-
PDF
- Split View
-
Views
-
Cite
Cite
Yasuhiro Ishimaru, Motofumi Suzuki, Takanori Kobayashi, Michiko Takahashi, Hiromi Nakanishi, Satoshi Mori, Naoko K. Nishizawa, OsZIP4, a novel zinc-regulated zinc transporter in rice, Journal of Experimental Botany, Volume 56, Issue 422, December 2005, Pages 3207–3214, https://doi.org/10.1093/jxb/eri317
Close - Share Icon Share
Abstract
Zinc (Zn) is an essential element for the normal growth of plants but information is scarce on the mechanisms whereby Zn is transported in rice (Oryza sativa L.) plants. Four distinct genes, OsZIP4, OsZIP5, OsZIP6, and OsZIP7 that exhibit sequence similarity to the rice ferrous ion transporter, OsIRT1, were isolated. Microarray and northern blot analysis revealed that OsZIP4 was highly expressed under conditions of Zn deficiency in roots and shoots. Real-time-PCR revealed that the OsZIP4 transcripts were more abundant than those of OsZIP1 or OsZIP3 in Zn-deficient roots and shoots. OsZIP4 complemented a Zn-uptake-deficient yeast (Saccharomyces cerevisiae) mutant, Δzrt1,Δzrt2, indicating that OsZIP4 is a functional transporter of Zn. OsZIP4-synthetic green fluorescent protein (sGFP) fusion protein was transiently expressed in onion epidermal cells localized to the plasma membrane. In situ hybridization analysis revealed that OsZIP4 in Zn-deficient rice was expressed in shoots and roots, especially in phloem cells. Furthermore, OsZIP4 transcripts were detected in the meristem of Zn-deficient roots and shoots. These results suggested that OsZIP4 is a Zn transporter that may be responsible for the translocation of Zn within rice plants.
Introduction
Zinc (Zn) is an essential nutrient that plays important roles in numerous physiological processes in plants, serving as a cofactor for many enzymes and as the key structural motifs in transcriptional regulatory proteins. A deficiency of Zn, therefore, decreases growth, but excess Zn has significant toxicity to biological systems through metal-based cytotoxic reactions. Therefore, the uptake and transport of Zn must be strictly regulated. Intracellular Zn homeostasis is achieved through the co-ordinated regulation of specific transporters engaged in Zn influx, efflux, and intracellular compartmentalization.
In the Arabidopsis thaliana genome, a large number of cation transporters potentially involved in metal ion homeostasis have been identified (Maser et al., 2001). Several members of the 15 Zinc-regulated transporters, Iron-regulated transporter-like Protein (ZIP) gene family (Guerinot, 2000) have been characterized and shown to be involved in metal uptake and transport in plants (Eide et al., 1996; Korshunova et al., 1999; Vert et al., 2001, 2002; Connolly et al., 2002). The ZIP proteins have been predicted to have eight transmembrane domains with their amino- and carboxyl-terminal ends situated on the outer surface of the plasma membrane (Guerinot, 2000). These proteins vary considerably in overall length, due to a variable region between the transmembrane domains (TM)-3 and TM-4, predicted to be on the cytoplasmic side providing a potential metal-binding domain rich in histidine residues. The most conserved region of these proteins lies in a variable region that has been predicted to form an amphipathic helix containing a fully conserved histidine that may form part of an intramembranous metal-binding site involved in transport (Guerinot, 2000). The transport function is eliminated when the conserved histidines or certain adjacent residues are replaced by mutation (Rogers et al., 2000).
ZIP1, ZIP3, and ZIP4 from Arabidopsis restore Zn uptake to the yeast (Saccharomyces cerevisiae) Zn-uptake mutant, Δzrt1,Δzrt2, and have been proposed to play a role in Zn transport (Grotz et al., 1998; Guerinot, 2000). ZIP1 and ZIP3 are expressed in roots in response to Zn deficiency, suggesting that they transport Zn from the soil to the plant, while ZIP4 is expressed both in the roots and shoots, suggesting that it transports Zn intracellularly or between plant tissues (Grotz et al., 1998; Guerinot, 2000). ZIP2 and ZIP4 rescue yeast mutants deficient in copper (Cu) transport, and ZIP4 is up-regulated in Cu-deficient roots (Wintz et al., 2003). ZRT1 and ZRT2 are high- and low-affinity Zn transporters, respectively (Eide, 1998; Guerinot, 2000). The proposed role of ZIP transporters in Zn nutrition has been further supported by the characterization of homologues from a number of plant species. For example, GmZIP1 has been identified in soybean (Glycine max) (Moreau et al., 2002), and functional complementation of Δzrt1,Δzrt2 yeast cells showed that GmZIP1 is highly selective for Zn, but not for iron (Fe) or manganese (Mn). GmZIP1 is expressed specifically in nodules, but not in roots, stems, or leaves, and the protein is localized to the peribacteroid membrane, suggesting a role in symbiosis. Ramesh et al. (2003) reported that OsZIP1 and OsZIP3 are also functional Zn transporters in rice (Oryza sativa) plants.
Previously, the OsIRT1 gene encoding a functional Fe2+ transporter that is homologous to Arabidopsis IRT1 (Bughio et al., 2002; Ishimaru et al., 2005) was isolated. OsIRT1 was highly up-regulated in Fe-deficient roots. OsIRT1 reversed the growth defect of YH003 (Δftr1,Δfet4,Δfre1) on Fe-depleted media.
In this report, the OsZIP4 gene, highly homologous to OsIRT1, was isolated and characterized and it was found that OsZIP4 encodes a Zn transporter localized to the plasma membrane and regulated by the plant's Zn status. In situ hybridization analysis revealed that OsZIP4 in Zn-deficient rice was expressed in shoots and roots, especially in phloem cells and the meristem.
Materials and methods
Plant material
Oryza sativa L. cv. Nipponbare was used for the microarray, northern blot, real time-PCR, and metal concentration analyses. Seeds were germinated for 3 d at room temperature on paper soaked with distilled water. After germination, the seedlings were transferred to a Saran net floating on distilled water in a growth chamber (day: 25 °C, 14 h of light at 320 μmol photons m−2 s−1; night: 10 h at 20 °C). After 3 d, 45 seedlings were transferred to a 20 l plastic container containing a nutrient solution with the following composition: 0.7 mM K2SO4, 0.1 mM KCl, 0.1 mM KH2PO4, 2.0 mM Ca(NO3)2, 0.5 mM MgSO4, 10 μM H3BO3, 0.5 μM MnSO4, 0.2 μM CuSO4, 0.5 μM ZnSO4, 0.05 μM Na2MoO4, and 0.1 mM Fe-EDTA. The ZnSO4 was omitted from the solution to induce Zn deficiency, as were the Fe, Mn, and Cu salts in order to induce deficiencies of these nutrients. The pH of the nutrient solution was adjusted daily to 5.5 with 1 M HCl, and the nutrient solution was renewed weekly. For the Zn-deficiency treatment, 2-week-old plants were transferred to nutrient solution without Zn and grown for 2 more weeks. To induce Fe, Mn, or Cu deficiency, 2-week-old plants were grown for 2 weeks in nutrient solutions without these respective nutrients.
Oligo DNA microarray analysis
A rice 22 K custom oligo DNA microarray kit (Agilent Technology, Tokyo, Japan), which contains 21 938 oligonucleotides synthesized based on the sequence data of the rice full-length cDNA project (http://cdna01.dna.affrc.go.jp/cDNA/) was used. Total RNA was extracted from shoots and roots using a RNeasy Plant Kit (Qiagen, Tokyo, Japan) according to the manufacturer's instructions; the yield and RNA purity were determined spectrophotometrically. The integrity of the RNA was checked using an Agilent 2100 Bioanalyser (Agilent Technology). Total RNA (200 ng) was labelled with Cy-3 or Cy-5 using an Agilent Low RNA Input Fluorescent Linear Amplification Kit (Agilent Technology). Fluorescent-labelled targets were hybridized to Agilent rice 22 K oligo DNA microarrays. The hybridization process was performed according to the manufacturer's instructions, and hybridized microarrays were scanned using an Agilent Microarray Scanner (Agilent Technology). Feature Extraction software (Agilent Technology) was used for the image analysis and data extraction processes.
PCR cloning of OsZIP4
The OsZIP4, OsZIP1, and OsZIP3 sequences, which are homologous to OsIRT1, were found on the Knowledge-based Oryza Molecular Biological Encyclopedia website (http://cdna01.dna.affrc.go.jp/cDNA/). A PCR-based cloning strategy was used to isolate OsZIP4, OsIRT1, OsZIP1, and OsZIP3. The primers used to amplify ORF were as follow; OsZIP4 forward (5′-CACCATGGACGCCATGAGGCAGAGCACGCG), OsZIP4 reverse (5′-TCATGCCCATATGGCAAGCAGAGACATCAT), OsIRT1 forward (5′-CACCGAATTCCGTACGGCATGGCGACGCCGCGGACACTGGT), OsIRT1 reverse (5′-ACAACTAATGGCGGCCGCTCACGCCCACTTGGCCATGACG), OsZIP1 forward (5′-CACCGGCGCAAGCTTCGACCATGGCCAGGA), OsZIP1 reverse (5′-GAAGCAAGTCTAGAACTAGGATGGATGGATC), OsZIP3 forward (5′-CACCATGGGAGCCAAGAAGCATACCTTGCA), and OsZIP3 reverse (5′-CTATGCCCATATGGCAAGCATTGACATCAG). The ORFs were amplified from a Zn- and Fe-deficient plant mixed cDNA library using these primers. The amplified fragment containing the OsZIP4 coding sequence was subcloned into pENTR/D-TOPO (Invitrogen, Carlsbad, CA). This pENTR/D-TOPO entry vector containing the OsZIP4 coding sequence was designated pENTR-OsZIP4. The pENTR-OsZIP4 was confirmed by sequencing. The other amplified fragments were inserted into pENTR/D-TOPO in the same manner. Isolated ORFs were sequenced using a Thermo Sequenase Cycle Sequencing kit (Shimadzu, Kyoto) and a DNA sequencer (DSQ-2000L; Shimadzu).
Northern blot analysis
Total RNA was extracted from roots and shoots, and 10 μg per lane were electrophoresed in 1.2% (w/v) agarose gels containing 0.66 M formaldehyde, transferred to Hybond-N+ membrane (Amersham, USA), and hybridized with probes at 65 °C according to the method of Mizuno et al. (2003). The amplified ORF of OsZIP4, OsIRT1, and OsZIP1 were used to prepare probes.
Quantitative real-time-PCR of OsZIP4, OsZIP1, and OsZIP3
Total RNA was treated with RNase-free DNase I (Takara, Tokyo, Japan) to remove contaminating genomic DNA. First-strand cDNA was synthesized using SuperScript II reverse transcriptase (Invitrogen) by priming with oligo-d(T)30. The fragment was amplified by PCR in a SmartCycler (Takara) with SYBR Green I and ExTaq™ Real-Time-PCR Version (Takara). The primers used for Real-Time-PCR were as follows; OsZIP4 forward (5′-GCGAAAGCAACAGTGATCATGGCGACTTTC), OsZIP4 reverse (5′-GCAGCTCTTGGTTGCTCTGAAGATCTCATG), OsZIP1 forward (5′-CTCTTCAAGTTCCTCGCCGTCCT), OsZIP1 reverse (5′-CGGCCACGATTAATGAATGGGGTG), OsZIP3 forward (5′-AATGTGCATAGCTCAACTGCCTT), and OsZIP3 reverse (5′-CAAAATCAAGCCTATCTGGGA). The primers used for internal control in RT-PCR were α-tubulin forward, (5′-TCTTCCACCCTGAGCAGCTC) and α-tubulin reverse (5′-AACCTTGGAGACCAGTGCAG). There was no genomic contamination and no differences of the internal control in each deficient condition (data not shown). The sizes of the amplified fragments were confirmed by gel electrophoresis and sequencing.
Determination of metal concentrations
The plants were dried for 1 week at 65 °C. The plants (30–50 mg) were then wet-ashed with 2 ml of 11 M HNO3 for 5 h at 150 °C. The metal concentrations were measured using inductively coupled plasma atomic emission spectrometry (SPS1200VR; Seiko, Tokyo, Japan) at wavelengths of 238.204 (Fe), 213.856 (Zn), 293.930 (Mn), and 324.754 (Cu) nm.
Yeast strains and growth media
The following strains of the yeast Saccharomyces cerevisiae were used in this study: CM3260 (parent strain) MATalpha trp1-63 leu2-3,112 gcn4-101 his3-609 ura3-52, YH003 MATalpha trp1-63 leu2-3,112 gcn4-101 his3-609 ura3-52 Δftr1::URA3 Δfre1::HIS Δfet3::TRP, CM-SMF MATalpha trp1-63 leu2-3,112 gcn4-101 his3-609 ura3-52 Δsmf1::URA3, CM-ZRT MATalpha trp1-63 leu2-3,112 gcn4-101 his3-609 ura3-52 Δzrt1::URA3 Δzrt2::HIS3, and FTRUNB1 MATalpha trp1-63 leu2-3,112 gcn4-101 his3-609 ura3-52 Δctr1::URA3. Yeast disruption mutants were made by homologous recombination. Yeast cells were grown in 1% yeast extract, 2% peptone, and 2% glucose (YPD) and synthetic defined medium (SD) supplied with the appropriate amino acids. Agar (2%) was added to obtain solid plate media (Sherman, 1991). For medium deprived of Fe, Zn, or Mn, respectively, 50 μM bathophenanthroline disulphonic acid (BPDS), 0.5 M ethylenediamine-N,N,N′,N′-tetraacetic acid (EDTA), or 50 mM ethylene glycol-bis-β-aminoethylether-N,N,N′,N′-tetraacetic acid (EGTA; Wako Pure Chemical Industries, Japan) were added. 1% yeast extract, 2% peptone, and 2% glycerol (YPG) medium were used with 10 μM Cu for Cu-deprived medium.
Functional expression in yeast
The yeast expression vector (pYH23) was kindly provided by Dr Hirotaka Yamaguchi (Kyushu National Agricultural Experiment Station) (Bughio et al., 2002). pYH23 has HindIII, PvuII, PstI, XhoI, SstI, XbaI, and NotI sites in the ADH1 expression cassette. The plasmid was digested with HindIII and XbaI, and the 1579 bp MultiSite Gateway Three-fragment (Invitrogen), containing an attR1 site at the 5′-end, the chloramphenicol resistance gene, the ccdB gene, and the attR2 site cassette, was inserted. This modified plasmid was designated pDESTADH as the destination vector. A subsequent attL substrate and attR substrate recombination reaction (Invitrogen) between the destination and entry vectors generated an expression clone containing the gene encoding pYH23- OsZIP4. The OsZIP4 and OsZIP3 ORFs were inserted into pYH23 in the same way. Yeast transformation was carried out using the Li-acetate transformation method (Gietz and Schiestl, 1995).
Construction of plasmid OsZIP4-sGFP and observation of OsZIP4-sGFP localization
Plasmid pUC18, containing the cauliflower mosaic virus (CaMV) 35S promoter-sGFP (S65T)-NOS3′ construct, was kindly provided by Dr Yasuo Niwa (University of Shizuoka). The construct had SalI and NcoI sites on the 3′ side of the CaMV 35S promoter. The NcoI site ‘CCATGG’ includes the initiation codon for sGFP. An annealed oligomer (5′-TCGAGATATCGGTACCAGATCTGAGCTCGAGGTCGA and 5′-CTAGTCGACCTCGAGCTCAGATCTGGTACCGATATC) was inserted into the NcoI and SalI site of CaMV 35S-sGFP (S65T)-NOS3′ to produce a new EcoRV (GATATC) site. The plasmid was digested with EcoRV, and the 1579 bp MultiSite Gateway Three-fragment (Invitrogen) was inserted. This modified plasmid was designated pDEST35S-sGFP as the destination vector. The ORF of OsZIP4 was amplified using two primers: 5′-CACCATGGACGCCATGAGGCAGAGCACGCG and 5′-TGCCCATATGGCAAGCAGAGACATCATCCC. The amplified fragment containing the OsZIP4 coding sequence was subcloned into pENTR/D-TOPO (Invitrogen). This pENTR/D-TOPO entry vector containing the OsZIP4 coding sequence was designated pENTR-OsZIP4. A subsequent attL substrate and attR substrate recombination reaction (Invitrogen) between the destination and entry vectors generated an expression clone containing the gene encoding 35S-OsZIP4-sGFP. Onion epidermal cells were transformed using the Biolistic PDS-1000/He Particle Delivery System (Bio-Rad, Tokyo, Japan), and the sGFP fluorescence occurred as described by Mizuno et al. (2003).
In situ localization
Oryza sativa L. cv. Nipponbare was grown with sufficient Zn for 2 weeks and plants transferred to nutrient solution without Zn for 10 d. Tissue from these plants was fixed in 4% (w/v) paraformaldehyde for 36 h, and was then dehydrated in an ethanol series. After dehydration, the tissue was infused with paraplast and then sectioned to 10 μm and mounted on slides. The OsZIP4 specific fragment of RT-PCR was subcloned into the pCR-TOPO vector (Invitrogen). This plasmid was used to generate sense and antisense probes for in situ hybridization. Sense and antisense probes were labelled with digoxigenin-11-UTP (Roche, Mannheim, Germany) according to the manufacturer's protocol. This plasmid was linearized with HindIII and transcribed with T7 RNA polymerase. After hydrolysis of the labelled probes and further tissue treatment, the slides were hybridized overnight at 42 °C and washed. The tissue was then incubated with anti-digoxigenin alkaline phosphatase conjugate (Roche) for 30 min at room temperature, and the antibody was detected with nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate after an overnight incubation. The results were visualized using an Axiophoto microscope (Carl Zeiss, Tokyo, Japan) following the manufacturer's instructions.z
Results
Isolation and phylogenic analysis of ZIP family transporters in rice plants
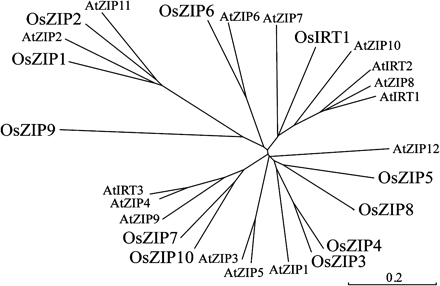
A search of the computer database Knowledge-based Oryza Molecular Biological Encyclopedia (http://cdna01.dna.affrc.go.jp/cDNA/) enabled four distinct genes of OsZIP to be identified and isolated, homologous with the previously identified OsIRT1: OsZIP4 (accession. no. AB126089), OsZIP5 (accession. no. AB126087), OsZIP6 (accession. no. AB126088), and OsZIP7 (accession. no. AB126090). A phylogenic analysis showed that OsZIPs differ in their amino acid sequences and in their relationship to other members of the ZIP family (Fig. 1). The OsZIP4, OsZIP5, OsZIP6, and OsZIP7 proteins show 54, 51, 33, and 50% identity with OsIRT1, respectively. They also show 58, 49, 44, and 35% identity to ZIP1 from A. thaliana, respectively. The rice genome database was searched for other ZIP-like genes, and three more were found: OsZIP8, OsZIP9, and OsZIP10. Therefore, it appears that rice plants have OsIRT1 and ten OsZIPs.
Unrooted phylogenic tree for the OsIRT1, OsZIPs, AtIRTs, and AtZIPs amino acid sequences for which ORFs are confirmed. Calculations were performed using the CLUSTAL W Neighbor–Joining method and the tree was visualized with TreeView.
Microarray analysis of OsZIP4, OsIRT1, OsZIP5, OsZIP6, OsZIP7, and OsZIP3
To understand their integrated regulation better, the expression patterns of known or potential metal transporters in rice roots and shoots in response to Zn deficiency were analysed using the 22 K microarray which contained six OsZIP family genes (Table 1). Of these, OsIRT1, OsZIP5, and OsZIP4 were up-regulated in Zn-deficient roots, and OsZIP4, OsZIP5, and OsZIP7 were up-regulated in Zn-deficient shoots. The induction ratio of OsZIP4 by Zn deficiency was the fourth highest in shoots and the fourteenth highest in roots of the 22 K genes on this array (data not shown).
Ratio of Zn-deficiency inducible OsZIP4, OsIRT1, and OsZIPs in Zn-deficient and Zn-sufficient roots and shoots
Gene . | Root . | Shoot . | Accession no. . |
|---|---|---|---|
| OsZIP4 | 4.74±0.75 | 20.88±5.66 | AB126089 |
| OsIRT1 | 2.12±0.28 | 1.42±0.11 | AB070226 |
| OsZIP5 | 1.72±0.36 | 3.78±0.95 | AB126087 |
| OsZIP6 | 0.79±0.09 | 1.79±0.20 | AB126088 |
| OsZIP7 | 1.44±0.17 | 4.57±1.02 | AB126090 |
| OsZIP3 | 1.07±0.20 | 0.39±0.02 | AY323915 |
Gene . | Root . | Shoot . | Accession no. . |
|---|---|---|---|
| OsZIP4 | 4.74±0.75 | 20.88±5.66 | AB126089 |
| OsIRT1 | 2.12±0.28 | 1.42±0.11 | AB070226 |
| OsZIP5 | 1.72±0.36 | 3.78±0.95 | AB126087 |
| OsZIP6 | 0.79±0.09 | 1.79±0.20 | AB126088 |
| OsZIP7 | 1.44±0.17 | 4.57±1.02 | AB126090 |
| OsZIP3 | 1.07±0.20 | 0.39±0.02 | AY323915 |
Microarray analysis was carried out to detect the induction of the expression of OsZIP4, OsIRT1, and OsZIPs in Zn-deficient roots compared with Zn-sufficient roots (–Zn Root), or in Zn-deficient shoots compared with Zn-sufficient shoots (–Zn Shoot). Data presented are the average of four biological replicates.
Ratio of Zn-deficiency inducible OsZIP4, OsIRT1, and OsZIPs in Zn-deficient and Zn-sufficient roots and shoots
Gene . | Root . | Shoot . | Accession no. . |
|---|---|---|---|
| OsZIP4 | 4.74±0.75 | 20.88±5.66 | AB126089 |
| OsIRT1 | 2.12±0.28 | 1.42±0.11 | AB070226 |
| OsZIP5 | 1.72±0.36 | 3.78±0.95 | AB126087 |
| OsZIP6 | 0.79±0.09 | 1.79±0.20 | AB126088 |
| OsZIP7 | 1.44±0.17 | 4.57±1.02 | AB126090 |
| OsZIP3 | 1.07±0.20 | 0.39±0.02 | AY323915 |
Gene . | Root . | Shoot . | Accession no. . |
|---|---|---|---|
| OsZIP4 | 4.74±0.75 | 20.88±5.66 | AB126089 |
| OsIRT1 | 2.12±0.28 | 1.42±0.11 | AB070226 |
| OsZIP5 | 1.72±0.36 | 3.78±0.95 | AB126087 |
| OsZIP6 | 0.79±0.09 | 1.79±0.20 | AB126088 |
| OsZIP7 | 1.44±0.17 | 4.57±1.02 | AB126090 |
| OsZIP3 | 1.07±0.20 | 0.39±0.02 | AY323915 |
Microarray analysis was carried out to detect the induction of the expression of OsZIP4, OsIRT1, and OsZIPs in Zn-deficient roots compared with Zn-sufficient roots (–Zn Root), or in Zn-deficient shoots compared with Zn-sufficient shoots (–Zn Shoot). Data presented are the average of four biological replicates.
Expression patterns of OsIRT1 and OsZIPs in various metal-deficient rice plants
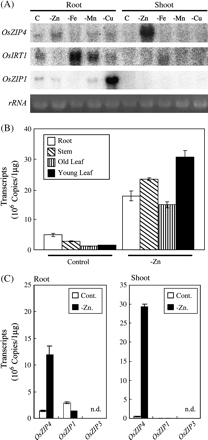
The abundance of OsZIP4, OsIRT1, and OsZIP1 transcripts were confirmed by northern blot analysis under Zn-, Fe-, Mn-, or Cu-deficient conditions (Fig. 2A). The level of OsZIP4 transcripts increased markedly in Zn-deficient shoots and roots, but a deficiency of other metals had little effect on the OsZIP4 transcript level. In quantitative real time (RT)-PCR, the expression of OsZIP4 was also highly induced by Zn deficiency in roots, stems, and leaves, and was the highest in young leaves (Fig. 2B).
Expression pattern of OsZIP4. (A) Northern blot analysis of the OsZIP4, OsIRT1 and OsZIP1 in the roots and leaves of rice plants grown under trace metal-deficient conditions. Total RNA (10 μg) extracted from plants grown in normal nutrient solution (control) (C) or under conditions of low zinc (–Zn), iron (–Fe), manganese (–Mn), or copper (–Cu) supply was blotted on each line. Ethidium bromide-stained rRNA is shown as a control for loading. (B) Quantification of OsZIP4 transcripts in each plant part. Total RNA was isolated from the roots, stems, old leaves, or young leaves of control or Zn-deficient plants, and RT-PCR was performed to monitor the amplification of each gene. Values represent the mean ±SD of the number of copies of transcripts in 1 μg of total RNA of these tissues in three reactions. (C) Quantification of OsZIP4, OsZIP1, and OsZIP3 transcripts under conditions of Zn deficiency. Total RNA was isolated from control or Zn-deficient plants, and RT-PCR was performed to monitor the amplification of each gene. The values represent the mean ±SD of the number of copies of transcripts in 1 μg of total RNA of the tissue in three reactions. n.d., not detected.
The expression pattern of OsIRT1 was consistent with previous results (Bughio et al., 2002). In contrast to previously published data for OsZIP1 (Ramesh et al., 2003), however, OsZIP1 was not up-regulated by Zn deficiency. Interestingly, OsZIP1 was up-regulated in Cu-deficient roots (Fig. 2A). OsZIP3 transcripts could not be detected in roots and shoots (data not shown). Consistent with northern blot analysis, RT-PCR revealed that OsZIP1 was down-regulated by Zn deficiency. It was found that OsZIP4 transcripts were much more abundant than those of OsZIP1 or OsZIP3 in Zn-deficient roots and shoots (Fig. 2C).
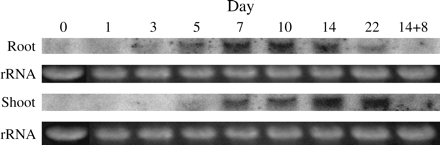
The time-course of OsZIP4 expression under Zn deficiency was examined by RNA gel-blot analysis using the OsZIP4 open reading frame (ORF) as a hybridization probe. This analysis revealed that the induction of OsZIP4 expression in roots does not parallel that in leaves (Fig. 3). OsZIP4 transcripts in roots were induced in 3 d and were abundant 7 d after transfer to culture lacking Zn. The corresponding values for the shoots were 5 d and 14 d. OsZIP4 transcripts were undetectable 8 d after the resupply of Zn.
Time-course of OsZIP4 mRNA abundance patterns in response to Zn-deficient growth conditions. Rice plants were grown for 2 weeks in normal nutrient solution and then transferred to Zn-deficient culture; roots and shoots were harvested 0, 1, 3, 5, 7, 10, 14, and 22 d after the transfer. The other plants were grown for 2 weeks in normal nutrient solution, transferred to Zn-deficient culture for 14 d, and transferred a second time to Zn-sufficient culture; roots and shoots were harvested 8 d after transfer (Day 14+8). RNA samples were prepared from each tissue sample and were used to prepare RNA gel blots. The OsZIP4 ORF was used to probe the RNA gel blot. Ethidium bromide-stained rRNA is shown as a control for loading.
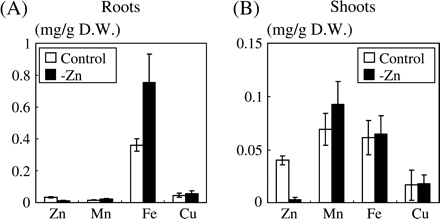
The concentrations of Zn, Mn, Fe, and Cu in Zn-deficient rice plants were determined to confirm the nutritional status of these plants. As expected, there was a significant decrease in Zn concentration (Fig. 4A, B). The Fe concentration in roots doubled in Zn-deficient roots, but there was no change in the concentrations of either Mn or Cu. In shoots, Zn deficiency caused no significant change in metal concentrations, except for the decrease of Zn (Fig. 4B). Similarly, metal concentrations of Fe-, Mn-, and Cu-deficient plants were analysed and it was confirmed that these plants were actually deficient in each metal (data not shown).
Metal concentrations in roots and shoots of plants grown under conditions of Zn sufficiency or under conditions designed to induce deficiency. The concentrations of Zn, Mn, Fe, and Cu in the roots (A) and leaves (B) are expressed as mg g−1 dry weight and are given as the mean ±SD of three replicates of each treatment.
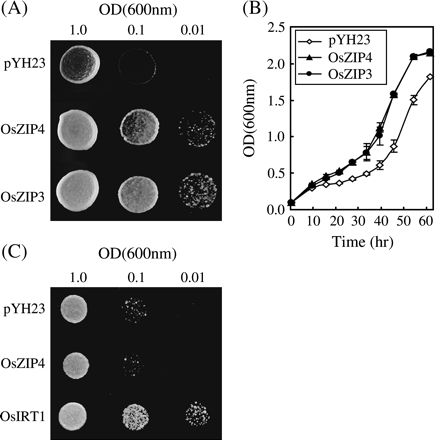
OsZIP4 reversed the growth defect of Zn-uptake mutants of yeast
The ability of OsZIP4 to restore the growth defect of CM-ZRT yeast strain (Δzrt1, Δzrt2) which possesses disrupted null mutations in the genes for the high-affinity Zn transporter was examined. The coding region of OsZIP4 was subcloned in the yeast expression vector pYH23 under the control of the ADH promoter. OsZIP3 was used as a positive control in the experiment. In a heterologous experiment using synthetic defined medium without Zn, both OsZIP4 and OsZIP3 restored the growth defect of CM-ZRT, whereas the pYH23 control did not (Fig. 5A, B).The ability of OsZIP4 to restore the growth defect of YH003 yeast strain (Δfet4,Δfre1,Δfet3) was also studied to examine the uptake of Fe. OsIRT1 was used as a positive control in the experiment. In a heterologous experiment using synthetic defined medium without Fe, OsIRT1 restored the growth defect of YH003, whereas the pYH23 control and OsZIP4 did not (Fig. 5C). An attempt was made to complement the yeast mutant strains CM-SMF (Δsmf1) and FTRUNB1 (Δctr1) to examine the uptake of Mn and Cu, but no significant effects on growth were observed by the expression of OsZIP4 (data not shown).
Complementation of the CM-ZRT yeast mutant by OsZIP4. (A) Serial dilutions of CM-ZRT cells transformed with the empty vector (pYH23) or the vector expressing OsZIP4 or OsZIP3 were placed onto SD medium without Zn. (B) Quantitative growth analysis of CM-ZRT cells expressing OsZIP4 or OsZIP3, or containing the empty pYH23 vector grown in SD medium. The data are the mean ±SE of three separate experiments, with a total of nine replicates. (C) Serial dilutions of YH003 cells transformed with the empty vector (pYH23) or the vector expressing OsZIP4 or OsIRT1 were placed onto SD medium without Fe.
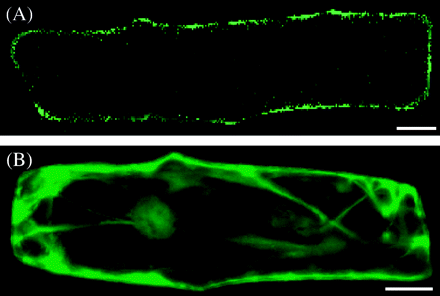
OsZIP4 is a transporter localized to the plasma membrane
The OsZIP4 protein fused to the N terminus of synthetic green fluorescent protein (sGFP) was expressed transiently under the control of the cauliflower mosaic virus (CaMV) 35S promoter in onion epidermis cells. The fluorescence of OsZIP4-sGFP was observed at the plasma membrane (Fig. 6A), while that of sGFP alone was localized to the cytoplasm and nucleus (Fig. 6B). This result indicates that OsZIP4 is a Zn transporter localized to the plasmamembrane.
Subcellular localization of transiently expressed OsZIP4-sGFP (A) or sGFP (B) fusion protein in onion epidermal cells observed using confocal laser scanning microscopy. Scale bars=20 μm.
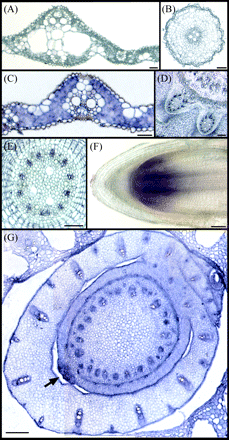
OsZIP4 is expressed in phloem cells of leaves and roots, and meristem
In situ hybridization experiments were performed in order to localize the transcripts of OsZIP4. OsZIP4 transcripts were present in all the vascular bundles and mesophyll cells of the leaves of Zn-deficient plants (Fig. 7C). In the stem, OsZIP4 was expressed mainly in phloem cells (Fig. 7D). In Zn-deficient roots, OsZIP4 expression was detected mainly in the vascular bundles, especially in the phloem cells (Fig. 7E). Furthermore, strong expression was detected in the root apical meristem, and in the region of shoot meristem (Fig. 7F, G).
In situ localization of OsZIP4 transcripts. In situ hybridization experiments were performed on transverse sections of a Zn-deficient rice using an OsZIP4 sense probe (A, B), or an OsZIP4 antisense probe (C–G). Transverse sections of the leaf (A), root (B), small vascular bundle (C), stem (D), and around shoot apex (G). Arrow indicates meristem. (E), Enlargement of the stele in the root. (F), Longitudinal section of the root tip. Scale bars=800 μm for (G); 400 μm for (A), (C), and (F); 100 μm for (B), and (D); and 50 μm for (E).
Discussion
These results suggest that OsZIP4 may function as a plasma membrane Zn-regulated Zn transporter responsible for the translocation of Zn. Recently, OsZIP1 and OsZIP3 were found to be rice Zn transporters induced by Zn deficiency (Ramesh et al., 2003). OsZIP1 and OsZIP3 expressed in the vascular bundles in shoots, and in the vascular bundles and epidermal cells in roots (Ramesh et al., 2003). In this experiment, however, expression of these genes was not induced by Zn deficiency (Fig. 2A, C). Moreover, the expression of OsZIP1 was increased dramatically in Cu-deficient roots (Fig. 2A), suggesting that OsZIP1 might be related to Cu deficiency in addition to the Zn transport system. By contrast, the expression of OsZIP4 was highly induced by Zn deficiency (Fig. 2A). The induction ratio of OsZIP4 under Zn deficiency in a microarray analysis was the fourth highest in shoots, and the transcript level in Zn deficiency was much higher than that of OsZIP1 and OsZIP3, especially in shoots (Fig. 2C). The expression of OsZIP4 was localized in both the vascular bundles and mesophyll cells of the Zn-deficient leaves, and mainly in the vascular bundles in Zn-deficient roots (Fig. 7), suggesting that OsZIP4 may not be responsible for Zn uptake from soil, but for Zn transport within the rice plant. This expression pattern of OsZIP4 is very similar to that of OsYSL2, which is a metal-nicotianamine complex transporter (Koike et al., 2004) and plays a role in the long-distance transport of metals in rice. It is noteworthy that the strong expression of OsZIP4 was detected in the root apical meristem and the shoot meristem. The roles of Zn in DNA and RNA metabolism, cell division, and protein synthesis are well documented (Uchiyama et al., 2002). A specific high requirement for Zn in the meristem would have caused the high expression of OsZIP4 in the meristem.
The expression of OsZIP4 in roots was increased in the early Zn-deficient stage, and the expression decreased gradually with prolonged Zn deficiency (Fig. 3). In this experiment, root and shoot growth continued for about 10 d after the start of the Zn-deficiency treatment (data not shown). Hence, it is likely that the expression of OsZIP4 in Zn-deficient roots increased until root growth stopped. By contrast, the induction of OsZIP4 in Zn-deficient shoots occurred after the induction of OsZIP4 in roots, and the expression of OsZIP4 in shoots was gradually increased by prolonged Zn deficiency. OsZIP4 was localized in mesophyll cells in Zn-deficient shoots (Fig. 7C). Zn deficiency not only increased the expression of OsZIP4 in young leaves, but also in old leaves. This suggests that OsZIP4 may be important for (i) the regulation of Zn supply in developing young leaves and (ii) in the long-distance transport of Zn from old to young leaves. It is possible that OsZIP4 is important in photosynthesis since carbonic anhydrase (CA) is a Zn-containing enzyme that catalyses the reversible conversion of CO2 to
In many cases, Zn-deficiency stress increases the Fe concentration in shoots, causing oxidative damage. For example, in tobacco (Nicotiana tabaccum) and barley (Hordeum vulgare), the Fe concentration in Zn-deficient plants is higher than that in control plants (Zhang et al., 1989; Kobayashi et al., 2003). However, the concentrations of Fe, Mn, and Cu in Zn-deficient rice shoots were not higher than those in the control plants (Fig. 4B). It was found that the heterologous expression of OsZIP4 in yeast had no effect on the growth of Fe- uptake mutant (Fig. 5C), Mn- or Cu-uptake mutants (data not shown). Therefore, OsZIP4 seems to be highly selective for Zn, but not for other metals. It is reported that OsZIP1 and OsZIP3 transport Zn but not Fe or Mn (Ramesh et al., 2003), and OsIRT1 transport Fe but not Cu (Bughio et al., 2002). These data suggest that ZIP family transporters in rice plants, including OsZIP4, might be transporters specific for one metal ion. If the OsZIP4 are not strictly regulated, one metal deficiency might easily cause an excess of another metal. Therefore, modifying OsZIP4 expression in rice plants may be widely applicable to creating transgenic plants that tolerate low Zn supply, while exhibiting no toxicity toward other metals due to excessive transport.
Yellow stripe 1 (YS1), the gene for the transporter protein from maize (Zea mays), which not only transports the Fe phytosiderophore complex, but also the Zn phytosiderophore complex, has been isolated and characterized (Curie et al., 2001; Schaaf et al., 2004). OsYSLs homologous to ZmYS1 have been found in rice plants (Koike et al., 2004). Further study may elucidate the function of each of these metal transport proteins (OsZIPs and OsYSLs), and how the expression of these proteins is regulated developmentally and spatially. Analysis of these genes will enhance the understanding of Zn nutrition, including Zn uptake from the soil, long-distance Zn transport within the plant, and Zn homeostasis within the cell.
Abbreviations: IRT, iron-regulated transporter; ZIP, zinc-regulated transporters; sGFP, synthetic green fluorescent protein.
We thank Dr P Blamey for assistance with English expression, Dr Nagamura and the Rice Genome Project (National Institute of Agrobiological Sciences, Tsukuba, Japan) for microarray analysis, and Dr Yoshimura, Dr Nagasaka, Ms Itai, and Dr Inoue for supporting our experiment.
References
Bughio N, Yamaguchi H, Nishizawa NK, Nakanishi H, Mori S.
Connolly EL, Fett JP, Guerinot ML.
Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL.
Eide D.
Eide D, Broderius M, Fett J, Guerinot ML.
Gietz RD, Schiestl RH.
Grotz N, Fox T, Connolly E, Park W, Guerinot ML, Eide D.
Hacisalihoglu G, Hart JJ, Vallejos CE, Kochian LV.
Ishimaru I, Suzuki M, Tsukamoto T, et al.
Kobayashi T, Yoshihara T, Jiang T, Goto F, Nakanishi H, Mori S, Nishizawa NK.
Koike S, Inoue H, Mizuno D, Takahashi M, Nakanishi H, Mori S, Nishizawa NK.
Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB.
Maser P, Thomine S, Schroeder JI, et al.
Mizuno D, Higuchi K, Sakamoto T, Nakanishi H, Mori S, Nishizawa NK.
Moreau S, Thomson RM, Kaiser BN, Trevaskis B, Guerinot ML, Udvardi MK, Puppo A, Day DA.
Ramesh SA, Shin R, Eide DJ, Schachtman DP.
Rogers EE, Eide DJ, Guerinot ML.
Schaaf G, Ludewig U, Erenoglu BE, Mori S, Kitahara T, von Wiren N.
Uchiyama Y, Hatanaka M, Kimura S, Ishibashi T, Ueda T, Sakakibara Y, Matsumoto T, Furukawa T, Hashimoto J, Sakaguchi K.
Vert G, Briat JF, Curie C.
Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, Curie C.
Wintz H, Fox T, Wu YY, Feng V, Chen W, Chang HS, Zhu T, Vulpe C.




Comments