-
PDF
- Split View
-
Views
-
Cite
Cite
Bunichi Ezaki, Kiyokuni Sasaki, Hideaki Matsumoto, Susumu Nakashima, Functions of two genes in aluminium (Al) stress resistance: repression of oxidative damage by the AtBCB gene and promotion of efflux of Al ions by the NtGDI1gene, Journal of Experimental Botany, Volume 56, Issue 420, October 2005, Pages 2661–2671, https://doi.org/10.1093/jxb/eri259
Close - Share Icon Share
Abstract
The functions of two genes whose expression provides tolerance to aluminium (Al) stress were investigated using plants and Saccharomyces cerevisiae (yeast): the Arabidopsis thaliana blue copper binding gene (AtBCB) and Nicotiana tabacumguanosine diphosphate (GDP) dissociation inhibitor gene (NtGDI1). To determine the localization of these proteins, each gene was fused to the green fluorescent protein (GFP) gene and introduced into onion epidermal cells. AtBCB was localized to cell membrane region and NtGDI1 to cytoplasm. Transgenic lines over-expressing the AtBCB gene showed constitutive lignin production in whole roots. By contrast, wild-type Arabidopsis (Ler) produced a negligible level of lignin and enhanced lignin production in the root-tip region by Al stress. Compared with Ler, the AtBCB-expressing lines showed a lower deposition of malon dialdehyde after Al stress. Microscopic observation of the Al-treated roots indicated that the deposition of lipid peroxides was clearly low in the area where lignin accumulated. It was proposed that lipid peroxidation caused by Al stress was diminished by the formation of lignin. Expression of the NtGDI1 gene in yeast complemented the temperature-sensitive phenotype of a sec19 mutant at 37 °C. This gene also complemented an Al-sensitive phenotype shown by the sec19 mutant at the permissive temperature of 32 °C. These results suggested that the yeast Sec19 vesicle transport system has a function in providing basal Al resistance in yeast by the export of Al ions. It was also proposed that over-expression of the NtGDI1 protein activates an Al efflux system that protects Arabidopsis against Al toxicity.
Introduction
Aluminium (Al) ions, especially Al3+, have a toxic effect on both plant and animal cells under low pH conditions. Inhibition of root growth is the major symptom of Al toxicity in plants, and is accompanied by an accumulation of Al ions in the cell walls of roots (see reviews by Matsumoto et al., 2003; Kochian et al., 2004). Al ions have been suggested to enhance peroxidation of phospholipids and proteins in cell membranes (Cakmak and Horst, 1991; Yamamoto et al., 2001). These interactions finally inhibit cell division and cell elongation in root tips. Many studies concerning Al resistance mechanisms in plants have been reported and the secretion of organic acid anions, such as malate, oxalate, citrate, and succinate, from root tips into soil is considered the most effective strategy (Delhaize et al., 1993; de la Fuente et al., 1997; Ma et al., 2001). Recently, the ALMT1 gene encoding a malate transporter was isolated from wheat. It was shown that this gene can confer Al tolerance to tobacco cells (Sasaki et al., 2004). However, it is clear that there are other Al resistance mechanisms in plants. Over-expression in Arabidopsis thaliana of 11 genes induced by Al stress has led to the identification of four genes that confer Al resistance: the AtBCB gene encoding an Arabidopsis blue cupper binding protein (Gysel et al., 1993; Richards et al., 1998), the parB gene encoding a tobacco glutathione-S-transferase (Takahashi and Nagata, 1992; Ezaki et al., 1995), the NtPox gene encoding a tobacco moderate anionic peroxidase (Ezaki et al., 1996), and the NtGDI1 gene encoding a tobacco GDP-dissociation inhibitor (Ezaki et al., 2000). The resistance mechanisms of these four genes appear not to involve the secretion of organic acid anions. In particular, the total enzyme activities of glutathione-S-transferase or peroxidase in transgenic lines expressing either the parB or NtPox genes were significantly higher than in a non-transformed control line (Ler) and lipid peroxidation caused by Al stress was repressed in these two transgenic lines. It was therefore concluded that over-expression of these two genes diminishes the oxidative damage caused by Al stress (Ezaki et al., 2001).
Influx and efflux experiments of Al ions in both Saccharomyces cerevisiae (yeast) transformants and Arabidopsis transgenic lines suggested that the AtBCB gene may suppress Al absorption, while over-expression of the NtGDI1 gene promotes a release of Al from the cytosol to the outside (Ezaki et al., 1999, 2001). However, precise resistance mechanisms of these two genes for Al stress have not been clarified completely. The BCB gene family has been related to various aspects of metabolism, such as (i) catalysing the transfer of electrons in photosynthesis (Gysel et al., 1993; Aram et al., 1998), (ii) lignification of the cell wall fraction (Drew and Gatehouse, 1994), and (iii) control of uptake of Fe, Mn, and Zn (Lin and Wu, 1994). The AtBCB gene is induced by Al (Richards et al., 1998) as well as by oxidative stress (Richards et al., 1998; Miller et al., 1999). The GDI gene family is involved in vesicle transport. Rab proteins represent a family of more than 30 different Ras-like GTPases that regulate the commitment of transport vesicles for targeting and/or fusion to specific acceptor membranes through a conformational change between the GTP and GDP forms (see reviews by Ferro-Novik and Novik, 1993; Pfeffer et al., 1995). Membrane targeting is accompanied by the displacement of GDI, followed by the exchange of bound GDP for GTP. GDIs have the capacity to deliver Rab proteins to their specific membrane-bound compartments and to retrieve Rabs from their fusion targets after they have completed a catalytic cycle. The GDI gene family has been well studied in yeast and mammalian cells. Garrett et al. (1994) have shown that depletion of yeast GDI using a regulated promoter led to multiple defects in protein transport and that the GDI1 gene is in fact allelic with an already identified pleiotropic secretory mutant gene, sec19. Ueda et al. (1996) reported that the expression of Arabidopsis AtGDI1 gene complemented the sec19 (gdi1) mutation.
In this study, the tolerance mechanisms of the two genes, AtBCB and NtGDI1, were characterized in detail using yeast and plants. Over-expression of AtBCB in the transgenic Arabidopsis caused an accumulation of lignin and a decrease of lipid peroxides. Al toxicity in yeast could be also ameliorated by a vesicle transport system in which the sec19 gene is involved. It was also supposed that the NtGDI1 gene has a similar function in plants. The results indicate that there are several different Al resistance mechanisms in plants and yeast in addition to the chelation of Al ions by organic acid anions.
Materials and methods
Growth conditions of plants and yeast cells
Arabidopsis thaliana ecotype Landsberg erecta (Ler, non-transgenic control line) and transgenic lines over-expressing the AtBCB or NtGDI1 genes (Ezaki et al., 2000) were grown under fluorescent light (approximately 50 μE m−2 s−1, 16/8 h light/dark) at 22 °C. A modified MS medium (Murashige and Skoog, 1962) in which MS salts and B5 vitamins were diluted six times (1/6 MS medium) was used (adjusted to pH 4.2). The 1/6 MS medium also contained 10 g l−1 sucrose as a carbon source.
Yeast sec19- temperature-sensitive mutant (ANS19-4) and its parental sec19+ wild-type strain (ANY21) were supplied by Dr Takashi Ueda (Riken, Japan) (Ueda et al., 1996). Low phosphate and magnesium (LPM) liquid medium or LPM agar plates (1.5% agar in LPM liquid medium) adjusted to pH 4.2 were used for yeast growth and metal sensitivity tests (MacDiarmid and Gardner, 1998). Yeast cells were grown at 22, 32, or 37 °C.
Construction of plasmids carrying green fluorescent protein (GFP) fusion genes and plant transformation
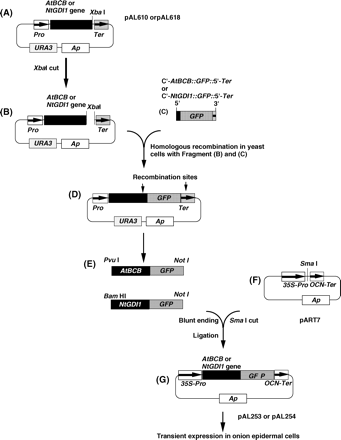
To confirm the localization of the AtBCB and NtGDI1 protein in plant cells, two plasmids (AtBCB∷GFP=pAL253 and NtGDI1∷GFP=pAL254) carrying fusions with the S65T-type GFP gene (Niwa et al., 1999) were constructed in this study (Fig. 1). Plasmids pAL610 or pAL618 carrying the AtBCB or NtGDI1 genes (Fig. 1A; Ezaki et al., 1999) were completely digested with XbaI at 37 °C for 3 h (Fig. 1B). Using AtBCB-F or NtGDI1-F as forward primers and pYES2Ter-R as a reverse primer, the GFP DNA fragment was amplified by PCR (Fig. 1C). The sequence of AtBCB-F, NtGDI1-F, and pYES2Ter-R are shown below.
AtBCB-F; 5′-GCTACTTTTCTGGTCGCTTTTGTTTCTGCTGTTGTTGCTCTCTTTATGAGAG GAGAACTTTTCACTGG-3′
NtGDI1-F; 5′-GTTCTTGACCTCAATGTGGATCTAAGTGCTGCTAGTGCCGCCGAAGAAAT GAGAGGAGAACTTTTCACTGG-3′
pYES2Ter-R; 5′-GGGAGGGCGTGAATGTAAGCGTGACATAACTAATTACATGATTTATTTGTATAGTT CATCCATGCCA-3′
Construction of the NtGDI1∷GFP and AtBCB∷GFP fusion genes. (A) to (G) represent starting, intermediate and end-products in the construction of GFP fusion plasmids. Plasmids pAL253, pAL254, and pAL255 were finally used for transient expression assays in onion epidermal cells.
The sequences in bold are derived from the 3′-terminal region of the AtBCB or NtGDI1 genes; the underlined sequences are from the GFP gene; the italic characters are from the 5′-terminator region of the CYC1 transcription terminator in pYES2. PCR was performed for 30 cycles (denaturing at 94 °C for 60 s, annealing at 55 °C for 60 s, and extension at 72 °C for 90 s). PCR products (C'-AtBCB∷GFP∷5′-Ter and C'-NtGDI1∷GFP∷5′-Ter) (Fig. 1C) were applied to a DNA sequencer to confirm their sequences. Fragments (Fig. 1B) and (Fig. 1C) were mixed and then introduced into yeast cells to cause a homologous recombination between the two fragments. Recombinant plasmids (Fig. 1D) were extracted from the yeast transformants and then amplified once in Escherichia coli. The fusion genes, AtBCB∷GFP and NtGDI1∷GFP, were obtained by complete digestion with PvuI/NotI or BamHI/NotI, respectively (Fig. 1E). These fragments were blunt-ended by T4 DNA polymerase and then ligated with SmaI-digested pART7 (Fig. 1F; Gleave, 1992). The constructed plasmids (Fig. 1G), pAL253 and pAL254, were finally used for transient expression assays. A control plasmid carrying only the GFP gene (pAL255) was also constructed in this study. According to the method described by Takumi et al. (1994), the constructed plasmids were introduced into onion epidermal cells by particle bombardment for transient expression.
Quantitative determination of lignin content in the root region
Cell wall fractions were prepared according to the method described by Tokunaga et al. (2005). Whole roots of 10-d-old plants (approximately 3 cm length) were washed twice with deionized water and homogenized with a homogenizer in 95% ethanol. After a spin down, the pellet was washed three times with 95% ethanol and twice with ethanol:hexane (1:2, v/v). The washed pellet was allowed to air-dry at 70 °C for 10 h. Lignin content was measured according to the method of Fukuda and Komamine (1982) with some modifications. Five mg of the air-dried samples suspended in a 1 ml aliquot of 25% acetyl bromide in acetic acid were treated at 70 °C for 30 min. After cooling down at 25 °C, 0.9 ml of 2 M NaOH, 5 ml of acetic acid, 0.1 ml of 7.5 M hydroxylamine hydrochloride, and 3 ml of glacial acetic acid were added. The 10 ml samples were centrifuged and the absorbance of the supernatant was measured at 280 nm to determine the lignin content.
Microscopic observations and detection of malon dialdehyde (MDA)
Morin staining and 2′,7′-dichlorofluorescein diacetate (H2DCFDA) staining for Al-treated or untreated plants were performed as described previously (Ezaki et al., 2000). The suitability of morin staining in Al localization is well known (Ezaki et al., 2000; Eticha et al., 2005). Detection of lignin was also performed according to the phloroglucinol staining (Monties, 1989). To monitor the precise localization of (i) Al ions, (ii) lipid peroxides, and (iii) lignin in the same roots, Al-treated roots were fixed on a glass slide, and stained with morin, H2DCFDA, and phloroglucinol in that order. Pictures were taken after each staining. Quantitative determination of MDA in Al-treated or untreated plants was according to the method described by Ono et al. (1995).
Yeast transformation and experimental condition of sensitivity tests
A Yep-type yeast expression vector, pYES2 (Smith et al., 1995) was used for transformation experiments in this study. Yeast cells were transformed with the vector DNA (pYES2) or the NtGDI1 gene (pAL618; Ezaki et al., 1999) using the lithium acetate method described by Gietz et al. (1992) and then used for growth tests at 22, 32, or 37 °C. In sensitivity tests, log phase yeast cells were diluted to 1/5, 1/25, 1/125, 1/625, and 1/3125 and then spotted on appropriate agar plates. Complementation of the sec19− mutation and sensitivity for Al stress were estimated by colony-forming ability on the plates after 3–4 d incubation. Final concentrations of 0–500 μM Al or 0–400 μM La were added to the LPM agar plates for individual sensitivity tests.
Uptake and release of Al ions by yeast cells
Log-phase yeast cells were grown in LPM medium at 32 °C, centrifuged, suspended in fresh LPM medium including a final concentration of 200 μM Al3+ and the culture was incubated at 32 °C for 2 h. The Al-treated cells were spun down and completely washed three times with deionized water to remove unabsorbed Al ions. These washed cells were suspended in fresh LPM medium and then grown at 32 °C for another 4 h (a total of 6 h). During the Al uptake period and Al release period, cells were harvested every 2 h (0, 2, 4, and 6 h) and washed completely as described above to remove unabsorbed Al ions from the surface of cells. The cell number of each sample was adjusted by OD600 nm value. Al-treated cell samples were decomposed by a treatment with acid mixture (HNO3 and H2SO4, 1:1, v/v) at 120 °C for 3 h and the Al content was determined by an atomic absorption spectrophotometer.
Results
Localization of the NtGDI1and AtBCB proteins in plant cells
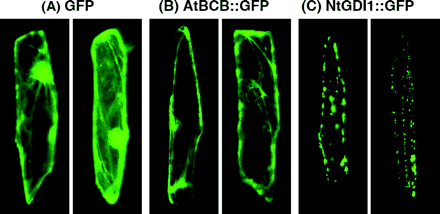
The computer program, PSORT (URL; http://psort.ims.u-tokyo.ac.jp/), predicted that the AtBCB protein and the NtGDI1 protein probably exist in the cell membrane and cytosol, respectively. To confirm these predictions, pAL253 (AtBCB∷GFP) and pAL254 (NtGDI1∷GFP) (Fig. 1) were introduced into onion epidermal cells for transient expression. As a control experiment, the plasmid carrying the GFP gene (pAL255) was also introduced into onion cells. Fluorescence microscope observation of the control transformants indicated that the fluorescent signal derived from the GFP gene was seen in the cytoplasm and in the nuclei (round shape) with a strong signal (Fig. 2A). Compared with these patterns, the transformed cells carrying the AtBCB∷GFP gene showed a lower signal in the cytoplasmic region, but a stronger signal in the cell membrane region (Fig. 2B). Fluorescent signals were observed as particles in the transformed cells carrying the NtGDI1∷GFP gene (Fig. 2C). When these particles were observed under simultaneous light and fluorescent conditions, all of the particles were detected in the cytoplasmic region and on the inside of a cell membrane (data not shown). No signal was detected in the nucleus in these two fusion constructs. These results are consistent with the prediction by PSORT.
Localization of the AtBCB∷GFP and the NtGDI1∷GFP fusion proteins by transient expression in onion epidermal cells. (A) Transformed cells carrying a control plasmid (pAL255) and expressing GFP protein. (B) Transformed cells carrying pAL253 and expressing AtBCB∷GFP protein. (C) Transformed cells carrying pAL254 and expressing NtGDI1∷GFP protein.
Accumulation of Al ions, oxidative peroxides and lignin in the root region by Al stress
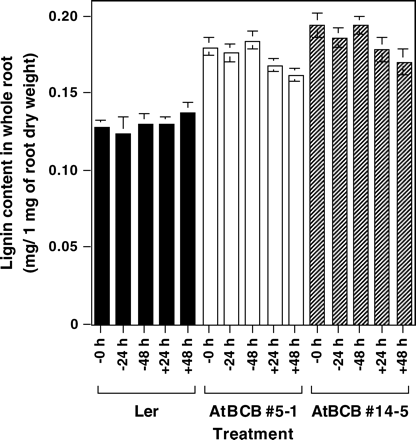
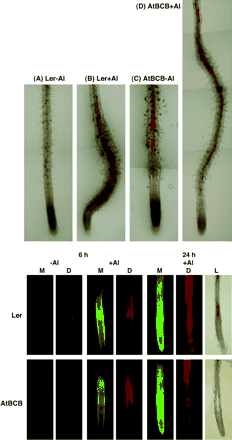
It has been reported that the AtBCB gene functions in electron transfer in photosynthesis and in lignin metabolism (Gysel et al., 1993; Drew and Gatehouse, 1994; Aram et al., 1998). To determine whether the AtBCB gene is actually related to lignin metabolism in plants, lignin content in Al-treated whole roots of a control line (Ler) and the transgenic plants over-expressing the AtBCB gene (AtBCB no. 5-1 and no. 14-5, Ezaki et al., 2000) was determined (Fig. 3). No significant difference in lignin content was seen between individual lines in the absence (−Al, 0, 24, and 48 h) or presence of Al (+Al, 24 and 48 h). However, lignin content in the two over-expressing lines was always higher than that in Ler (e.g. approximately 1.4 times higher). This result strongly suggested that the AtBCB gene is related to lignin sysnthesis, and that over-expression of the gene induces deposition of excess lignin in roots. Furthermore, lignin content in root tips was observed by microscope after phloroglucinol staining, which specifically detects lignin formation (Fig. 4, upper). Untreated roots of Ler showed no staining, while Al-treated roots were stained red, especially in the swollen region and its neighbouring area. In the transgenic lines over-expressing the AtBCB gene, red areas due to lignin formation were observed independent of the Al treatment. These results suggest that lignin formation constitutively occurs in the root tip region as well as in the whole root in these lines. The results of the AtBCB no. 5-1 line are shown in Fig. 4. This result also suggested that the AtBCB gene is involved in the biosynthesis of lignin. The red areas were mainly seen in the central cylinder zone of these two lines, but not at the surface of the roots.
Lignin content in the whole root region of Ler and the AtBCB over-expressing transgenic lines. −, +; treatments under 0 μM or 100 μM Al condition. Whole root region of 10-d-old plants grown in 1/6 MS medium (pH 4.2) were used for quantitative determination of lignin content. Three independent experiments were performed and the results are shown as average values ±SE.
Biological changes caused by Al stress in a control line (Ler) and a transformant over-expressing the AtBCB gene (AtBCB no. 5-1). Upper: accumulation of lignin in the root tip region by Al stress. Root tip region of the two lines were exposed to 0 μM (−Al) or 100 μM Al (+Al) for 2 d and then stained with phloroglucinol. Red zones in (B), (C) and (D) showed the accumulation of lignin. Bottom: accumulation of Al ions, lipid peroxides, and lignin in the same root tip region in response to Al stress. Root region of the two lines exposed to 0 μM Al treatment (−Al) for 0 h or 100 μM Al treatment (+Al) for 6 h and 24 h were stained with morin (M) and then stained with H2DCFDA (D) to detect the localization of Al ions and lipid peroxides, respectively. The same samples were subsequently stained with phloroglucinol as the third staining to detect lignin (L). (Data for the samples exposed to the 24-h Al treatment only are shown here.)
What is the function of the lignin deposition in Al-treated roots? To address this question, localization of (i) Al ions, (ii) lipid peroxides, and (iii) lignin were determined in the same Al-treated roots by morin, H2DCFDA, and phosphoglucinol, respectively (Fig. 4 bottom). Untreated roots (0 μM Al treatment) of the control line and the AtBCB over-expressing line showed very low fluorescent signals by morin or H2DCFDA staining. Both of the lines treated with 100 μM Al for 6 h showed morin-specific fluorescent signals in their root tips. A deposition of peroxides derived from Al stress (H2DCFDA staining) was also observed in almost the same area where the Al ions accumulated. In this stage, a deposition of lignin in Al-treated roots of the control line was not observed, but a small amount of lignin was visible in the AtBCB over-expression line (data not shown). After exposure to 100 μM Al treatment for 24 h, stronger morin-specific fluorescent signals were observed in wider areas of both lines, indicating that many more Al ions were incorporated during the 24 h. A more severe and wider staining pattern by H2DCFDA was also observed in the same roots of both lines, but the area was no longer consistent with the staining pattern by morin. These results indicated that the oxidative damage had probably spread out to surrounding areas at this stage. Deposition of lignin was clearly seen in both lines after 24 h exposure to Al stress. It is notable that the areas accumulating high amounts of lignin showed a low accumulation of reactive peroxides in the same roots.
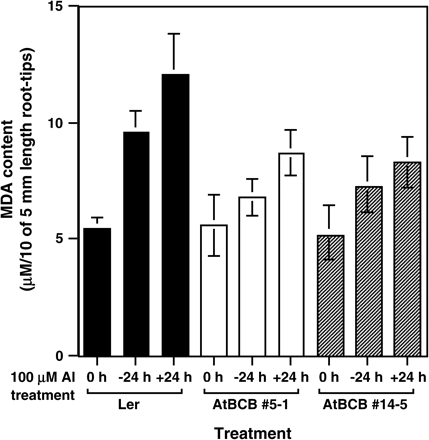
Accumulation of lipid peroxides in the root was also estimated by MDA, which is a final product of lipid peroxidation (Fig. 5). There was no difference in the content of MDA among the three plants (Ler, AtBCB no. 5-1 and no. 14-5) without Al (0 h). All of these plants showed an increase of MDA after an exchange to a fresh medium (−24 h) and another additional increase of MDA after Al stress (+24 h). However, compared with the control line, transgenic lines over-expressing the AtBCB gene clearly showed a lower increase of MDA after these two treatments (−24 h and +24 h).
Amount of lipid peroxides caused by Al treatment in root tip region. Lipid peroxides in ten root-tip sections (5 mm in length) of the control line (Ler, black bars) and the two transgenic plants over-expressing the AtBCB gene (no. 5-1, white bars, and no. 14-5, hatched bars) were determined after Al treatment (100 μM for 24 h) and the content was estimated as MDA. Error bars were calculated from three independent experiments.
Yeast has a Sec19-dependent resistance system for Al toxicity
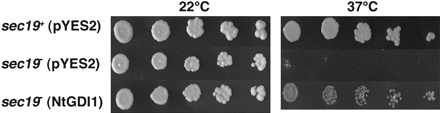
The sec19− yeast mutant was isolated as a temperature-sensitive mutant of a vesicle transport system; the mutant is active at 22 °C, but inactive at 37 °C (Ueda et al., 1996). These authors also reported that the Arabidopsis AtGDI1 gene can complement the temperature-sensitive phenotype of the sec19− mutation in yeast cells. These results led to the hypothesis that the NtGDI1 gene (83% identity in amino acid sequence to AtGDI1) can also complement the sec19− mutation and has a similar function to the sec19 gene in yeast. The wild-type sec19+ cells carrying pYES2 could form colonies at 22 °C and 37 °C, while the sec19− mutant carrying pYES2 formed colonies at 22 °C, but not at 37 °C (Fig. 6). On the contrary, the sec19− mutant carrying the NtGDI1 gene could partially form colonies at the both temperatures, like the sec19+cells. This result indicated that the NtGDI1 gene could complement the temperature-sensitive phenotype of the sec19− mutation.
Complementation test of the yeast sec19− mutation on agar plates. Log phase cells of three yeast transformants, sec19+ strain (ANY21) carrying pYES2, sec19− mutant (ANS19-4) carrying pYES2 and sec19− mutant carrying the NtGDI1 gene were serially diluted with LPM medium and then spotted on LPM agar plates. Plates were incubated at 22 °C or 37 °C for 3–4 d.
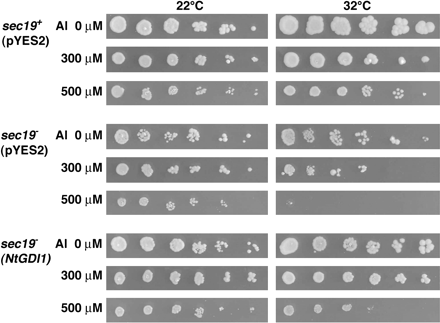
To determine whether the sec19 gene is related to an Al resistance mechanism in yeast, Al sensitivity of the sec19− mutant was investigated. A preliminary experiment indicated that the sec19− mutant carrying pYES2 cannot form colonies at 37 °C, but formed almost similar-sized colonies at 32 °C as at 22 °C (Fig. 7, 0 μM Al condition). Al sensitivity tests for the sec19− mutant were therefore performed at both the permissive temperatures (22 °C and 32 °C). As the Al concentration increased from 0 μM to 500 μM, the sec19+ wild type strain carrying pYES2 showed a slight growth inhibition. However, there was no difference in colony size between 22 °C and 32 °C. The sec19− mutant carrying pYES2 showed a similar Al sensitivity to the sec19+ strain at 22 °C, but was more sensitive at 32 °C, where it formed much smaller colonies in 300 μM Al and no colonies at 500 μM Al. This result indicates that the Sec19 protein is related to an Al resistance mechanism in yeast.
Sensitivity test of yeast transformants for Al stress. Three yeast transformants described in Fig. 6 were serially diluted with LPM medium and then spotted onto LPM agar plates adjusted to pH 4.2 including 0, 300, or 500 μM Al. Plates were incubated at 22 °C or 32 °C for 3–4 d.
NtGDI1 protein can ameliorate Al toxicity by a secretion system
Another Al sensitivity test was performed for the sec19− mutant carrying the NtGDI1 gene (Fig. 7). The temperature-dependent, Al-sensitive phenotype of the sec19− mutant was partially recovered by the NtGDI1 gene and the transformant carrying this gene could form colonies under 500 μM Al stress. Furthermore spontaneous ura− segregants isolated from the transformant carrying pAL618 showed a similar Al sensitivity as the parental sec19− mutant (ANS19-4) again (data not shown). This result provided further support for the conclusion that the NtGDI1 gene ameliorates Al toxicity in the sec19− mutant.
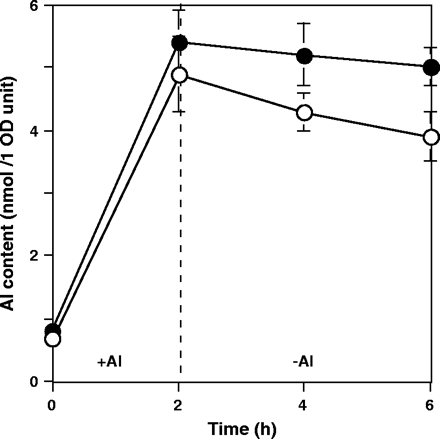
To confirm the function of the Sec19 protein in Al resistance mechanism, Al content of the sec19− mutant and the sec19+ parental strain during both Al uptake and Al release were determined at 32 °C (Fig. 8). The sec19− mutant showed almost the same Al uptake to the wild-type strain (2 h), while it showed a slower decrease of Al content than the sec19+ parental strain during the Al release period (4 h). These results suggested that the mutated Sec19 protein has an impaired function in vesicle transport as compared to the wild-type Sec19 protein at 32 °C.
The amount of absorbed and released Al ions in the sec19+ strain and the sec19− mutant. White circles, sec19+ strain (ANY21) carrying pYES2; black circles, sec19− mutant (ANS19-4) carrying pYES2. +Al, 200 μM Al treatment for 2 h uptake; −Al, 4 h treatment without Al as a release period. All treatments were performed at 32 °C. Al contents of the two transformants were measured from three independent experiments and shown as average values ±SE.
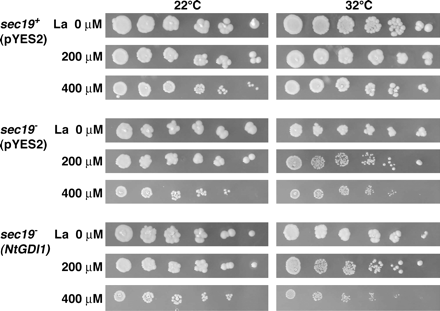
To determine whether the Sec19-dependent resistance mechanism is specific for Al stress or not, a sensitivity test for another trivalent cation, lanthanium (La) was also performed (Fig. 9). The sec19+ strain carrying pYES2 showed a similar colony formation capability from 0–400 μM La treatment at 22 °C and 32 °C. The sec19− mutant carrying pYES2 showed a growth inhibition under 400 μM La toxicity at both temperatures. Compared with 22 °C, a slight growth inhibition was also detected in 200 μM La at 32 °C. These results indicate that the sec19 gene was partially related to a La resistance mechanism in yeast. While the sec19− mutant carrying the NtGDI1 gene showed a similar La sensitivity to the sec19− mutant carrying pYES2. This result indicated that the NtGDI1 gene could not complement the sec19− mutation for La toxicity in yeast. Arabidopsis transgenic plants over-expressing the NtGDI1 gene (NtGDI1 no. 5-11 and no. 9-11; Ezaki et al., 2000) showed a similar sensitivity to Ler (control line; non-transformant) in La toxicity (0–500 μM La treatment) (data not shown), consistent with the results shown in yeast transformants.
Sensitivity test of yeast transformants for La stress. Three yeast transformants described in Fig. 6 were serially diluted and then spotted on LPM agar plates including 0, 200, or 400 μM La. Plates were incubated at 22 °C or 32 °C for 3–4 d.
Discussion
In this study, the functions of the AtBCB and NtGDI1 genes in Al stress were characterized. Microscopic observation of AtBCB∷GFP and NtGDI1∷GFP fusion proteins suggested that the AtBCB protein is located in a cell membrane rather than in the cytoplasm and that the NtGDI1 protein apparently exists in/on small particles in a cytoplasmic fraction. Although the results support the prediction by PSORT on the subcellular localization of both proteins, final proof will depend on cell fractionation or other approaches.
The BCB gene family has been related to various aspects of cell metabolism, including lignification of the cell wall fraction (see Introduction). The transgenic lines over-expressing the AtBCB gene constitutively deposit a higher level of lignin in their whole root region than Ler. This result is consistent with that of Drew and Gatehouse (1994), which strongly suggested that the Arabidopsis AtBCB protein is related to lignin metabolism. The result of phloroglucinol staining in the root-tip region also supported the relation between the AtBCB gene and lignin synthesis. Perhaps a more interesting point was that most of the areas where lignin was accumulated showed a lower accumulation of lipid peroxides after a 24 h Al treatment in both tested lines, Ler and AtBCB no. 5-1. Moreover, the transgenic plants accumulated less MDA than Ler in root tips after Al stress. A simple explanation is that toxic peroxide species induced by Al stress were diminished by being consumed in lignin metabolism, because reactive oxygen species (including H2O2 and phenoxyl radicals) are used as triggers and/or intermediate substrates of electron transfer in the chain reaction of lignin formation (Ward et al., 2003). Recently, phenolic compounds such as flavonoids, alkaloids, terpenoids, and glycosides, were reported to form strong complexes with Al ions and were implicated in internal Al detoxification in tea and other Al-accumulating species (Ofei-Manu et al., 2001). Kidd et al. (2001) also reported a correlation between the rate of Al-stimulated root exudation of the flavonoids catechin and quercetin, and differential Al tolerance in three maize genotypes; the correlation was higher than that with Al-activated organic acids exudation. They suggested that Al-activated exudation of phenolics may play an important role in the detoxification of Al in the rhizosphere surrounding the root apex of maize plants. Kato (2001) also suggested the possibility that lignin can form a complex with Al ions. These results suggested that lignin formation in response to Al toxicity is one of the protective mechanisms. Consumption of Al-stress-induced lipid peroxides, such as H2O2 and various phenoxyl radicals, by electron transfer during lignin formation may be more important for establishing an Al resistance mechanism than the deposition of lignin. A previous report suggested that Al-resistant yeast transformants over-expressing the AtBCB gene also showed Al resistance and a lower accumulation of Al ions within the cells compared with a control strain (Ezaki et al., 1999). It is supposed that a similar consumption of Al-induced lipid peroxides by the electron transfer system probably occurs in this yeast transformant.
It has already been suggested that the NtGDI1 protein probably functions in an efflux of cytoplasmic Al ions in both yeast and Arabidopsis (Ezaki et al., 1999, 2001). Several results shown in this study support the concept. (i) The NtGDI1∷GFP fusion protein in plant cells was mainly found to be associated with small particles in the cytoplasm or near the inner surface of a cell membrane. The yeast Sec19 protein and many GDI family proteins exist mainly as a free form in the cytosol and an attached form adjacent to membranes in a vesicle transport process (Garrett et al., 1994; Pfeffer et al., 1995). Microscopic observation of the fusion protein is not direct evidence that those small particles are actual vesicles in the transport system, but is consistent with the deduced function of those particles. (ii) The yeast sec19− mutants showed increased sensitivity to Al and also showed a reduced capability for Al efflux. Since the Sec19 protein functions as a vesicle transport system in yeast (Garrett et al., 1994), it is suggested that this system can transport toxic Al ions from the cytoplasm to the outside. (iii) The tobacco NtGDI1 gene could complement not only the lethal phenotype of the temperature-sensitive sec19 mutant, but also the Al-sensitive phenotype. This result indicates that the NtGDI1 gene in plants has a similar function to the sec19 gene in yeast against Al toxicity. From these results, it is supposed that a functional secretory system is required rapidly and efficiently to respond to Al toxicity with an appropriate up-regulation of transport capacity through de novo synthesis of proteins in plants and yeast. The NtGDI1 gene, as well as the yeast sec19 gene, is probably involved in the functional secretory system. The possibility cannot completely be excluded that the lower Al content of yeast cells expressing the NtGDII gene could be due to displacement of Al from the apoplasm (including cell walls) as a consequence of the increased efflux of Al-complexing substances from the cell.
The La sensitivity test suggested that the vesicle transport system mediated by the Sec19 protein functions for the La resistant mechanism in yeast, while the transformant carrying the tobacco NtGDI1 gene showed almost similar La sensitivity to the sec19− mutant. This result indicates that the NtGDI1 gene cannot complement the sec19− mutation for La stress. The transgenic Arabidopsis over-expressing the NtGDI1 gene also showed a similar La sensitivity to Ler (control non-transformant line). These results suggested that the NtGDI1 protein is related to the resistance mechanism for Al, but not La. A simple explanation for these results is that the functions of the Sec19 protein in yeast are not completely the same as those of NtGDI1 protein in plants. Or there may be a difference in metal selectivity between both proteins.
This work describes two novel mechanisms by which cells can become tolerant to Al. One involves lignin biosynthesis; it was suggested that various reactive oxygen species are prevented from damaging membranes by their utilization in lignin biosynthesis. The second appears to involve a vesicular pathway for Al secretion from the cell. To make the former mechanism clearer, the theory of lignin synthesis as a sink for ROS or an Al chelator should be tested in future work by feeding low concentrations of phenolic compounds that act as precursors for peroxidase-mediated polymerization to the roots in the presence and absence of Al. The obtained results will show the actual function of lignin synthesis in the Al-resistance mechanism.
We thank Ms Kanako Akashi and Ms Tomomi Sugao for their technical assistance and Professor Richard C Gardner (Auckland University, New Zealand) for his revision and fruitful comments for our manuscript. We also thank Dr Takashi Ueda (RIKEN, Japan) for his kind supply of yeast strains (ANS19-4 and ANY21). This work received financial support from the Ministry of Education, Culture, Sports, Science, and Technology (Grant-in-Aid for Scientific Research (C) (2) no. 13660066 to BE and Grant-in-Aid for Scientific Research (C) (2) no. 16580046 to BE), JSPS Joint Project under Japan–US Cooperative Science Program (to BE), Japan–Korea Basic Science Cooperation Program (to BE), and the Program for Promotion of Basic Research Activities for Innovative Biosciences (to HM).
References
Aram MN, Chad I, Michael GH, John HP, Gregory W, Reinhold GH, Joan SV.
Cakmak I, Horst WJ.
Delhaize E, Ryan PR, Randall PJ.
de la Fuente JM, Ramirez-Rodriguez V, Cabrera-Ponce JL, Herrera-Estrella L.
Drew LE, Gatehouse JA.
Eticha D, Staß A, Horst WJ.
Ezaki B, Gardner RC, Ezaki Y, Matsumoto H.
Ezaki B, Katsuhara M, Kawamura M, Matsumoto H.
Ezaki B, Sivaguru M, Ezaki Y, Matsumoto H, Gardner RC.
Ezaki B, Tsugita S, Matsumoto H.
Ezaki B, Yamamoto Y, Matsumoto H.
Ferro-Novik S, Novik P.
Fukuda H, Komamine A.
Garrett MD, Zahner JE, Cheney CM, Novick PJ.
Gietz D, Jean AS, Woods RA, Schiestl RH.
Gleave AP.
Gysel AV, Montagu MV, Inzé D.
Kato Y.
Kidd PS, Llugany M, Poschenrieder C, Gunse B, Barcelon J.
Kochian LV, Hoekenga OA, Pineros MA.
Lin SL, Wu L.
Ma JF, Ryan PR, Delhaize E.
MacDiarmid CW, Gardner RC.
Matsumoto H, Yamamoto Y, Ezaki B.
Miller JD, Richard NA, Eva JP.
Monties B.
Murashige T, Skoog F.
Niwa Y, Hirano T, Yoshimoto K, Shimizu M, Kobayashi H.
Pfeffer SR, Dirac-Svejstrup AB, Soldati T.
Ono K, Yamamoto Y, Hachiya A, Matsumoto H.
Ofei-Manu P, Wagatsuma T, Ishikawa S, Tawaraya K.
Richards KD, Schott EJ, Sharma YK, Davis KR, Gardner RC.
Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan P, Delhaize E, Matsumoto H.
Smith FW, Hawkesford MJ, Prosser IM, Clarkson DT.
Takahashi Y, Nagata T.
Takumi S, Otani M, Shimada T.
Tokunaga N, Sakakibara N, Umezawa T, Ito Y, Fukuda H, Sato Y.
Ueda T, Anai T, Tsukaya H, Harata A, Uchimiya H.
Ward G, Hadas Y, Bilkis I, Dosoretz CG.




Comments