-
PDF
- Split View
-
Views
-
Cite
Cite
Salim Al-Babili, Tran Thi Cuc Hoa, Patrick Schaub, Exploring the potential of the bacterial carotene desaturase CrtI to increase the β-carotene content in Golden Rice, Journal of Experimental Botany, Volume 57, Issue 4, March 2006, Pages 1007–1014, https://doi.org/10.1093/jxb/erj086
Close - Share Icon Share
Abstract
To increase the β-carotene (provitamin A) content and thus the nutritional value of Golden Rice, the optimization of the enzymes employed, phytoene synthase (PSY) and the Erwinia uredovora carotene desaturase (CrtI), must be considered. CrtI was chosen for this study because this bacterial enzyme, unlike phytoene synthase, was expressed at barely detectable levels in the endosperm of the Golden Rice events investigated. The low protein amounts observed may be caused by either weak cauliflower mosaic virus 35S promoter activity in the endosperm or by inappropriate codon usage. The protein level of CrtI was increased to explore its potential for enhancing the flux of metabolites through the pathway. For this purpose, a synthetic CrtI gene with a codon usage matching that of rice storage proteins was generated. Rice plants were transformed to express the synthetic gene under the control of the endosperm-specific glutelin B1 promoter. In addition, transgenic plants expressing the original bacterial gene were generated, but the endosperm-specific glutelin B1 promoter was employed instead of the cauliflower mosaic virus 35S promoter. Independent of codon optimization, the use of the endosperm-specific promoter resulted in a large increase in bacterial desaturase production in the T1 rice grains. However, this did not lead to a significant increase in the carotenoid content, suggesting that the bacterial enzyme is sufficiently active in rice endosperm even at very low levels and is not rate-limiting. The endosperm-specific expression of CrtI did not affect the carotenoid pattern in the leaves, which was observed upon its constitutive expression. Therefore, tissue-specific expression of CrtI represents the better option.
Introduction
Carotenoids are a group of isoprenoid pigments which are widely distributed in nature. They are synthesized by all photosynthetic organisms and some non-photosynthetic bacteria and fungi. Carotenoids protect the photosynthetic apparatus from photo-oxidation and represent structural components of light-harvesting antenna and reaction-centre complexes. Carotenoids confer their colour to many flowers and fruits, contributing substantially to plant–animal communication (Cunningham and Gantt, 1998; Hirschberg, 2001; Fraser and Bramley, 2004). In addition, some 9-cis-epoxycarotenoids serve as precursors of the phytohormone abscisic acid (Schwartz et al., 1997).
All vitamin A is derived from carotenoids. This essential compound is either taken up directly from animal foodstuffs or in the form of certain carotenoids with provitamin A activity, such as β-carotene. β-Carotene is then converted into retinal via a cleavage reaction, which is catalysed by an oxygenase. The corresponding enzyme has been identified from Drosophila melanogaster, chicken, and mammals including humans (for a review, see Giuliano et al., 2003). Recently, biochemical chracterization as well as the molecular structure of a related retinal-forming enzyme from the cyanobacterium Synechocystis have been described (Kloer et al., 2005; Ruch et al., 2005).
The provitamin A supply is critical in many parts of the world. Golden Rice (Ye et al., 2000), a rice genetically modified to synthesize provitamin A in the endosperm, is under product development to supplement classical interventions. Vitamin A deficiency (VAD)-dependent diseases prevail in developing countries, affecting millions (Underwood, 2000; UNICEF, 2000). VAD can result in permanent blindness (Chong and Scheufele, 2002) and impair the immune system, thereby exacerbating infectious diseases and leading to thousands of deaths, especially during early childhood (Mayne, 1996). VAD also contributes to anaemia by interfering with iron bioavailability (for a review, see Haschke and Javaid, 1991).
Golden Rice was developed based on the initial finding that the precursor geranyl geranyl diphosphate (GGPP), produced in wild-type rice endosperm, can be utilized by the daffodil enzyme phytoene synthase (PSY), when the latter is supplemented by transformation (Burkhardt et al., 1997). Subsequently, cDNA was introduced into rice under the control of the endosperm-specific promoter Gt1 together with the Erwinia uredovora carotene desaturase (CrtI) gene equipped with a transit peptide sequence, under the control of the constitutive cauliflower mosaic virus 35S promoter (CaMV35S promoter). This construct yielded yellow-coloured grains containing β-carotene and xanthophylls (Ye et al., 2000). The transformation with lycopene β-cyclase (β-LCY) was found unnecessary because β-carotene also formed in its absence. Recently, evidence has been presented which explains this phenomenon as a result of the functional expression of the endogenous enzymes responsible, carotene isomerase (CRTISO), β-LCY, and β-carotene hydroxylase (Schaub et al., 2005).
Golden Rice was originally produced by transformation of the Japonica variety Taipei 309 (Ye et al., 2000), and the technology was subsequently shown to be functional in different cultivars of rice that are relevant in Asia (Datta et al., 2003; Hoa et al., 2003). However, the prototype technology used needed improvement because of a 1.6 μg g−1 carotenoid ceiling in rice grains. Although an ex ante study targeting the Philippines indicated the presence of beneficial effects of these events (Zimmermann and Qaim, 2002), the enhancement of the provitamin A content appeared desirable to satisfy the dietary requirements better.
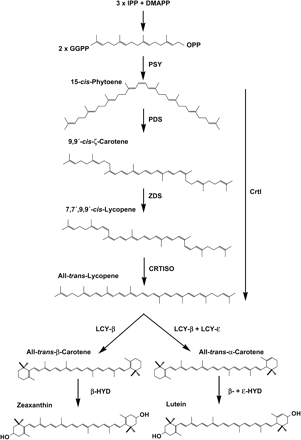
Because rate-limiting steps in the carotenoid biosynthetic pathway in rice endosperm were unknown, the two obvious options that needed to be considered for optimization are phytoene synthase, which carries out the first committed step in carotenogenesis, and the bacterial desaturase (CrtI), which introduces four double bonds and forms the red-coloured lycopene. Lycopene is the substrate for cyclization by β-LCY, yielding β-carotene (Fig. 1).
Scheme of carotenoid biosynthesis in relation to Golden Rice. The first committed step in the carotenoid biosynthesis is catalysed by the phytoene synthase (PSY), which mediates the condensation of two molecules of geranyl geranyl diphosphate (GGPP) to yield 15-cis-phytoene. In plants, the three enzymes, phytoene desaturase (PDS), ζ-carotene desaturase (ZDS), and carotene isomerase (CRTISO), are required to convert this colourless compound into the red carotene all-trans-lycopene. Thereby, the conjugated system of phytoene is extended through the introduction of four double bonds, catalysed by the two desaturases, leading to the intermediate prolycopene (7,7‘,9,9’-cis-lycopene). Prolycopene is then converted by CRTISO into all-trans-lycopene. It was also supposed that CRTISO acts on the desaturation-intermediate 7,9,9‘-cis-neurosporene yielding 9’-cis-neurosporene, and that all-trans-lycopene is directly formed from 7‘,9’-cis-lycopene (not shown; see Isaacson et al., 2004). The bacterial desaturase CrtI substitutes for the three plant enzymes by performing the complete desaturation sequence with all-trans intermediates. The cyclization of lycopene by the lycopene-β-cyclase (β-LCY) and the lycopene-ε-cyclase (ε-LCY) leads to α-carotene. β-Carotene is formed by β-LCY. The hydroxylation of these cyclic carotenes results in the formation of the xanthophylls zeaxanthin and lutein. The reaction is catalysed either by the β-carotene hydroxylase (β-HYD) or by β-HYD and the ε-carotene hydroxylase (ϵ-HYD), respectively. Wild-type rice endosperm is capable of synthesizing GGPP in the amyloplasts and contains functional cyclases and hydroxylases. Therefore, only two enzymes, PSY and CrtI, have to be supplemented to form β-carotene.
The discovery of the bacterial carotenoid gene clusters from Erwinia herbicola and Erwinia uredovora represented a breakthrough in carotenoid research, which enabled the identification of several carotenogenic enzymes from plants and cyanobacteria. In addition, the Erwinia genes were successfully used to enhance or modify the carotenoid content of several crops like rice (Ye et al., 2000), tomato (Romer et al., 2000), canola (Ravanello et al., 2003), and potato (Ducreux et al., 2005). From a biotechnological point of view, the employment of Erwinia carotene desaturase CrtI appears very advantageous because it substitutes for the three plant enzymes, phytoene desatursae, ζ-carotene desaturase, and carotene cis-trans-isomerase (CRTISO; see Fig. 1). However, it was shown that the constitutive expression of this enzyme does lead to alterations in the carotenoid pattern in leaves reducing the relative amount of lutein (Romer et al., 2000; Schaub et al., 2005). Such changes may affect the photosynthetic performance under high-light conditions (Lokstein et al., 2002).
This is a report on the approaches made to improve CrtI expression in rice endosperm. Originating from bacteria, CrtI's codon usage adaptation to match that of rice could result in improved translation. In addition, the expression of CrtI under the control of an endosperm-specific instead of the constitutive CaMV35S promoter was also expected to increase the enzyme amount in the endosperm. Endosperm-specific CrtI expression also avoids alterations in the carotenoid pattern observed in leaves upon constitutive expression. Accordingly, DNA constructs were made and the carotenoid content determined in the endosperm and leaves of stably transformed rice plants. The second option for optimization, targeting phytoene synthase, has been dealt with by others (Paine et al., 2005).
Materials and methods
Construction of pCarNew
To isolate the endosperm-specific glutelin B1 promoter (GluB1 promoter; Takaiwa et al., 1991) from pUC18-GluB, the vector was digested with BamHI, filled-in using T4-DNA polymerase, and treated with HindIII. The GluB1-fragment was then ligated into the filled-in XbaI and HindIII sites of pUCET4 (Misawa et al., 1993), replacing the original CaMV 35S promoter in the CrtI expression cassette, thereby yielding the vector pGCrtI. pCarNew was constructed in two steps. First, the tissue-specific CrtI-expression cassette was isolated from pGCrtI using EcoRI and HindIII and ligated into the corresponding sites of pMCA1380, a derivative of pCAMBIA1380 (Cambia, Canberra, Australia) encoding phosphomannose isomerase as a selection marker (Lucca et al., 2001), yielding the binary vector pBCrtI. Finally, pCarNew was constructed by ligating a HindIII fragment encoding the PSY-expression cassette from pCaCar (Hoa et al., 2003) into the corresponding site of pBCrtI.
Construction of pFun3
The modified TP-CrtI gene was synthesized by Entelechon GmbH, Regensburg, Germany. It was excised from pPCR-CrtI using NotI and EcoRI. Prior to EcoRI digestion, a T4-DNA polymerase treatment was performed to fill-in the Not1-site. The fragment obtained was then cloned into SmaI/EcoRI-treated pUC18-GluB, yielding pFun1. A SalI/EcoRI fragment encoding the endosperm-specific promoter GluB1 and the synthetic TP-CrtI was isolated from pFun1 and ligated into the corresponding sites of pMCA1390, a derivative of pCAMBIA1390 (Cambia, Canberra, Australia) encoding phosphomannose isomerase as a selection marker (Lucca et al., 2001), yielding the binary vector pFun2. Finally, pFun3 was constructed by ligating a SalI fragment encoding the PSY expression cassette from pBaal2 (Ye et al., 2000) into the corresponding site of pFun2.
Western blot analysis
About 200 mg of polished rice grains was incubated at 98 °C in 1 ml of sample buffer [65 mM TRIS-HCl, pH 6.75, 4% (w/v) SDS, 10% (v/v) β-mercaptoethanol, 20% (v/v) glycerol] for 10 min. Subsequently, 60 μl of the highly viscous samples were applied to SDS-PAGE using chopped-off tips. Applied protein amounts were verified by Coomassie Brilliant Blue staining of a gel run in parallel. For immunodetection, anti-CrtI antibodies were raised in mice against a purified His-tagged CrtI-fusion protein expressed in Escherichia coli cells (S Al-Babili, unpublished results). The antibodies were then affinity-purified according to the method of Smith and Fisher (1984). For detection, horseradish peroxidase-coupled secondary anti-mouse antibodies and the ECL system (Amersham Biosciences Europe, Freiburg, Germany) were employed according to the manufacturer's protocol. PSY was detected using affinity-purified antibodies (Schledz et al., 1996).
Plant materials and transformation
Immature embryos of the cultivar Taipei 309 were inoculated with A. tumefaciens strain LBA 4404 (Hoekema et al., 1984). The transformation experiments were performed according to Aldemita and Hodges (1996). The procedures for selection and regeneration, as described by Lucca et al. (2001), were followed. Mannose-resistant rice plants were transferred to soil and grown in the greenhouse at 28 °C (day) and 21 °C (night) and 80% relative humidity.
Carotenoid extraction and analysis
Dehusked seeds were polished for 1 min using a commercial grain polisher (Kett, Tokyo, Japan), thereby removing the embryos. Polished seeds (0.5 g) were ground for 1 min to a fine powder using a Micro-Dismembrator (Braun, Melsungen, Germany). The powder in the Teflon capsules was resuspended in 2 ml of acetone at room temperature and transferred to a glass test tube, and, as an internal standard, 200 μg of tocopherol acetate (Sigma, Deisenhofen, Germany) was added from an appropriate acetone stock solution. This suspension was sonicated for 10 s, centrifuged, and the supernatant collected. The extraction procedure was repeated with another 2 ml of acetone to ensure complete carotenoid extraction. The combined extracts were dried in a Vortex-Evaporator (Haake-Buchler; Saddle Brook, NJ, USA) and resuspended in 500 μl of acetone. Carotenoid amounts were determined by HPLC using the internal plus an external standard (echinenone, kindly provided by Hoffmann-La Roche, Basel, Switzerland). Samples were dried and dissolved in 30 μl of chloroform, of which 10 μl was injected into the HPLC system as described (Hoa et al., 2003). Following the same procedure, carotenoid extraction from rice leaves was performed using about 5 mg of lyophilized and ground material.
Results and discussion
CrtI protein level is low in Golden Rice
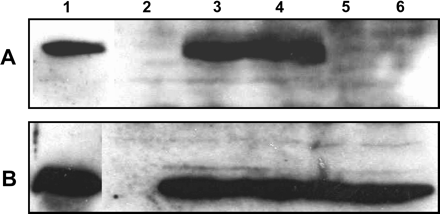
Golden Rice, as initially described (Ye et al., 2000), as well as some later versions (Datta et al., 2003; Hoa et al., 2003), rely on the expression of TP-CrtI, the fusion gene encoding a carotene desaturase from Erwinia uredovora equipped with the pea (Pisum sativum) RuBisCO small subunit transit peptide (Misawa et al., 1993). In all of these versions, CrtI was under the control of the constitutive CaMV 35S promoter. To determine protein levels, western blot analyses of several endosperm samples from transgenic lines previously described (Hoa et al., 2003) were performed using affinity-purified anti-CrtI antibodies. Examples are given in Fig. 2, lanes 5 and 6. In all samples, the CrtI protein was found to be below detection level, albeit the observed accumulation of β-carotene implied the presence of the corresponding enzymatic activity. It had been shown previously that the expression of PSY alone did not result in the formation of coloured carotenoids (Burkhardt et al., 1997), calling for an exogenous desaturase. By contrast, western blot analyses of PSY in the same samples gave strong signals. This indicated that CrtI expression in these earlier versions of Golden Rice may be too low effectively to convert phytoene into coloured carotenoids. The high expression levels observed with PSY are most likely caused by the employment of the endosperm-specific promoter Gt1, which may be more active in the endosperm than the CaMV 35S promoter. Alternatively, inefficient translation may be considered to be caused by differences in codon usage between Erwinia and plants. Incorporating rice endosperm codon usage into CrtI gene sequences may increase its expression level in this tissue as shown, for instance, for Bacillus thuringiensis genes cryI(A)b and cryI(A)c, where codon adaption dramatically increased the corresponding protein levels in tomato, tobacco, and rice plants (Perlak et al., 1991; Cheng et al., 1998), and in maize endosperm cell cultures (Sardana et al., 1995). Similarly, the codon optimization of a bacterial (1,3-1,4)-β-glucanase resulted in a significant increase of the corresponding protein level in barley (Jensen et al., 1996).
Western blot analysis of rice endosperm. Lane 1, positive control with total protein extract (10 μg) of CrtI-expressing E. coli cells (A) and of daffodil chromoplasts (B); lane 2, wild type; lane 3, transformed with pCarNew (CarNew E4-4, T1), lane 4 with pFun3 (Fun3 E3-5, T1), and lanes 5 and 6 with pCaCar (Hoa et al., 2003; lines CaCar 48-67-8-7, T3, and 29–35CR 13, T1). Detection was performed with anti-CrtI antibodies (A) and anti-PSY antibodies (B).
Codon optimization of TP-CrtI
Based on the sequences of six rice storage proteins, 10 kDa prolamin (X84649), 13 kDa prolamin (S39468), and the glutelins (X14568, AB016501, M17513, and M28159), the codon probabilities were determined and compared with those of TP-CrtI. As can be seen in Table 1, the TP-CrtI codon usage differed markedly from that in rice genes. For instance, the 20 codons for histidine in TP-CrtI are represented seven times by CAC and 13 times by CAU, corresponding to a frequency of 35% and 65%, respectively. By contrast, the inverse ratio was found in the selected rice genes. Similar differences were obtained by comparing codon ratios of lysine and phenylalanine. Therefore, a synthetic TP-CrtI with adapted codon usage was made which was 73.8% identical to the original sequence. A comparison of the frequency of codons in the original TP-CrtI and the synthetic gene is given in Table 1.
Codon frequency of selected storage genes (I), TP-CrtI (II), and the synthetic gene (III)
Amino acid . | Codon . | I . | II . | III . |
|---|---|---|---|---|
| A | GCA | 25.00% | 32.65% | 26.53% |
| GCU | 25.00% | 10.20% | 24.48% | |
| GCG | 20.00% | 30.61% | 20.40% | |
| GCC | 30.00% | 26.53% | 28.57% | |
| C | UGU: | 30.00% | 60.00% | 20.00% |
| UGC | 70.00% | 40.00% | 80.00% | |
| D | GAU | 45.00% | 61.53% | 46.15% |
| GAC | 55.00% | 38.46% | 53.84% | |
| E | GAA | 25.00% | 53.84% | 23.07% |
| GAG | 75.00% | 46.15% | 76.92% | |
| F | UUU | 40.00% | 70.00% | 40.00% |
| UUC | 60.00% | 30.00% | 60.00% | |
| G | GGA | 25.00% | 8.51% | 23.40% |
| GGU | 20.00% | 23.4% | 19.14% | |
| GGG | 15.00% | 12.76% | 14.89% | |
| GGC | 40.00% | 55.32% | 42.55% | |
| H | CAU | 35.00% | 65.00% | 35.00% |
| CAC | 65.00% | 35.00% | 65.00% | |
| I | AUA | 20.00% | 14.28% | 19.04% |
| AUU | 30.00% | 66.66% | 33.33% | |
| AUC | 50.00% | 19.04% | 47.61% | |
| K | AAA | 20.00% | 52.38% | 19.05% |
| AAG | 80.00% | 47.61% | 80.95% | |
| L | UUA | 10.00% | 15.51% | 10.34% |
| UUG | 20.00% | 8.62% | 20.68% | |
| CUA | 10.00% | 5.17% | 10.34% | |
| CUU | 20.00% | 10.34% | 20.68% | |
| CUG | 15.00% | 46.55% | 15.51% | |
| CUC | 25.00% | 13.79% | 22.41% | |
| M | AUG | 100.00% | 100.00% | 100.00% |
| N | AAU | 45.00% | 52.95% | 47.05% |
| AAC | 55.00% | 47.05% | 52.95% | |
| P | CCA | 35.00% | 14.81% | 33.33% |
| CCU | 25.00% | 22.22% | 25.92% | |
| CCG | 20.00% | 33.33% | 18.51% | |
| CCC | 20.00% | 29.62% | 22.22% | |
| Q | CAA | 55.00% | 25.00% | 54.16% |
| CAG | 45.00% | 75.00% | 45.83% | |
| R | AGA | 20.00% | 12.90% | 19.35% |
| AGG | 30.00% | 6.45% | 29.03% | |
| CGA | 0.00% | 0.00% | 0.00% | |
| CGU | 15.00% | 25.80% | 16.12% | |
| CGG | 15.00% | 16.12% | 16.12% | |
| CGC | 20.00% | 38.70% | 19.35% | |
| S | AGU | 20.00% | 16.66% | 19.44% |
| AGC | 25.00% | 16.66% | 25.00% | |
| UCA | 10.00% | 19.44% | 11.11% | |
| UCU | 15.00% | 16.66% | 16.66% | |
| UCG | 15.00% | 11.11% | 13.88% | |
| UCC | 15.00% | 19.44% | 13.88% | |
| T | ACA | 20.00% | 15.15% | 21.21% |
| ACU | 30.00% | 21.21% | 30.30% | |
| ACG | 10.00% | 36.36% | 9.09% | |
| ACC | 40.00% | 27.27% | 39.39% | |
| V | GUA | 15.00% | 5.00% | 15.00% |
| GUU | 25.00% | 25.00% | 25.00% | |
| GUG | 30.00% | 30.00% | 30.00% | |
| GUC | 30.00% | 40.00% | 30.00% | |
| W | UGG | 100.00% | 100.00% | 100.00% |
| Y | UAU | 40.00% | 52.38% | 38.10% |
| UAC | 60.00% | 47.61% | 61.90% |
Amino acid . | Codon . | I . | II . | III . |
|---|---|---|---|---|
| A | GCA | 25.00% | 32.65% | 26.53% |
| GCU | 25.00% | 10.20% | 24.48% | |
| GCG | 20.00% | 30.61% | 20.40% | |
| GCC | 30.00% | 26.53% | 28.57% | |
| C | UGU: | 30.00% | 60.00% | 20.00% |
| UGC | 70.00% | 40.00% | 80.00% | |
| D | GAU | 45.00% | 61.53% | 46.15% |
| GAC | 55.00% | 38.46% | 53.84% | |
| E | GAA | 25.00% | 53.84% | 23.07% |
| GAG | 75.00% | 46.15% | 76.92% | |
| F | UUU | 40.00% | 70.00% | 40.00% |
| UUC | 60.00% | 30.00% | 60.00% | |
| G | GGA | 25.00% | 8.51% | 23.40% |
| GGU | 20.00% | 23.4% | 19.14% | |
| GGG | 15.00% | 12.76% | 14.89% | |
| GGC | 40.00% | 55.32% | 42.55% | |
| H | CAU | 35.00% | 65.00% | 35.00% |
| CAC | 65.00% | 35.00% | 65.00% | |
| I | AUA | 20.00% | 14.28% | 19.04% |
| AUU | 30.00% | 66.66% | 33.33% | |
| AUC | 50.00% | 19.04% | 47.61% | |
| K | AAA | 20.00% | 52.38% | 19.05% |
| AAG | 80.00% | 47.61% | 80.95% | |
| L | UUA | 10.00% | 15.51% | 10.34% |
| UUG | 20.00% | 8.62% | 20.68% | |
| CUA | 10.00% | 5.17% | 10.34% | |
| CUU | 20.00% | 10.34% | 20.68% | |
| CUG | 15.00% | 46.55% | 15.51% | |
| CUC | 25.00% | 13.79% | 22.41% | |
| M | AUG | 100.00% | 100.00% | 100.00% |
| N | AAU | 45.00% | 52.95% | 47.05% |
| AAC | 55.00% | 47.05% | 52.95% | |
| P | CCA | 35.00% | 14.81% | 33.33% |
| CCU | 25.00% | 22.22% | 25.92% | |
| CCG | 20.00% | 33.33% | 18.51% | |
| CCC | 20.00% | 29.62% | 22.22% | |
| Q | CAA | 55.00% | 25.00% | 54.16% |
| CAG | 45.00% | 75.00% | 45.83% | |
| R | AGA | 20.00% | 12.90% | 19.35% |
| AGG | 30.00% | 6.45% | 29.03% | |
| CGA | 0.00% | 0.00% | 0.00% | |
| CGU | 15.00% | 25.80% | 16.12% | |
| CGG | 15.00% | 16.12% | 16.12% | |
| CGC | 20.00% | 38.70% | 19.35% | |
| S | AGU | 20.00% | 16.66% | 19.44% |
| AGC | 25.00% | 16.66% | 25.00% | |
| UCA | 10.00% | 19.44% | 11.11% | |
| UCU | 15.00% | 16.66% | 16.66% | |
| UCG | 15.00% | 11.11% | 13.88% | |
| UCC | 15.00% | 19.44% | 13.88% | |
| T | ACA | 20.00% | 15.15% | 21.21% |
| ACU | 30.00% | 21.21% | 30.30% | |
| ACG | 10.00% | 36.36% | 9.09% | |
| ACC | 40.00% | 27.27% | 39.39% | |
| V | GUA | 15.00% | 5.00% | 15.00% |
| GUU | 25.00% | 25.00% | 25.00% | |
| GUG | 30.00% | 30.00% | 30.00% | |
| GUC | 30.00% | 40.00% | 30.00% | |
| W | UGG | 100.00% | 100.00% | 100.00% |
| Y | UAU | 40.00% | 52.38% | 38.10% |
| UAC | 60.00% | 47.61% | 61.90% |
Codon frequency of selected storage genes (I), TP-CrtI (II), and the synthetic gene (III)
Amino acid . | Codon . | I . | II . | III . |
|---|---|---|---|---|
| A | GCA | 25.00% | 32.65% | 26.53% |
| GCU | 25.00% | 10.20% | 24.48% | |
| GCG | 20.00% | 30.61% | 20.40% | |
| GCC | 30.00% | 26.53% | 28.57% | |
| C | UGU: | 30.00% | 60.00% | 20.00% |
| UGC | 70.00% | 40.00% | 80.00% | |
| D | GAU | 45.00% | 61.53% | 46.15% |
| GAC | 55.00% | 38.46% | 53.84% | |
| E | GAA | 25.00% | 53.84% | 23.07% |
| GAG | 75.00% | 46.15% | 76.92% | |
| F | UUU | 40.00% | 70.00% | 40.00% |
| UUC | 60.00% | 30.00% | 60.00% | |
| G | GGA | 25.00% | 8.51% | 23.40% |
| GGU | 20.00% | 23.4% | 19.14% | |
| GGG | 15.00% | 12.76% | 14.89% | |
| GGC | 40.00% | 55.32% | 42.55% | |
| H | CAU | 35.00% | 65.00% | 35.00% |
| CAC | 65.00% | 35.00% | 65.00% | |
| I | AUA | 20.00% | 14.28% | 19.04% |
| AUU | 30.00% | 66.66% | 33.33% | |
| AUC | 50.00% | 19.04% | 47.61% | |
| K | AAA | 20.00% | 52.38% | 19.05% |
| AAG | 80.00% | 47.61% | 80.95% | |
| L | UUA | 10.00% | 15.51% | 10.34% |
| UUG | 20.00% | 8.62% | 20.68% | |
| CUA | 10.00% | 5.17% | 10.34% | |
| CUU | 20.00% | 10.34% | 20.68% | |
| CUG | 15.00% | 46.55% | 15.51% | |
| CUC | 25.00% | 13.79% | 22.41% | |
| M | AUG | 100.00% | 100.00% | 100.00% |
| N | AAU | 45.00% | 52.95% | 47.05% |
| AAC | 55.00% | 47.05% | 52.95% | |
| P | CCA | 35.00% | 14.81% | 33.33% |
| CCU | 25.00% | 22.22% | 25.92% | |
| CCG | 20.00% | 33.33% | 18.51% | |
| CCC | 20.00% | 29.62% | 22.22% | |
| Q | CAA | 55.00% | 25.00% | 54.16% |
| CAG | 45.00% | 75.00% | 45.83% | |
| R | AGA | 20.00% | 12.90% | 19.35% |
| AGG | 30.00% | 6.45% | 29.03% | |
| CGA | 0.00% | 0.00% | 0.00% | |
| CGU | 15.00% | 25.80% | 16.12% | |
| CGG | 15.00% | 16.12% | 16.12% | |
| CGC | 20.00% | 38.70% | 19.35% | |
| S | AGU | 20.00% | 16.66% | 19.44% |
| AGC | 25.00% | 16.66% | 25.00% | |
| UCA | 10.00% | 19.44% | 11.11% | |
| UCU | 15.00% | 16.66% | 16.66% | |
| UCG | 15.00% | 11.11% | 13.88% | |
| UCC | 15.00% | 19.44% | 13.88% | |
| T | ACA | 20.00% | 15.15% | 21.21% |
| ACU | 30.00% | 21.21% | 30.30% | |
| ACG | 10.00% | 36.36% | 9.09% | |
| ACC | 40.00% | 27.27% | 39.39% | |
| V | GUA | 15.00% | 5.00% | 15.00% |
| GUU | 25.00% | 25.00% | 25.00% | |
| GUG | 30.00% | 30.00% | 30.00% | |
| GUC | 30.00% | 40.00% | 30.00% | |
| W | UGG | 100.00% | 100.00% | 100.00% |
| Y | UAU | 40.00% | 52.38% | 38.10% |
| UAC | 60.00% | 47.61% | 61.90% |
Amino acid . | Codon . | I . | II . | III . |
|---|---|---|---|---|
| A | GCA | 25.00% | 32.65% | 26.53% |
| GCU | 25.00% | 10.20% | 24.48% | |
| GCG | 20.00% | 30.61% | 20.40% | |
| GCC | 30.00% | 26.53% | 28.57% | |
| C | UGU: | 30.00% | 60.00% | 20.00% |
| UGC | 70.00% | 40.00% | 80.00% | |
| D | GAU | 45.00% | 61.53% | 46.15% |
| GAC | 55.00% | 38.46% | 53.84% | |
| E | GAA | 25.00% | 53.84% | 23.07% |
| GAG | 75.00% | 46.15% | 76.92% | |
| F | UUU | 40.00% | 70.00% | 40.00% |
| UUC | 60.00% | 30.00% | 60.00% | |
| G | GGA | 25.00% | 8.51% | 23.40% |
| GGU | 20.00% | 23.4% | 19.14% | |
| GGG | 15.00% | 12.76% | 14.89% | |
| GGC | 40.00% | 55.32% | 42.55% | |
| H | CAU | 35.00% | 65.00% | 35.00% |
| CAC | 65.00% | 35.00% | 65.00% | |
| I | AUA | 20.00% | 14.28% | 19.04% |
| AUU | 30.00% | 66.66% | 33.33% | |
| AUC | 50.00% | 19.04% | 47.61% | |
| K | AAA | 20.00% | 52.38% | 19.05% |
| AAG | 80.00% | 47.61% | 80.95% | |
| L | UUA | 10.00% | 15.51% | 10.34% |
| UUG | 20.00% | 8.62% | 20.68% | |
| CUA | 10.00% | 5.17% | 10.34% | |
| CUU | 20.00% | 10.34% | 20.68% | |
| CUG | 15.00% | 46.55% | 15.51% | |
| CUC | 25.00% | 13.79% | 22.41% | |
| M | AUG | 100.00% | 100.00% | 100.00% |
| N | AAU | 45.00% | 52.95% | 47.05% |
| AAC | 55.00% | 47.05% | 52.95% | |
| P | CCA | 35.00% | 14.81% | 33.33% |
| CCU | 25.00% | 22.22% | 25.92% | |
| CCG | 20.00% | 33.33% | 18.51% | |
| CCC | 20.00% | 29.62% | 22.22% | |
| Q | CAA | 55.00% | 25.00% | 54.16% |
| CAG | 45.00% | 75.00% | 45.83% | |
| R | AGA | 20.00% | 12.90% | 19.35% |
| AGG | 30.00% | 6.45% | 29.03% | |
| CGA | 0.00% | 0.00% | 0.00% | |
| CGU | 15.00% | 25.80% | 16.12% | |
| CGG | 15.00% | 16.12% | 16.12% | |
| CGC | 20.00% | 38.70% | 19.35% | |
| S | AGU | 20.00% | 16.66% | 19.44% |
| AGC | 25.00% | 16.66% | 25.00% | |
| UCA | 10.00% | 19.44% | 11.11% | |
| UCU | 15.00% | 16.66% | 16.66% | |
| UCG | 15.00% | 11.11% | 13.88% | |
| UCC | 15.00% | 19.44% | 13.88% | |
| T | ACA | 20.00% | 15.15% | 21.21% |
| ACU | 30.00% | 21.21% | 30.30% | |
| ACG | 10.00% | 36.36% | 9.09% | |
| ACC | 40.00% | 27.27% | 39.39% | |
| V | GUA | 15.00% | 5.00% | 15.00% |
| GUU | 25.00% | 25.00% | 25.00% | |
| GUG | 30.00% | 30.00% | 30.00% | |
| GUC | 30.00% | 40.00% | 30.00% | |
| W | UGG | 100.00% | 100.00% | 100.00% |
| Y | UAU | 40.00% | 52.38% | 38.10% |
| UAC | 60.00% | 47.61% | 61.90% |
Rice transformation of TP-CrtI under the control of GluB1
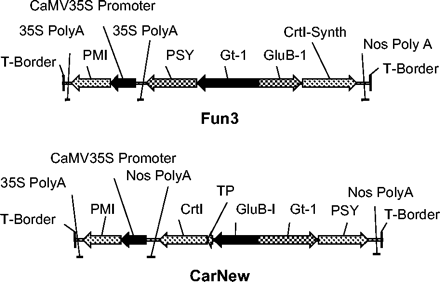
Different sets of A. tumefaciens-mediated transformation experiments were performed using immature embryos of Japonica variety Taipei 309, relying on phosphomannose isomerase (PMI, Positech®) as the selection marker. The TP-CrtI expression was driven by the endosperm-specific promoter GluB1 in all experiments. This promoter had been employed previously to express ferritin in rice endosperm (Goto et al., 1999). Transformations were performed using the binary vectors pCarNew or pFun3 harbouring the original TP-CrtI or the codon-optimized gene, respectively. Both vectors contained the daffodil PSY-cDNA under the control of the endosperm-specific promoter Gt1. The vectors used are schematically represented in Fig. 3.
Scheme of the T-insert of pCarNew and pFun3. PMI, phosphomannose isomerase; PSY, phytoene synthase; CrtI, Erwinia uredovora phytoene desaturase; TP, a fragment encoding RuBisCo small subunit transit peptide; CrtI-Synthetic, codon-optimized TP-CrtI; Gt-1, glutelin 1 promoter; GluB-I, glutelin B1 promoter; Nos, nopaline synthase.
Analysis of T1 seeds
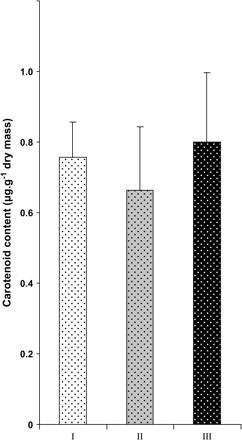
First, T1 seeds were phenotypically selected by visible colour after polishing. After confirming the presence of the transgene by Southern blot analyses (data not shown), segregating seed populations with notably yellow grains (18 events out of 44 obtained with pCarNew, and 20 events out of 48 obtained with pFun3), were subjected to carotenoid analysis. Randomly selected grains from these segregating T1 populations were extracted, and the carotenoid amount and pattern were determined by HPLC. The carotenoid amounts in the T1 seeds of pCarNew and pFun3 transformants were in similar ranges, 0.2–0.9 and 0.2–1 μg g−1, respectively. The values observed did not surpass the amounts obtained from seeds transformed with pCaCar containing the original TP-CrtI under the control of the constitutive CaMV 35S promoter (Hoa et al., 2003). In addition, significant changes were not observed in the carotenoid pattern of the pCarNew and pFun3 seeds in comparison to the one found in the endosperm of pCaCar transformants. The average carotenoid contents of the best five events from each transformation are given in Fig. 4.
Carotenoid analysis of Taipei 309 T1 seeds. Wild-type seeds were carotenoid-free. The values represent the average of total carotenoid amounts of the best five lines from transformation experiments performed using pCaCar (I; Hoa et al., 2003), pCarNew (II), and pFun3 (III). Dry mass refers to fully mature dry seeds.
CrtI is not rate-limiting in Golden Rice endosperm
To verify that the use of GluB-1 and the synthetic TP-CrtI accomplished the anticipated increase of the CrtI protein amount, endosperm samples from pCaCar, pCarNew, and pFun3 transformants were analysed by western blotting using affinity-purified anti-CrtI and, in a control, anti-PSY antibodies. Figure 2 depicts selected events exhibiting the best performance with respect to carotenoid content. As can be seen in lanes 3 and 4, the employment of the GluB-1 instead of the CaMV35S promoter resulted in a dramatic increase in CrtI protein abundance. This increase was largely independent of the codon usage, as shown in lane 3 representing a pCarNew transformant. By contrast, CrtI was below detection level in pCaCar endosperm. In these events, the expression of PSY was as high as in the previous pCaCar events (lanes 5 and 6), as expected.
The present data indicate that the Erwinia enzyme CrtI is very active in rice endosperm. Even protein amounts below the detection level were capable of processing all the phytoene formed by phytoene synthase into coloured carotenoids. Phytoene did not accumulate and, assuming that phytoene is not degraded by an unknown mechanism, it is apparently completely desaturated by CrtI. Accordingly, this suggests that the improvement of the CrtI protein level is less relevant to increase the β-carotene content in rice endosperm. This is further corroborated by recent studies that indicate that the rate-limiting step is evidently represented by phytoene synthase (Paine et al., 2005).
Endosperm-specific CrtI expression does not alter the carotenoid pattern in rice leaves
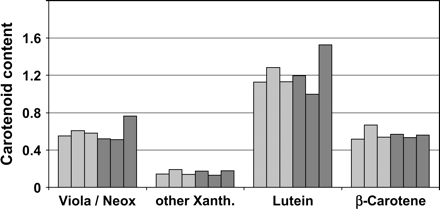
It was shown that the constitutive expression of CrtI led to a decrease in lutein, which was partially compensated by an increase in the number of β-carotene-derived xanthophylls in the leaves of tomato (Romer et al., 2000) and rice (Schaub et al., 2005). This alteration is not mirrored by a change in the expression of the respective lycopene ε- or β-cyclases but correlated, in an event-dependent manner, with the CrtI-protein amount. It was assumed that this effect is most likely due to changes at the level of the cis–trans isomerism of lycopene (Schaub et al., 2005). This change in xanthophyll composition may have a negative impact on the phytosynthetic capacity under high-light conditions (Lokstein et al., 2002). Endosperm-specific expression of CrtI may not lead to such alteration in the carotenoid pattern of the leaves. To prove this assumption, leaves of homozygote rice transformants specifically expressing CrtI endosperm were analysed. The events used were generated and kindly provided by Syngenta through transformation with daffodil PSY and CrtI, both under the control of the glutelin 1 promoter (as given in Al-Babili and Beyer, 2005). As can be seen in Fig. 5, no significant differences in xanthophyll composition between azygote and homozygote plants were detected. These results suggest that the observed alterations in the carotenoid pattern of rice leaves constitutively expressing CrtI do not occur when an endosperm-specific promoter is employed. Thus, the endosperm-specific expression of this bacterial enzyme is the preferred option. CrtI-dependent alteration of the xanthophyll pattern may be a general effect that can be avoided by restricting the expression to the non-green target tissue.
Carotenoid pattern of leaves obtained from homozygote lines expressing CrtI under the control of the endosperm-specific Gt1 promoter. Dark grey, the homozygote lines (from left to right) are 146.5.48, 309.731, and 652.11.22; light grey, the corresponding azygotes. Carotenoid content is given in mg g−1 dry mass. By contrast to plants expressing Crt constitutively (Schaub et al., 2005), the alteration in the carotenoid pattern of the leaves did not occur.
This work was supported by The Rockefeller Foundation, New York, and the HarvestPlus programme (www.harvestplus.org). We would like to thank Professor F Takaiwa, National Institute of Agrobiological Sciences, Tsukuba, Japan, for kindly providing the plasmid pUC18-GluB. We thank Dr Randall Cassada for correcting the English version of the manuscript and Dr Jorge Mayer for valuable discussions.
References
Al-Babili S, Beyer P.
Aldemita RR, Hodges TK.
Burkhardt PK, Beyer P, Wünn J, Kloti A, Armstrong GA, Schledz M, von Lintig J, Potrykus I.
Cheng X, Sardana R, Kaplan H, Altosaar I.
Chong M, Scheufele DA.
Cunningham FX, Gantt E.
Datta K, Baisakh N, Oliva N, et al.
Ducreux LJ, Morris WL, Hedley PE, Shepherd T, Davies HV, Millam S, Taylor MA.
Fraser PD, Bramley PM.
Goto F, Yoshihara T, Shigemoto N, Toki S, Takaiwa F.
Giuliano G, Al-Babili S, von Lintig J.
Hirschberg J.
Hoa TT, Al-Babili S, Schaub P, Potrykus I, Beyer P.
Hoekema A, Roelvink PW, Hooykaas PJJ, Schilperoort RA.
Isaacson T, Ohad I, Beyer P, Hirschberg J.
Jensen LG, Olsen O, Kops O, Wolf N, Thomsen KK, von Wettstein D.
Kloer DP, Ruch S, Al-Babili S, Beyer P, Schulz GE.
Lokstein H, Tian L, Polle JE, DellaPenna D.
Lucca P, Ye X, Potrykus I.
Misawa N, Yamano S, Linden H, de Felipe MR, Lucas M, Ikenaga H, Sandmann G.
Paine JA, Shipton CA, Chaggar S, et al.
Perlak FJ, Fuchs RL, Dean DA, McPherson SL, Fischhoff DA.
Ravanello MP, Ke D, Alvarez J, Huang B, Shewmaker CK.
Romer S, Fraser PD, Kiano JW, Shipton CA, Misawa N, Schuch W, Bramley PM.
Ruch S, Beyer P, Ernst H, Al-Babili S.
Sardana R, Dukiandijiev S, Giband M, Cheng X, Cowan K, Sauder C, Altosaar I.
Schaub P, Al-Babili S, Beyer P.
Schledz M, Al-Babili S, von Lintig J, Kleinig H, Rabbani S, Beyer P.
Schwartz SH, Tan BC, Gage DA, Zeevaart JA, McCarty DR.
Smith DE, Fisher PA.
Takaiwa F, Oono K, Wing D, Kato A.
Underwood BA.
Ye X, Al-Babili S, Klöti A, Zhang J, Lucca P, Beyer P, Potrykus I.
Zimmermann R, Qaim M.




Comments