-
PDF
- Split View
-
Views
-
Cite
Cite
Machiraju S. Ravi Shankar, A. N. Aravindan, Preet M. Sohal, Harbir S. Kohli, Kamal Sud, Krishan L. Gupta, Vinay Sakhuja, Vivekanand Jha, The prevalence of tuberculin sensitivity and anergy in chronic renal failure in an endemic area: tuberculin test and the risk of post-transplant tuberculosis, Nephrology Dialysis Transplantation, Volume 20, Issue 12, December 2005, Pages 2720–2724, https://doi.org/10.1093/ndt/gfi141
Close - Share Icon Share
Abstract
Background. Cutaneous sensitivity to the tuberculin antigen is thought to indicate latent tuberculosis infection (LTBI). Some guidelines suggest treating end-stage renal disease (ESRD) patients for LTBI on the basis of tuberculin positivity. The prevalence of tuberculin sensitivity and cutaneous anergy in Indian patients with ESRD and the utility of the tuberculin test for predicting post-transplant tuberculosis are not known.
Methods. We prospectively studied cutaneous tuberculin reactivity and anergy in 108 ESRD patients and 100 age- and sex-matched healthy controls. Mumps antigen and tetanus toxoid were used as control antigens. Patients who failed to react to all antigens were classified as anergic. Seventy-nine patients underwent living donor kidney transplants and were followed-up for approximately 2 years.
Results. About 44% of ESRD patients and 66% of controls showed tuberculin reactivity (P = 0.0018). The prevalence of anergy was significantly higher in the ESRD population (44% vs 16%, P<0.001). The haemoglobin, serum albumin and creatinine values were significantly higher amongst the tuberculin-reactor ESRD patients. Tuberculin positivity and anergy rates in a subgroup of well-nourished ESRD patients were similar to the control population. Four patients developed tuberculosis after transplantation. Tuberculin test had a sensitivity of 50% and a specificity of 52% for post-transplant tuberculosis.
Conclusions. In comparison to healthy controls, tuberculin reactivity rates are lower and anergy rates higher in Indian ESRD patients. There is a significant relationship between markers of nutritional status (haemoglobin, albumin and creatinine) and cutaneous reactivity. Pre-transplant Mantoux positivity has low sensitivity and specificity for predicting post-transplant tuberculosis.
Introduction
The prevalence of tuberculosis in India is estimated to be over 5000 per million population. The average annual infection risk is 1.7%, and one patient dies of tuberculosis every minute [1]. Poor living conditions, overcrowding and malnutrition (related to low socio-economic status) and a rising HIV-positive population contribute to the high prevalence. The risk is increased amongst chronic renal failure (CRF) patients [2,3], and goes up further when these patients undergo transplantation and are put on immunosuppressive therapy [4,5]. Most cases are encountered in the first year after transplant, hence are likely to represent progression from latent infection to active disease. Identifying these cases is likely to positively impact the morbidity and mortality associated with this infection.
Traditionally, cutaneous sensitivity to the tuberculin antigen (Mantoux test) is used to detect latent tuberculosis infection [6–8]. Centers for Disease Control and Prevention, USA (CDC) recommends annual tuberculin skin testing for CRF patients [9], but whether positive patients should receive treatment is not settled. Reactivity to this antigen depends upon several factors, including the load of infection in the community and the status of cell-mediated immunity in the individual.
Despite the high incidence of this infection amongst dialysis and transplant patients, there are no data on the prevalence of tuberculin sensitivity or cutaneous anergy in Indian end-stage renal disease (ESRD) patients. Also, the predictive value of tuberculin positivity in development of tuberculosis in either dialysis patients or renal transplant recipients in endemic areas such as India remains unknown. This prospective study was designed to (a) determine the prevalence of cutaneous tuberculin sensitivity and anergy amongst CRF patients and healthy controls, (b) identify the factors associated with tuberculin sensitivity and (c) assess the value of the tuberculin test in predicting the development of post-transplant tuberculosis.
Patients and methods
The study protocol was cleared by the Institute Ethics Committee. Patients were recruited from the haemodialysis unit of the Postgraduate Institute of Medical Education and Research, a large 1200-bed tertiary care hospital in north India. All ESRD patients who came to the dialysis unit over an 8-month period were evaluated. A significant proportion of these patients had been referred to our unit for kidney transplantation. All patients underwent a thorough clinical evaluation to look for evidence of active tuberculosis. Besides demographic data, information was obtained regarding etiology and duration of renal failure, known immunodeficiency states including HIV infection, family history and history of recent exposure to cases of tuberculosis. Patients with active or recently treated tuberculosis and those with acute intercurrent infections were excluded. Laboratory values that were recorded included haemoglobin, blood urea, serum creatinine, albumin, protein, bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase. A chest X-ray was taken in all patients.
A control population consisting of first or second degree relatives of the patients was also studied. All control subjects were asked about any past history of tuberculosis or contact with a recent case. A thorough physical examination was done and a chest X-ray obtained in all subjects. Those with active tuberculosis or past treated disease were not included.
Skin tests were done after taking appropriate consent from all subjects. Cutaneous sensitivity to tuberculin was assessed using purified protien derivative (PPD) reagent (RT23 with tween 80) obtained from BCG Vaccine Laboratory, Guindy, Chennai. The National Tuberculosis Institute (NTI) Guidelines for Tuberculin Testing were followed [10]. One and two tuberculin units (TU) were injected intradermally in the forearm of controls and ESRD patients, respectively. Injections were made away from scars and at least 3 cm from any visible blood vessel. If a wheal was raised after the injection, the intradermal injection was considered appropriate. The test was read after 72 h, and the diameter of the area of induration was recorded. The test was recorded as positive if the indurated area was ≥10 mm.
Mumps antigen and tetanus toxoid were the other reagents used for assessment of anergy. Both were injected in the forearm at least 3 cm away from each other and read after 72 h. Tests were considered positive if the induration was ≥3 mm. Anergy was defined as lack of response to all antigens.
A total of 79 patients underwent living donor transplantation. Post-transplant immunosuppression consisted of cyclosporine 8 mg/kg/day in two divided doses and titrated to a trough level of 200–300 ng/ml for the first 3 months, 150–200 ng/ml until 6 months and 100–150 ng/ml thereafter. Azathioprine was administered at 1–1.5 mg/kg/day, and prednisolone at 0.5 mg/day tapered down to 0.15–0.2 mg/kg/day by 6 months. Five patients received induction therapy using the IL-2 receptor antagonist basiliximab, and 15 received mycophenolate mofetil at 1.5–2 g/day in place of azathioprine. Rejection was diagnosed and treated according to standard criteria. None of the patients received isoniazid prophylaxis. The diagnosis of tuberculosis was made by demonstration of acid-fast bacilli or caseating granulomas; and complete response to anti-tubercular therapy in patients with a clinical picture consistent with tuberculosis. Cyclosporine treated patients were treated with a rifampicin-free antitubercular protocol described earlier [4].
Groups were analyzed using Student's t-test for continuous variables and chi-square test for categorical data. A P-value of <0.05 was considered significant.
Results
A total of 137 ESRD patients were evaluated. Twenty-nine patients were excluded for the following reasons: past history of treated tuberculosis (9), inadequate work-up (8), failure to give consent for skin test (5), detection of active tuberculosis during evaluation (4) and death during evaluation period (3); 108 patients were recruited in the study.
The ages of these patients studied ranged from 18 to 60 years, with a mean of 37.75±11.8 years. Males constituted 72.2% of the study population. Chronic glomerulonephritis was the underlying disease in 28 patients, followed by chronic interstitial nephritis (20), diabetic nephropathy (14), hypertensive nephrosclerosis (8) and autosomal dominant polycystic kidney disease (3). The cause could not be ascertained in the remaining 35 cases. The majority of the patients were from a low to middle socio-economic backgrounds. Two patients were on continuous ambulatory peritoneal dialysis, while the rest were receiving regular haemodialysis. The interval between the initiation of dialysis and skin tests ranged from 2 months to 1.5 years (mean 4.3±2.6 months). Over 75% of patients had spent less than 6 months on dialysis.
The skin tests were also performed in 100 controls. None of the controls had either active or old history of tuberculosis. The average age of the controls was 36.63±9.9 (range: 18 to 58) years. Thirty-seven were renal donors, whereas the remaining 63 were first or second degree relatives of the ESRD patients. There was no difference in the mean age or sex ratio between the patient and control populations.
Prevalence of tuberculin positivity and anergy in CRF patients and controls
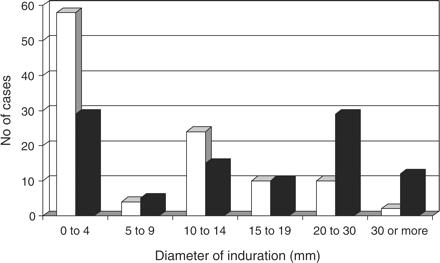
The Mantoux test was positive in a total of 46 (44%) CRF patients and 66 (66%) controls (P = 0.0018). The distribution of induration in response to the tuberculin antigen in patients and controls is shown in Figure 1. Cutaneous reactivity to at least one of the other two antigens was noted in 43 (40%) ESRD patients and 60 (60%) controls (P = 0.004). The proportion of Mantoux-positive subjects who tested negative to the control antigens was similar in the CRF and control groups (29 and 27%, respectively), suggesting that when cutaneous reactivity was absent, it was not specific to a particular antigen. A total of 46 ESRD patients (44%) and 16 controls (16%) were classified as anergic (P<0.001).
Distribution of ESRD patients (open bars) and controls (solid bars) according to the diameter of induration 72 h after intradermal PPD injection.
Factors associated with tuberculin reactivity and anergy in ESRD patients
Table 1 shows the results of risk factor analysis with a positive tuberculin test in CRF patients. A strong relationship was noted with haemoglobin, serum albumin and creatinine levels. Values of all three parameters were significantly higher in patients who exhibited cutaneous reactivity to the tuberculin antigen.
Factors associated with tuberculin sensitivity in ESRD patients
| Parameter . | Mantoux positive (n = 46) . | Mantoux negative (n = 62) . | P-value . |
|---|---|---|---|
| Age (years) | 37.8±13.4 | 37.5±9.3 | 0.44 |
| Sex ratio M:F | 36:10 | 42:20 | 0.23 |
| Basic renal disease | 0.57 | ||
| Diabetic nephropathy | 5 | 9 | |
| Non-diabetic kidney disease | 41 | 53 | |
| Duration of disease (months) | 4.1±2.6 | 4.4±1.9 | 0.51 |
| History of BCG vaccination | 30 (65%) | 46 (74%) | 0.31 |
| Prior steroid/immunosuppressive drug use | 2 (6.5%) | 2 (3.2%) | 0.76 |
| Background | 0.10 | ||
| Rural | 22 | 37 | |
| Urban | 24 | 25 | |
| Haemoglobin (g/dl) | 8.7±1.8 | 7.4±1.6 | <0.001 |
| Serum albumin (g/dl) | 3.6±0.6 | 3.0±0.5 | <0.001 |
| Serum creatinine (mg/dl) | 10.9±3.7 | 8.3±3.0 | <0.001 |
| HBsAg and/or anti-HCV positivity | 12 (26%) | 13 (21%) | 0.53 |
| Parameter . | Mantoux positive (n = 46) . | Mantoux negative (n = 62) . | P-value . |
|---|---|---|---|
| Age (years) | 37.8±13.4 | 37.5±9.3 | 0.44 |
| Sex ratio M:F | 36:10 | 42:20 | 0.23 |
| Basic renal disease | 0.57 | ||
| Diabetic nephropathy | 5 | 9 | |
| Non-diabetic kidney disease | 41 | 53 | |
| Duration of disease (months) | 4.1±2.6 | 4.4±1.9 | 0.51 |
| History of BCG vaccination | 30 (65%) | 46 (74%) | 0.31 |
| Prior steroid/immunosuppressive drug use | 2 (6.5%) | 2 (3.2%) | 0.76 |
| Background | 0.10 | ||
| Rural | 22 | 37 | |
| Urban | 24 | 25 | |
| Haemoglobin (g/dl) | 8.7±1.8 | 7.4±1.6 | <0.001 |
| Serum albumin (g/dl) | 3.6±0.6 | 3.0±0.5 | <0.001 |
| Serum creatinine (mg/dl) | 10.9±3.7 | 8.3±3.0 | <0.001 |
| HBsAg and/or anti-HCV positivity | 12 (26%) | 13 (21%) | 0.53 |
Factors associated with tuberculin sensitivity in ESRD patients
| Parameter . | Mantoux positive (n = 46) . | Mantoux negative (n = 62) . | P-value . |
|---|---|---|---|
| Age (years) | 37.8±13.4 | 37.5±9.3 | 0.44 |
| Sex ratio M:F | 36:10 | 42:20 | 0.23 |
| Basic renal disease | 0.57 | ||
| Diabetic nephropathy | 5 | 9 | |
| Non-diabetic kidney disease | 41 | 53 | |
| Duration of disease (months) | 4.1±2.6 | 4.4±1.9 | 0.51 |
| History of BCG vaccination | 30 (65%) | 46 (74%) | 0.31 |
| Prior steroid/immunosuppressive drug use | 2 (6.5%) | 2 (3.2%) | 0.76 |
| Background | 0.10 | ||
| Rural | 22 | 37 | |
| Urban | 24 | 25 | |
| Haemoglobin (g/dl) | 8.7±1.8 | 7.4±1.6 | <0.001 |
| Serum albumin (g/dl) | 3.6±0.6 | 3.0±0.5 | <0.001 |
| Serum creatinine (mg/dl) | 10.9±3.7 | 8.3±3.0 | <0.001 |
| HBsAg and/or anti-HCV positivity | 12 (26%) | 13 (21%) | 0.53 |
| Parameter . | Mantoux positive (n = 46) . | Mantoux negative (n = 62) . | P-value . |
|---|---|---|---|
| Age (years) | 37.8±13.4 | 37.5±9.3 | 0.44 |
| Sex ratio M:F | 36:10 | 42:20 | 0.23 |
| Basic renal disease | 0.57 | ||
| Diabetic nephropathy | 5 | 9 | |
| Non-diabetic kidney disease | 41 | 53 | |
| Duration of disease (months) | 4.1±2.6 | 4.4±1.9 | 0.51 |
| History of BCG vaccination | 30 (65%) | 46 (74%) | 0.31 |
| Prior steroid/immunosuppressive drug use | 2 (6.5%) | 2 (3.2%) | 0.76 |
| Background | 0.10 | ||
| Rural | 22 | 37 | |
| Urban | 24 | 25 | |
| Haemoglobin (g/dl) | 8.7±1.8 | 7.4±1.6 | <0.001 |
| Serum albumin (g/dl) | 3.6±0.6 | 3.0±0.5 | <0.001 |
| Serum creatinine (mg/dl) | 10.9±3.7 | 8.3±3.0 | <0.001 |
| HBsAg and/or anti-HCV positivity | 12 (26%) | 13 (21%) | 0.53 |
We found a similar association to reactivity with control antigens as well. Patients who reacted positively to controls had higher haemoglobin (9.3 vs 7 gm/dl, P<0.001), albumin (3.8 vs 2.8 gm/dl, P<0.001) and creatinine (11.9 vs 7.7 mg/dl, P<0.001) values.
Amongst a subgroup of well-nourished patients (haemoglobin >8 g/dl and serum albumin >3.5 g/dl; n = 30, the Mantoux test was positive in 19 (63%), a figure close to the control population (P>0.05), and only four (13%) exhibited cutaneous anergy, again similar to the healthy controls. On the other hand, the prevalence of Mantoux positivity was significantly lower (34%) in malnourished patients.
Predictive value of the Mantoux test for post-transplant tuberculosis
A total of 79 patients underwent transplantation, and have now been followed-up for a mean duration of 23.34 (range 20–30) months. Fifty-nine (75%) received kidneys from a first degree relative, 17 (21%) from spouses and three (4%) from second degree relatives. The pre-transplant Mantoux test was positive in 38 of these cases. A total of 18 patients, 10 of whom were Mantoux positive before transplant, developed acute rejection. All, however, responded to standard anti-rejection therapy.
A total of four patients developed tuberculosis during the follow-up period. All four presented with prolonged fever, two had pulmonary infiltrates and showed acid-fast bacilli on bronchoalveolar lavage, one presented with exudative pleural effusion and showed caseating granuloma on pleural biopsy, whereas the last patient showed generalized lymphadenopathy which yielded caseating granulomas and acid fast bacilli on needle aspiration. Two out of these four patients were Mantoux positive before transplant. One had exhibited cutaneous anergy. The Mantoux test had a sensitivity and specificity of 50% and 52%, respectively, in predicting post-transplant tuberculosis. The positive predictive value of a test was only 5%, whereas the negative predictive value was 95%. The sensitivity increased to 75% upon excluding the single anergic patient from the calculation.
All patients received a rifampicin-free antitubercular regimen, and responded well to treatment. Of the 29 who did not undergo transplantation, 25 were lost to follow-up, two continued on continuous ambulatory peritoneal dialysis and two remained on long-term haemodialysis. None of the four developed tuberculosis.
Discussion
We found an overall Mantoux positivity rate of 44% in our dialysis patients. This figure is higher than most published reports [6,7,11,12], reflecting the dependence of hypersensitivity on prior exposure to the organism. The infection is rampant in India and disease occurs fairly frequently, whereas in the West, the infection itself is fairly uncommon and disease even less frequent. However, this figure was substantially lower than the positivity observed in the simultaneously tested control population drawn from the same background as the ESRD patients. The Mantoux positivity amongst the healthy population in this study is higher than the only previous population survey from India [13]. According to this 30-year-old report, 30% of the general population (25% females and 35% males) were Mantoux positive. A recent Indian study found a 78% Mantoux positivity rate amongst adults [14]. Studies conducted in the West have also reported high Mantoux positivity rates among Asian immigrants. In a survey conducted at Minnesota, 98% of Tibetan immigrants from India and Nepal exhibited tuberculin reactivity [15]. Cauthen et al. [16] noted 35–55% tuberculin positivity amongst Asian immigrants. In an ongoing study of Mantoux testing in the general population at our institute, the positivity rate exceeds 50% (unpublished data). The possible reasons for this increase could be multiple, including emergence of organisms resistant to commonly used drugs such as isoniazid and rifampicin, a rapidly increasing HIV population, and overall improvement in nutritional status.
In order to investigate whether there was a generalized impairment of cell-mediated immunity (anergy), we looked for cutaneous reactivity to mumps and tetanus antigens. This is the first study from India to systematically evaluate the cutaneous hypersensitivity response to different antigens in the ESRD population. The antigens used for anergy testing in different studies have included tetanus toxoid, mumps and/or candida. Variability is reported in reactivity to different antigens; Woeltje et al. [7] used candida and tetanus, and noted higher reactivity with the former, whereas more patients showed induration to mumps antigen than to candida in another series [11]. Our choice of antigens was based on issues of availability and cost.
Compared to the control population, the anergy rate (44%) was higher in our CRF patients. This figure exceeds those in most published reports (19–40%) [6,7,17], and therefore cannot be simply explained by the immunosuppressive effect of chronic renal failure. Wauters et al. [6] failed to find a correlation between cutaneous tuberculin sensitivity and immunological pathways known to be abnormal in uraemia. Several factors have been thought to influence the hypersensitivity response to these antigens. These include increasing age, male gender, immunosuppressive drug use, liver disease, smoking and peptic ulcer disease [6,7].
An important finding of this study was that the lower Mantoux positivity and higher anergy rates were limited to the subgroup of patients with low haemoglobin and albumin values. Those with preserved values showed reaction rates similar to that observed in the healthy control population, indicating that factors other than uraemia impact on cutaneous reactivity. Serum albumin is an easily available marker of overall health and nutrition. An association between lower serum albumin and anergy in dialysis patients has been documented [7]. Compared to the non-reactors, tuberculin reactors also had higher serum creatinine values, indicating better preserved muscle mass and nutrition. Patients from lower socio-economic strata start dialysis late, use less erythropoietin and are generally malnourished. Akiyama et al. [18] found a direct correlation between dietary protein intake and cutaneous reactivity. We did not measure dietary protein intake. Serum albumin is also a negative phase reactant, and lower values indicate a state of inflammation. It is thought that the proinflammatory state of dialysis contributes to lower skin reactivity; this issue needs further study. In addition to the higher likelihood of exposure to tuberculosis in the community, another reason for preserved cutaneous reactivity in our well-nourished ESRD patients could be the relatively short duration of dialysis. Most patients in the Western reports have been on dialysis for several years.
In addition to latent M. tuberculosis infection, tuberculin sensitivity is also influenced by prior infection with other non-tuberculous environmental mycobateria (highly prevalent in India and other tropical countries) and BCG-induced sensitivity. The Mantoux test protocol is designed to minimize the false-positive results due to these factors. The dose of PPD antigen used for testing varies from place to place; 2 TU is used in Europe, 10 in UK and Australia, whereas 5 TU is the norm in the United States. The use of higher doses has been shown to reduce the test specificity in India, and hence the NTI recommends 1 TU for population testing [10]. A dose of 2 TU is used in immunosuppressed individuals and that is the reason we chose this dose for ESRD patients. Another strategy used to increase the positivity rates in countries with a low community prevalence of tuberculosis is repeating the test in those showing a negative result to the first injection [19]. There is no fixed protocol for such multi-step testing, and 1–4 repeat injections have been used in different studies. However, the NTI reckons that this ‘boosting’ results from recall of sensitivity induced by BCG vaccination or infection with environmental bacteria, and does not indicate infection with M. tuberculosis. Repeating the test in the Indian population is recommended only when the initial injection or reading is unsatisfactory [10]. In a study amongst approximately 2500 South-east Asians, Cauthen et al. [16] also confirmed that the boosting phenomenon was linked with reactivity to non-tuberculous mycobacterial antigens. Data on the effect of BCG on Mantoux reaction is inconsistent. We did not find any influence of BCG vaccination status on the test results. In a recent study of over 5000 European subjects, prior BCG vaccination influenced skin test results of ≤18 mm in diameter among persons <40 years old, but was not a significant factor in individuals >40 years old [19].
The American Thoracic Society and European Renal Association recommend isoniazid prophylaxis in PPD positive cases who undergo kidney transplantation, mostly on the basis of data generated in other patient groups. However, the value of this test in predicting post-transplant tuberculosis has not been prospectively studied. This is the first study to do so, and did not show a high incidence in Mantoux-positive cases over a 2 year follow-up period. In fact, 95% of Mantoux-positive cases did not develop tuberculosis during the period of observation, and the test had a low sensitivity (50%), specificity (52%) and positive predictive value (5%). As the risk of tuberculosis is highest during the first year after transplantation [4,5], it is unlikely that such an association will be documented with longer follow-up. The test sensitivity increased to 75% upon excluding the anergic patient from the calculation. Since anergic patients are expected to have a negative test even in the presence of latent tuberculosis infection, the possibility that this test would be useful for predicting post-transplant tuberculosis in non-anergic uraemic individuals cannot be ruled out. The number of patients who developed post-transplant tuberculosis was relatively small, however, and hence the conclusions need to be validated in a larger number of cases.
In conclusion, compared to an age and sex-matched control population drawn from the same background, Indian ESRD patients exhibit a significantly lower frequency of tuberculin reactivity and increased rates of cutaneous anergy. This difference is predominantly confined to malnourished individuals. The utility of the Mantoux test for prediction of post-transplant tuberculosis needs to be examined in larger studies.
Conflict of interest statement. None declared.
References
Chadha VK. Epidemiological situation of tuberculosis in India.
Aladren MJ, Vives PJ, Celorrio JM. Diagnosis and prevention of tuberculosis in hemodialysis patients. a new old problem?
Erkoc R, Dogan E, Sayarlioglu H et al. Tuberculosis in dialysis patients, single centre experience from an endemic area.
Sakhuja V, Jha V, Varma PP, Joshi K, Chugh KS. The high incidence of tuberculosis among renal transplant recipients in India.
John GT, Shankar V, Abraham AM, Mukundan U, Thomas PP, Jacob CK. Risk factors for post-transplant tuberculosis.
Wauters A, Peetermans WE, Van den Brande P et al. The value of tuberculin skin testing in haemodialysis patients.
Woeltje KF, Mathew A, Rothstein M, Seiler S, Fraser VJ. Tuberculosis infection and anergy in hemodialysis patients.
Sester M, Sester U, Clauer P et al. Tuberculin skin testing underestimates a high prevalence of latent tuberculosis infection in hemodialysis patients.
Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). Targeted tuberculin testing and treatment of latent tuberculosis infection.
Smirnoff M, Patt C, Seckler B, Adler JJ. Tuberculin and anergy skin testing of patients receiving long-term hemodialysis.
Fang HC, Chou KJ, Chen CL et al. Tuberculin skin test and anergy in dialysis patients of a tuberculosis-endemic area.
Tuberculosis in a rural population of South India: a five-year epidemiological study.
Chakraborty AK, Suryanarayana HV, Murthy VV, Murthy MS, Shashidhara AN. Prevalence of tuberculosis in a rural area by an alternative survey method without prior radiographic screening of the population.
Truong DH, Hedemark LL, Mickman JK, Mosher LB, Dietrich SE, Lowry PW. Tuberculosis among Tibetan immigrants from India and Nepal in Minnesota, 1992–1995.
Cauthen GM, Snider DE Jr, Onorato IM. Boosting of tuberculin sensitivity among Southeast Asian refugees.
Woeltje KF, Kilo CM, Johnson K, Primack J, Fraser VJ. Tuberculin skin testing of hospitalized patients.
Akiyama M, Numata A, Imagawa A. Influence of protein intake on phytohemagglutinin skin test in patients undergoing maintenance hemodialysis.





Comments