inhibitor, suggest that molecular profiling of treated tumors could be more predictive of outcome on a particular treatment than molecular profiling of tumors before treatment. However, it is also plausible that 2 weeks of treatment could affect the expression of some of the key factors known to be associated with prognosis (e.g., the progesterone receptor), which would weaken their predictive values if they were measured after several weeks of treatment. We plan to test the hypothesis that molecular prognostication is improved when conducted on tumors after short-term endocrine treatment as a primary aim of the Peri-Operative Endocrine Treatment for Individualizing Care trial, a large randomized trial of 2-week perioperative treatment with an aromatase inhibitor or not in women with primary hormone receptor–positive breast cancer. If in that trial expression of Ki67 or other molecular indices are found to have clinically significant value for predicting recurrence-free survival, only a modest change in clinical practice with minimal resource implications would be required to deliver this type of predictive testing on a routine basis, by initiating adjuvant endocrine treatment 2 weeks before surgery rather than afterward.
M. Dowsett receives grant funding and honoraria for lecturing and attending advisory board meetings from AstraZeneca (manufacturer of Arimidex). I. E. Smith receives honoraria for lecturing and attending advisory board meetings from AstraZeneca. A. Skene conducted research supported by AstraZeneca and received educational grants and advisory panel professional fess from AstraZeneca.
The conduct of the IMPACT trial and the collection of pathology and biomarker data used in this work were supported by AstraZeneca. The follow-up and statistical analyses required for this research were supported by funding from The Royal Marsden Hospital. Neither of these funding sources was involved in the design or conduct of this analysis, the writing of the manuscript or the decision to submit the manuscript for publication, or the interpretation of the data.
The authors are grateful to the numerous investigators in the IMPACT trial for their collaborative support of these studies.
References
Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials.
Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, Griffith C, et al.; IMPACT Trialists. Short-term changes in Ki67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival.
Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial.
Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer J-U, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial.
Mainwaring PN, Ellis PA, Detre S, Smith IE, Dowsett M. Comparison of in situ methods to assess DNA cleavage in apoptotic cells in patients with breast cancer.
Locker AP, Birrell K, Bell JA, Nicholson RI, Elston CW, Blamey RW, et al. Ki67 immunoreactivity in breast carcinoma: relationships to prognostic variables and short term survival.
Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki67 in early breast cancer.
MacKay A, Dixon JM, Urruticoechea A, Dexter T, Iravani M, Fenwick K, et al. Molecular determinants of aromatase inhibitor sensitivity in primary breast cancer.
Van de Vijver MJ, He YD, Van't Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer.
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer.