-
PDF
- Split View
-
Views
-
Cite
Cite
Mamoru Okamoto, J. John Vidmar, Anthony D. M. Glass, Regulation of NRT1 and NRT2 Gene Families of Arabidopsis thaliana: Responses to Nitrate Provision, Plant and Cell Physiology, Volume 44, Issue 3, 15 March 2003, Pages 304–317, https://doi.org/10.1093/pcp/pcg036
Close - Share Icon Share
Abstract
Four low-affinity (NRT1), and seven high-affinity (NRT2) nitrate transporter gene homologues have been identified in Arabidopsis thaliana. We investigated the transcript abundances of all eleven genes in shoot and root tissues in response to the provision of 1 mM NO3–, using relative quantitative RT-PCR. Based upon this criterion, genes were classified as nitrate-inducible, nitrate-repressible, or nitrate-constitutive. AtNRT1.1, 2.1, and 2.2 were strongly induced by NO3–, peaking at 3–12 h and subsequently declining. By contrast AtNRT2.4 showed only modest induction both in shoots and roots. Expression of AtNRT2.5, one of the nitrate-repressible genes, was strongly suppressed by nitrate provision in both roots and shoots. The last group, characterized by a constitutive expression pattern, included AtNRT1.2, 1.4, 2.3, 2.6, and 2.7. Correlation coefficients between 13NO3– influx from 100 µM and 5 mM [NO3–], suggest that high- and low-affinity transport systems are mediated primarily by AtNRT2.1 and AtNRT1.1, respectively. Functional roles for the other members of these families remain uncertain.
(Received September 23, 2002; Accepted December 27, 2002)
Introduction
Molecular evidence has documented the existence of two families of genes encoding NO3– transporters among eukaryotes, namely the NRT1 and NRT2 families. These families are thought to correspond to the earlier defined physiological categories of low- and high-affinity NO3– transporters (Crawford and Glass 1998, Forde 2000). In Arabidopsis thaliana, four members of the AtNRT1 family, including AtNRT1.1 (originally CHL1), AtNRT1.2 (originally NTL1), AtNRT1.3 (originally NTP3), and AtNRT1.4 (originally NTP2) have been described (Tsay et al. 1993, Hatzfeld and Saito 1999, Huang et al. 1999, Forde 2000), although only AtNRT1.1 and AtNRT1.2 have been the subject of detailed functional analysis. Although AtNRT1.1 mutants have been demonstrated to exhibit reduced NO3– uptake in the concentration range characteristic of low-affinity transport when grown on NH4NO3 (Doddema and Telkamp 1979, Touraine and Glass 1997), there still remained a substantial nitrate influx suggesting that other members of the NRT1 family might contribute to low-affinity transport in wild-type plants or, at the very least, that they might compensate for disruption of AtNRT1.1 in mutant plants. Furthermore when these same AtNRT1.1 mutants were grown on KNO3, there was no significant reduction of low-affinity transport (Touraine and Glass 1997). These observations raise doubts concerning the exclusive role of AtNRT1.1 in low-affinity NO3– uptake that have not been satisfactorily resolved. Representatives of the high-affinity nitrate transporter (AtNRT2) family, AtNRT2.1 and AtNRT2.2, were first isolated by use of degenerate primers based upon the Aspergillus and Chlamydomonas sequences (Zhuo et al. 1999), and by a differential display method (Filleur and Daniel-Vedele 1999). The Arabidopsis genome project unveiled five more members of the family (The Arabidopsis Genome Initiative 2000). Preliminary experiments showed that all seven members were expressed in roots of this plant (Glass et al. 2001), but no details of the time-course of their responses to nitrate provision were presented in this study. Recently, six members of this family were analyzed in various organs including leaves, flowers, and stem of Arabidopsis at different developmental stages when plants were grown on 0.5 mM and 10 mM nitrate (Orsel et al. 2002). The recent demonstration by Filleur et al. (2001), that a T-DNA disruption of AtNRT2.1 and AtNRT2.2 reduced high-affinity NO3– uptake by 63%, provides strong evidence that one or both of these genes are involved in high-affinity transport in this species. However, although AtNRT2.1 expression showed a strong correlation with 13NO3– influx (Zhuo et al. 1999), the role of AtNRT2.2 as a potential contributor to high-affinity influx remains unresolved. Likewise, despite claims that the Arabidopsis low-affinity nitrate transporter (NRT1.1) plays a major role in high-affinity nitrate uptake (Wang et al. 1998), the plants used for these experiments were grown on high levels of nitrogen, guaranteed to suppress expression of AtNRT2.1. The purpose of the present study was therefore to characterize all eleven members of the AtNRT1 and AtNRT2 families, particularly with respect to their responses to nitrate provision, by means of an integrated use of physiological and molecular analyses, with the ultimate goal of elucidating the functions of each of these nitrate transporters.
Nitrate uptake has been classified according to kinetic criteria as occurring as a result of three discrete transport systems: a constitutive high-affinity transport system (cHATS), an inducible high-affinity transport system (iHATS), and a low-affinity transport system (LATS) (Glass and Siddiqi 1995, Crawford and Glass 1998, Forde 2000). The cHATS and iHATS typically operate in the range of 10–250 µM NO3–, while the LATS only becomes evident above these concentrations. At such concentrations total uptake rates for NO3– are the sum of these three transporter activities (Siddiqi et al. 1990, Glass et al. 1992). Despite this physiological classification into discrete high- and low-affinity transporters, NO3– uptake by AtNRT1.1 mutants, exhibiting defective LATS, was also reduced in the HATS range (<250 µM), and it has been suggested that the so-called LATS transporters may function as dual-affinity transporters (Wang et al. 1998, Liu et al. 1999).
The first high-affinity nitrate transporter gene, NrtA (originally called crnA), was isolated from a filamentous fungus, Aspergillus nidulans (Unkles et al. 1991, Unkles et al. 2001). Mutants of A. nidulans mutant with defective NrtA and NrtB (a homologous gene) are incapable of absorbing NO3– even at 200 mM. This information, together with the determination of Km values of 11 and 108 µM, respectively, for NO3– influx by NrtA and NrtB transporters, establishes the critical role of these two genes in encoding high-affinity NO3– transporters (Unkles et al. 2001). In Chlamydomonas reinhardtii there are also two high-affinity NO3– transporters and these also exhibit differences in Km values of the same magnitude as observed in A. nidulans (Galvan et al. 1996).
It is well established that AtNRT2.1 is induced by NO3– (Filleur and Daniel-Vedele 1999, Lejay et al. 1999, Zhuo et al. 1999, Gansel et al. 2001), as are NRT2 genes in barley (Trueman et al. 1996, Vidmar et al. 2000), N. plumbaginifolia (Quesada et al. 1997, Krapp et al. 1998), soybean (Amarasinghe et al. 1998), and tomato (Ono et al. 2000). The transcript abundances of AtNRT2.1 and the patterns of high-affinity nitrate influx under different conditions of N provision showed high correlations, suggesting that AtNRT2.1 might be primarily responsible for iHATS activity (Lejay et al. 1999, Zhuo et al. 1999). This conclusion was substantiated by the recent finding that a T-DNA mutant, lacking AtNRT2.1 and a part of 2.2, lost 63% of high-affinity NO3– uptake capacity compared to the wild-type plants, while LATS transport was unaffected (Cerezo et al. 2001, Filleur et al. 2001). Characterizations of other members of the NRT2 family (i.e. AtNRT2.3–2.7) and their functional determination await investigation.
The first member of a family of putative low-affinity nitrate transporter genes, AtNRT1.1 (originally designated CHL1), was isolated from an Arabidopsis mutant which was resistant to chlorate, a toxic NO3– analogue (Tsay et al. 1993). Other members of this family have been identified by homology searches using the AtNRT1.1 sequence (Hatzfeld and Saito 1999, Huang et al. 1999, Forde 2000). Interestingly, the latest findings suggested that AtNRT1.1 might also be involved in organogenesis (Guo et al. 2001).
Significant portions of incoming NO3– proceed from roots to shoots to be reduced and metabolized and/or stored (Marschner 1995). Indeed, in some species virtually all nitrate reduction occurs in leaf tissue, and hence NO3– absorption by leaf cells is also of crucial importance. Yet, there is little information concerning NO3– transport systems in shoots. Although loading of NO3– into xylem vessels is considered to be passive, the absorption of NO3– from the leaf apoplast by leaf mesophyll cells is necessarily against the electrochemical potential gradient (Glass and Siddiqi 1995). This NO3– flux might be anticipated to involve LATS based upon typical values of xylem NO3– (Glass and Siddiqi 1995). Notwithstanding the importance of absorbing NO3– into leaf cells, expression levels of AtNRT1.1, 1.2, and 2.1 in shoots were shown to be significantly lower than those in roots (Tsay et al. 1993, Huang et al. 1999, Zhuo et al. 1999). However, Guo et al. (2001) showed strong expression of AtNRT1.1 in shoots using GFP/GUS fusion lines. The foregoing emphasizes the importance of NO3– transport systems in shoots.
Therefore, in the present study we have characterized the expression patterns of 11 AtNRT family members in both roots and shoots, in response to the provision of NO3– following a 7-day period of NO3– deprivation. Semi-quantitative analyses of transcript abundances were obtained by use of RT-PCR. 13NO3– influx studies at high and low external NO3– concentrations were performed in parallel in order to attempt to correlate patterns of individual gene expression with patterns of high- and low-affinity NO3– influx.
Results
Gene structure of the AtNRT2 family
Besides the first two AtNRT2 genes (AtNRT2.1, and 2.2) cloned experimentally (Filleur and Daniel-Vedele 1999, Zhuo et al. 1999), five more homologues were retrieved by a homology search against the database generated by the genome project (Table 1). Three genes (i.e. AtNRT2.1, 2.2, and 2.5) are located on chromosome 1, where AtNRT2.1 and 2.2 are closely located in a tail-to-tail configuration (Zhuo et al. 1999). AtNRT2.3, 2.4, and 2.7 were found on chromosome 5, with AtNRT2.3 and 2.4 in tandem 3.8 kb apart (Forde 2000). Seven AtNRT2 members encoding predicted proteins consisting of 493–539 amino acids, typical membrane proteins, belong to the nitrate/nitrite porter (NNP) family, which is a subfamily of the major facilitator superfamily (MFS) (Pao et al. 1998). AtNRT2.1, 2.2 and 2.4 are highly homologous at the protein level, sharing 87% and 82% identity between AtNRT2.1 and 2.2, or 2.1 and 2.4, respectively (Table 1). AtNRT2.3 and 2.6 proteins also show high homology, with values of 89% identity and 93% similarity between the two. Membrane-spanning regions and topologies were analyzed with prediction programs including HMMTOP (Tusnady and Simon 1998), MEMSTAT (Jones et al. 1994), TMHMM (Sonnhammer et al. 1998), TMpred (Hofmann and Stoffel 1992), and Toppred2 (Claros and von Heijne 1994). Although it is generally accepted that the MFS has 12 trans-membrane-spanners (TMS) and a cytoplasmic N-terminal, predicted TMS numbers varied from 9 to 12 (data not shown). However, the average numbers settled between 11 and 12 (Fig. 1A).
The middle regions of the proteins have even higher degrees of similarities, whereas N- and C-termini, which mostly consist of hydrophilic residues, share less homology within the family (Fig. 1A). In TMS5 all AtNRT2 family members have a sequence, [AG]-G-W-[GA]-[ND]-x-G, which is highly related to a signature motif found in the NNP family (Trueman et al. 1996, Pao et al. 1998). Another conserved sequence, R-[PA]-x-G-G-x-x-[SA]-D, was identified in the region between TMS8 and TMS9 (Fig. 1A-C1). This is not only found within plant members of the NRT2 family but it is also well conserved in all known NNP family members including NrtA in A. nidulans (Unkles et al. 1991), YNT1 in Pichia angusta (Hansenula polymorpha) (Perez et al. 1997), and narK from E. coli (Rowe et al. 1994). Half of this sequence (G-x-x-[SA]-D) is closely related to a part of the MFS specific sequence motif, G-[RKPATY]-L-[GAS]-[DN]-[RK]-[FY]-G-R-[RK]-[RKP]-[LIVGST]-[LIM], which is located between TMS2 and TMS3 (Pao et al. 1998). It is notable that the longest conserved sequence, F-G-M-R-G-R-L-W, was found at the beginning of TMS9 in all AtNRT2 members (Fig. 1A-C2). This sequence is also well conserved in other photosynthetic species including C. reinhardtii, and Chlorella sorokiniana (data not shown). AtNRT2.7 protein, the least homologous among the seven AtNRT2 members, has the longest hydrophilic loop predicted between TMS6 and TMS7 (Fig. 1A). This long loop is more evident in NrtA (Unkles et al. 1991) and YNT1 (Perez et al. 1997).
Some protein kinase C (PKC) phosphorylation sites (motif: [ST]-x-[RK]), and casein kinase 2 (CK2) phosphorylation sites (motif: [ST]-x-x-[DE]), were found in the cytosolic domains (Fig. 1A). The PKC sites before TMS11 and at the N- and C-termini were well conserved, whereas most of the CK2 sites were restricted to both termini with less conservation. It is intriguing that the conserved threonine residue in the N-terminus (i.e. Thr-16 in AtNRT2.1) is a phosphorylation site of both PKC and CK2 (Fig. 1A). N-glycosylation sites, where the consensus pattern is (N-{P}-[ST]-{P}), were found among TMS1 and TMS2 in five of the proteins (Fig. 1A). Neither PSORT (Nakai and Kanehisa 1992) nor SignalP (Nielsen et al. 1999) were able to find N-terminal signal sequences within the AtNRT2 members.
Gene structure of the AtNRT1 family
The gene structures of AtNRT1.1 (CHL1) and 1.2 (NTL1) had been characterized previously (Tsay et al. 1993, Huang et al. 1999), and a brief characterization of AtNRT1.3 (NTP3) and 1.4 (NTP2) was also reported earlier (Hatzfeld and Saito 1999). Briefly, among the four AtNRT1 members, sequence identities (between 34 and 51% at the protein level), were not particularly high (Table 1). Although they are considered to be nitrate transporters, the AtNRT1 family also belongs to a larger peptide transporter family (PTR, also called POT), which contains 51 members (Steiner et al. 1995, Pao et al. 1998, http://www.cbs.umn.edu/arabidopsis). Two PTR signature sequences: PTR1: [GA]-[GAS]-[LIVMFYWA]-[LIVM]-[GAS]-D-x-[LIVMFYWT]-[LIVMFYW]-G-x3-[TAV]-[IV]-x3-[GSTAV]-x-[LIVMF]-x3-[GA] between TMS2 and TMS3, and PTR2: [FYT]-x2-[LMFY]-[FYV]-[LIVMFYWA]-x-[IVG]-N-[LIVMAG]-G-[GSA]- [LIMF]-[FYT]-x2-[LMFY]-[FYV]-[LIVMFYWA]-x-[IVG]-N-[LIVMAG]-G-[GSA]-[LIMF] within TMS5, are well conserved among the four AtNRT1 members (Fig. 1B). AtNRT1 members share only 3–10% identities with members of the AtNRT2 family at the protein level. The PTR family was not included within the MFS because of insufficient homology between the two families (Pao et al. 1998). However, PTR members exhibit some features in common with those of MFS. The four AtNRT1 members possess sequences of 592 amino acids on average, which are 66 residues longer than those of AtNRT2 proteins, and they are also predicted to have 12 TMS (Fig. 1B). The AtNRT1 members commonly have cytosolic N- and C-termini and relatively long cytosolic loops, consisting of approximately 100 amino acids, between TMS6 and TMS7 (Fig. 1B). In the loops at least one phosphorylation site was found in each protein. A conserved PKC site was also found at the end of TMS6 (Fig. 1B). Putative N-glycosylation sites were found between TMS1 and TMS2 of AtNRT1.1 and 1.2. Because predicted TMS1 of AtNRT1.2, M-34 to A-53, is shifted upstream compared to AtNRT1.1, the N-54 becomes a possible glycosylation site (Fig. 1B).
Expression of two NRT families by relative quantitative RT-PCR
A unique feature of nitrate transport is that NO3– is both a substrate for transport and also for the induction of NO3– transport systems at the gene and at the physiological levels (Glass and Siddiqi 1995, Forde 2000). Therefore, as the first step in characterizing members of the two NO3– transporter families, their responses to induction by NO3– were examined by means of time-course experiments. Five-week-old Arabidopsis plants that had been grown on 0.5 mM NH4NO3 were N deprived for 7 d in order to de-induce NO3– transport (Siddiqi et al. 1989). The starved plants were then transferred to nutrient solution supplemented with 1 mM NO3– for up to 3 d. Gene expression in response to this renewed NO3– provision was analyzed using a relative quantitative RT-PCR method.
Designing the gene specific primer is the first and perhaps the most essential step of the entire RT-PCR method. Table 2 shows the gene specific primer sets used in this study. The primers were designed either to flank half of one exon and the other half on the next exon to eliminate genomic DNA amplification, or to flank a region that contains intron(s) to indicate genomic DNA contamination (i.e. genomic DNA would be amplified in larger product size than that of cDNA due to intron(s)). The product sizes were more than 300 bp since the internal standard (18S ribosomal RNA) primer sets produced 315 bp fragments. The specificities of each set of primers were stringently confirmed by the sizes and sequences of the products. To analyze gene products quantitatively, optimum amount of template RNA and cycle number, which provided linear range of gene amplification, were carefully determined for all 11 genes. The optimization was also carried out in shoots and roots differently because expression levels of most NRT genes were exceedingly different among the tissues (Table 2, see Materials and Methods). For example, in shoots AtNRT2.1 was amplified from 250 ng of total RNA and 28 cycles of PCR, whereas root tissue required only 25 ng of RNA and 21 cycles to achieve a similar level of PCR product (Table 2). These numbers were used to estimate relative expression levels between shoots and roots (Fig. 2). Expression levels of all NRT2 genes, with the exceptions of AtNRT2.7, were higher in roots than shoots, whereas NRT1 genes were either more highly expressed in shoots, or at similar levels in roots and shoots (AtNRT1.2) (Fig. 2). Based upon the smaller amounts of RNA and/or fewer numbers of PCR cycles used to obtain a similar signal, AtNRT2.1, 2.2, 1.2, and 1.3 (Table 2, Fig. 3, 4) were considered to be more highly expressed than all other genes whether in roots or shoots. Despite diverse levels of transcript abundances it was possible to detect expression patterns of all eleven NO3– transporter genes. From the responses to NO3– exposure, genes were categorized into three groups, namely nitrate-inducible, nitrate-repressible, and nitrate-constitutive.
Nitrate-inducible genes
Three genes in this category, AtNRT2.1, AtNRT2.2 and AtNRT1.1 showed very strong inductions (>3- to 5-fold increases) in root tissues following exposure to 1 mM NO3–, while other genes showed only modest increases in roots or shoots under the same conditions. In the AtNRT2 family AtNRT2.1 showed the strongest induction by NO3– in roots (Fig. 3A, C). By 3 h of NO3– provision, the expression level had reached >5-fold that of 0 h plants. This peak was sustained for up to 24 h, followed by a gradual reduction to about half of the peak level by 72 h. In shoots, on the other hand, there was only a small enhancement in the expression of AtNRT2.1 after 3 h of exposure to NO3–, followed by rapid down-regulation after 6 h, until by 24 h the level was lower than it had been at 0 h. Transcript level in shoots was less than 1% of that observed in roots based on the fact that shoot PCR required seven times the number of PCR cycles and 10 times more RNA template than root PCR (Fig. 3B, Table 2). AtNRT2.2 was also induced by NO3– both in roots and shoots. This was more pronounced in roots where 4-fold increases in transcript abundance were evident by 3 h. However, after this peak, expression levels were quickly down-regulated and returned to a value that was close to the original level by 24 h (Fig. 3A, B). This rapid down-regulation was different from that observed for AtNRT2.1, although the expression level of AtNRT2.2 was initially similar to AtNRT2.1 as we described earlier. AtNRT2.2 in shoots as well as AtNRT2.4 in both in shoots and roots showed similar expression patterns which were characterized by modest increases culminating in peaks at 3 h followed by declines that stabilized by 24 h (Fig. 3).
AtNRT1.1 (CHL1) also showed a strong induction by NO3– in roots. The induction peaked rapidly, as early as the third hour of NO3– provision, and reached a maximum level that was 2.5 times that of the 0 h. This high level of transcript was sustained for from 12 h to 48 h (Fig. 4A, C). The expression of AtNRT1.1 in shoots showed a similar pattern to that of AtNRT2.4, namely a small spike at 3 h, followed by a return to a level that was as low as the original level (Fig. 4B). Interestingly, unlike AtNRT2.1, the expression level of AtNRT1.1 was equal to, or even higher in shoots than roots considering both the PCR cycle numbers and RNA template provided (Fig. 2, 4).
There were three genes, AtNRT1.3, 1.4, and 2.3, which were induced by nitrate only in shoots (Fig. 3B, 4B). The expression patterns of those genes in the roots will be described later. AtNRT2.3 was induced slowly, reaching peak expression that was 90% higher than at time 0 h, only after 48 h of NO3– provision (Fig. 3B). AtNRT1.3 expression level was also gradually induced, increasing to more than 2-fold at 48 h, although there was a slight decline during the first 6 h. However, there was subsequently a significant down-regulation, which began at 48 h, reducing transcript abundance to a level that was similar to that at 24 h (Fig. 4B). Another NRT1 family member, AtNRT1.4 also had a slow induction pattern, reaching the plateau after 48 h, a level that was maintained for the next 48 h (Fig. 4B).
Nitrate-repressible genes
In contrast to NO3–-inducible genes, there were some genes whose transcript abundances were actually reduced by nitrate provision. AtNRT2.5 conformed to this pattern in both roots and shoot, while this pattern was only observed in roots for AtNRT1.3. Highest levels of AtNRT2.5 were evident prior to the provision of nitrate (0 h). By 3–6 h after exposure to 1 mM NO3–, transcript abundances had declined to 50% (shoots) and 25% (roots) of the 0 h values (Fig. 3). Although both shoots and roots showed a similar response pattern, roots responded more rapidly than shoots. It is also notable that the transcript abundance in roots was more than 100 times higher than that of the shoots at 0 h (Table 2, Fig. 3). AtNRT1.3 also revealed a repressible pattern in the roots, although gene expression was induced by NO3– in the shoots (Fig. 4). The initial response to nitrate provision was as rapid as that of AtNRT2.5 in the roots. However, after 6 h, expression level decreased, reaching a value that was about 30% of the initial (0 h) value by 48 h (Fig. 4A).
Nitrate-constitutive genes
The third group of nitrate transporter genes is described as constitutively expressed. A characteristic pattern of this group is that substantial transcript abundance was already present even under NO3–-starved conditions (0 h), and the expression levels did not change substantially during 72 h of exposure to NO3–. AtNRT2.6 and 2.7 in the NRT2 family, and AtNRT1.2 in the NRT1 family showed such a constitutive expression pattern both in shoots and roots, wherein the fluctuations of expression levels were less than ±50% (Fig. 3, 4). Interestingly, the expression level of AtNRT2.7 in the shoot was higher than that in the roots (Table 2, Fig. 3). This is unique in the AtNRT2 family because all other members had greater transcript abundances in roots (Fig. 2, Orsel et al. 2002). Although AtNRT1.2 expression levels in shoots was ~10% of those in roots, this gene was among the most highly expressed of shoot-expressed genes. Therefore, overall, this is one of the most highly expressed nitrate transporter genes throughout the plant during all stages of our investigation (Table 2, Fig. 2).
As mentioned earlier, AtNRT2.3 showed a nitrate-inducible pattern in the shoots, but its expression in roots was essentially constitutive, although a slight down-regulation was observed during the first 6 h (Fig. 3A). The expression of AtNRT1.4 in the roots also showed a constitutive pattern (Fig. 4A). This insensitivity to NO3– did not match its shoot expression where the gene was induced by NO3– provision (Fig. 4B). Similar to AtNRT2.7, 1.1, and 1.3AtNRT1.4 was the fourth gene whose expression levels were higher in shoots than in roots at some time points (Fig. 2).
NO3– influx by HATS and LATS
Nitrate influx by Arabidopsis roots was measured at low (100 µM) and high (5 mM) external NO3–concentrations, representative of the high- and low-affinity NO3– transporter systems (HATS, and LATS, respectively), using 13NO3– at intervals of time after the initial exposure to 1 mM NO3–. Prior to exposure to 1 mM NO3– there was already a substantial HATS influx of 2.5 µmol (g FW)–1 h–1 at 100 µM NO3–. This is considered to be due to the cHATS (Fig. 5A). After provision of 1 mM NO3– in the external media, influx via the HATS increased continuously for 6 h, peaking at a value that was 2.5 times the constitutive value. After 12 h, the NO3– flux steadily decreased down to 2 µmol (g FW)–1 h–1 by 72 h, a value that was similar to the original (0 h) value (Fig. 5A). Fig. 5B shows that nitrate influx from media containing 5 mM external NO3– also increased initially and subsequently declined. Both HATS and LATS are considered to contribute to NO3– influx at 5 mM (Glass et al. 1992, Lejay et al. 1999). To estimate LATS-mediated influx as distinct from the HATS influx, flux values due to the HATS activity were subtracted from those measured at 5 mM, corresponding to both HATS and LATS influx, presuming that the root influx from 100 µM was close to the Vmax for HATS influx, and that the HATS contribution remained unchanged at the two external concentrations (Fig. 5C). This “corrected” value for LATS influx was initially <1 µmol (g FW)–1 h–1 (at 0 h) but showed a rapid induction after NO3– provision, reaching a peak of 8 µmol (g FW)–1 h–1 at 24 h. This was followed by a slow down-regulation (Fig. 5C). In summary, the cHATS influx (0 h plants) was higher than the constitutive LATS (cLATS). Induction of both HATS and LATS by NO3– was rapid, but times of peak activities and flux values were different. Furthermore, down-regulation in HATS appeared earlier and faster than that in LATS.
Correlation coefficients for HATS and LATS 13NO3– influx values and expression levels of the NRT2 and NRT1 families of genes are shown in Table 3. Only NRT2.1 and NRT1.1 gave significant positive values (0.74 and 0.88, respectively) that were significant at the P = 0.05 level of probability. While other genes gave significant correlations (e.g. AtNRT2.3 and AtNRT2.5 with HATS and LATS influx, respectively), the correlation coefficient was negative.
Discussion
AtNRT families and high- and low-affinity nitrate transport system
iHATS transport
It is evident from the foregoing results, that not all members of the NRT2 family of genes can be characterized as nitrate-inducible. On the basis of correlations between physiological patterns of NO3– influx and corresponding patterns of gene expression, it has been suggested that the NRT2.1 protein probably plays a major role in inducible HATS in Arabidopsis (Lejay et al. 1999, Zhuo et al. 1999), N. plumbaginifolia (Krapp et al. 1998), soybean (Amarasinghe et al. 1998), and barley (Trueman et al. 1996, Vidmar et al. 2000). Our present data also showed that AtNRT2.1 transcript abundance in roots was significantly correlated (r = 0.74, P = 0.05) with the temporal pattern of 13NO3– influx in the HATS range following provision of NO3– to NO3–-deprived plants (Fig. 3A, 5A). No other gene gave a significant correlation with the HATS flux (Table 3). Recently, atnrt2, a T-DNA knockout mutant in which AtNRT2.1 and the 3′ end of AtNRT2.2 were deleted, has been characterized (Cerezo et al. 2001, Filleur et al. 2001). 15NO3– influx associated with iHATS activity of this Arabidopsis mutant was reduced to 27% of that of wild-type plants, while the LATS activity was relatively intact (Filleur et al. 2001). Although the data presented did not allow the authors to categorically distinguish which of the two genes, AtNRT2.1 or AtNRT2.2, was the predominant contributor for iHATS at this stage, they were, nevertheless, able to conclude that these genes represent the major players (Corezo et al., 2001). Our findings support this conclusion and further, as shown in Fig. 3, suggest that the pattern of AtNRT2.1 expression more closely corresponds with the pattern of iHATS influx. AtNRT2.2 expression pattern matched the iHATS profile only during the first 3 h (Fig. 3A, 5A). Subsequently AtNRT2.2 transcript abundance was rapidly reduced compared to AtNRT2.1. Thus, AtNRT2.1 appears to be a more likely candidate for iHATS influx.
Despite the identification of two distinct families of genes, NRT1 and NRT2, encoding low- and high-affinity NO3– transporters, respectively, it has been suggested that AtNRT1.1 (CHL1) should receive the status of a dual-affinity nitrate transporter (Crawford and Glass 1998, Wang et al. 1998, Liu et al. 1999). This proposal is based on two observations: (1) defective NO3– transport by both the HATS and LATS in AtNRT1.1 mutants grown on NH4NO3 (but not on KNO3), and (2) heterologous expression of AtNRT1.1 in Xenopus oocytes resulted in both HATS and LATS activities (Touraine and Glass 1997, Wang et al. 1998, Liu et al. 1999). Since our observations were also based on NH4NO3-grown plants, AtNRT1.1 protein might also be involved in the measured iHATS activity (Fig. 4A, 5A).
cHATS transport
Fig. 5A shows that Arabidopsis plants have substantial cHATS activity even in uninduced plants (i.e. 2.5 µmol (g FW)–1 h–1), as was the case in soybean plants (Amarasinghe et al. 1998) and in Steptoe barley (King et al. 1993). On the other hand, other barley varieties and spruce tend to have relatively small cHATS capacities (Siddiqi et al. 1990, Kronzucker et al. 1995, Glass and Siddiqi 1995). Despite the disruption of AtNRT2.1 and AtNRT2.2 in the atnrt2 mutant (Filleur et al. 2001), cHATS activity was almost the same as that of the wild type in uninduced plants, suggesting that AtNRT2.1 and AtNRT2.2 make no contribution to cHATS activity. In NO3–-treated plants, this cHATS was even slightly increased by NO3– treatment (Cerezo et al. 2001, Filleur et al. 2001), consistent with earlier reports in barley and white spruce (Aslam et al. 1992, Kronzucker et al. 1995).
cHATS candidates might be expected to satisfy two criteria: (1) relatively high transcript abundance prior to exposure to NO3–, and (2) modest up-regulation of this transcript following induction by NO3–, the latter based on the documented increase of cHATS influx following this treatment (Aslam et al. 1992, Kronzucker et al. 1995, Cerezo et al. 2001). Although several members of the NRT2 family, e.g. AtNRT2.3, 2.4, 2.5, 2.6 and 2.7 satisfy the first criterion, only AtNRT2.4 and 2.7 meet the second criterion. Given that AtNRT2.4 expression level decreased to 50% by 12 h after NO3– provision, AtNRT2.7 might be a viable candidate for the cHATS activity (Fig. 3A). Wang and Crawford (1996) isolated a mutant, which was impaired only in the cHATS transport in roots of Arabidopsis. It will be interesting to see if this mutation is located in one of the above-proposed candidates.
LATS transport
Low-affinity transporter systems were originally thought to be either constitutive or repressible on the basis of flux analysis in barley (Glass and Siddiqi 1995). However, the AtNRT1.1 (CHL1) gene, considered to encode a low-affinity NO3– transporter in Arabidopsis, was induced by nitrate (Tsay et al. 1993). These contradictory findings may have been resolved by the demonstration that Arabidopsis possessed both an inducible- and a constitutively expressed member of the NRT1 family (Huang et al. 1996, Lejay et al. 1999, Liu et al. 1999). Our present findings confirm this, and also suggest that one member of the NRT1 family is nitrate-repressible. However, the function of a nitrate-repressible NRT1 gene is unclear at present given that our physiological evidence demonstrates only cLATS and iLATS (Fig. 5B, C). As expected, AtNRT1.1 showed a NO3–-inducible expression pattern in roots, and the pattern corresponded well with the observed LATS activity (Fig. 4A, 5C, Table 3). Other members of the NRT1 family were either constitutively (AtNRT1.2 and 1.4), or repressively expressed (AtNRT1.3), indicating that these three transporters are unlikely to be major contributors to the iLATS. Rather AtNRT1.2 and/or AtNRT 1.4 may encode the cLATS.
According to Liu and Tsay (personal communications) the AtNRT1.1 (CHL1), a putative dual-affinity transporter, switches between high and low affinity through protein phosphorylation. This surprising finding adds even greater complexity to the existing situation vis á vis the 11 (seven NRT2 and four NRT1) putative nitrate transporters. In the present study, although at the transcriptional level AtNRT2.1 and AtNRT1.1 behaved similarly, there were distinct differences between patterns of HATS and LATS influx: (1) the iHATS peaked at 18 h earlier than the LATS, (2) the iHATS influx was reduced to the original influx value after 72 h of NO3– exposure, whereas LATS remained considerably higher (5.1 µmol (g FW)–1 h–1) than its original value (<1 µmol (g FW)–1 h–1) even after 72 h (Fig. 5A, C). Thus at the physiological and molecular levels, the two nitrate transport systems (low- and high-affinity) not only co-exist but they appear to be differently regulated. Nevertheless, both HATS and LATS have constitutive and inducible systems.
A role for NO3–-repressible genes
A surprising finding was that some members of the NRT1 and NRT2 families showed no increase of expression following exposure to NO3–, but rather were subject to rapid down-regulation (Fig. 3, 4). Thus transcript abundances of AtNRT2.5 in both roots and shoots, and of AtNRT1.3 in roots, showed their highest levels when plants had been deprived of NO3– for 7 d, and tissue NO3– was at its lowest concentration. Of the many NO3– fluxes that might be down regulated on re-supplying NO3–, one possibility is the transfer of NO3– from storage pools (such as the vacuole) to the cytoplasm. However, we find no evidence of the appropriate signal peptides that would indicate such a vacuolar transport function. Thus the role of these repressible genes remains unclear.
Shoot and root differences and similarities
Our study using RT-PCR clearly showed that all 11 AtNRT genes were expressed in roots and shoots, whereas previous reports have claimed that expression levels of nitrate transporters in the shoots were either extremely low or undetectable. This applies specifically to AtNRT2.1 (Filleur and Daniel-Vedele 1999, Zhuo et al. 1999), AtNRT2.2 (Zhuo et al. 1999), AtNRT1.1 (Tsay et al. 1993), and AtNRT1.2 (Huang et al. 1999). Our successful demonstration of shoot expression of these genes, though in low abundance, is probably due to the greater sensitivity of the RT-PCR method (Orsel et al. 2002), but might also result from differences in plant growth conditions among different laboratories. In previous reports plants were grown in sterilized systems where sucrose was added in the media (Tsay et al. 1993, Huang et al. 1999, and Zhuo et al. 1999). Sucrose additions to growth media have been documented to produce significant effects on N- and C-metabolisms at both the physiological and molecular levels (Hanisch et al. 1981, Sheen 1990, Krapp and Stitt 1995, Lejay et al. 1999).
Although the patterns of, and extents of, expression levels of the genes were variable, we grouped all members into three categories in order to organize the information generated. Most of those genes investigated had similar expression patterns in roots and shoots. However, in specific cases, such as AtNRT2.3, and AtNRT1.3, expression patterns were quite different between roots and shoots (Fig. 3, 4). Orsel et al. (2002) reported that AtNRT2.3 expression level in shoots was higher than that of in roots in vegetative (29-day-old) plants. However, leaves turned into prime targets in flowering (45-day-old) plants (Orsel et al. 2002). The present study, using vegetative stage plants (6-week-old) revealed that AtNRT2.3 was predominantly expressed in roots, suggesting that an apparent shift in preferential expression might occur at a late vegetative stage.
Roots are considered to be the main organ for nitrogen uptake by terrestrial plants, and have consequently been the focus of attention in studies of NO3– transport. By contrast, only a few studies have reported on ion transport by shoot tissue. Nitrate translocated to shoots is released from vascular tissue to the leaf apoplasm before being re-absorbed by leaf cells. The cytosolic nitrate concentration in leaves varies from zero to 30 mM depending on growth conditions and techniques of nitrate measurement (Siddiqi and Glass 2002). In consideration of the thermodynamics of NO3– transport, even when apoplasmic [NO3–] is in the high mM range (>20 mM), the extent of the membrane electrical potential across the cell membrane (~150–200 mV) dictates that the NO3– re-absorption step in leaves would be active, requiring a source of free energy (presumably the proton motive force) and appropriate nitrate transporters (Glass 1988, Glass et al. 1992, Glass and Siddiqi 1995). Based upon typical analyses of apoplasmic [NO3–] as stated above, leaf re-absorption would most likely be mediated by low-affinity nitrate transporters. However, in Limium and Bromus, the concentrations of leaf apoplastic nitrate varied from 0.11 mM to 2.38 mM in N-deficient and N-replete plants, respectively (J. Schjoerring, personal communication). This suggests the possibility that as apoplasmic NO3– declines from mM to µM concentrations, as for example under field conditions as external supplies of NO3– are depleted, high-affinity transport systems may be necessary to scavenge apoplasmic NO3– from the latter range of concentration. Both the NRT1 and NRT2 transporters may participate in the re-absorption of NO3– by leaf cells.
At present, the functions of shoot NRT1 and NRT2 transporters are largely unknown, and further physiological analysis, such as NO3– uptake by leaf tissues, and additional genetic analysis, including spatial NRT gene expression patterns, and characterization of deficient and/or over expressed lines of NRT genes, will be helpful to understand the precise role of individual NRT gene in both root and shoot.
Materials and Methods
Plant growth condition
Arabidopsis plants (ecotype Columbia) were grown hydroponically under non-sterile conditions as described in earlier papers (Gibeaut et al. 1997, Lejay et al. 1999). Briefly, 1/4- inch thick Styrofoam was fitted to an 8-liter container as a floating platform to support plant growth. Each platform contained 30 holes (diameter 1.5 cm) covered with nylon mesh at the bottom surface and these holes were filled with clean sand. Seeds were imbibed in a cold room at 4°C for 3–5 d, and sown directly on the moistened sand in the platform. The nutrient solution which was used to support plant growth contained: 1 mM KH2PO4, 0.5 mM MgSO4, 0.25 mM CaSO4, 20 µM Fe-EDTA, 25 µM H3BO3, 2 µM ZnSO4, 2 µM MnSO4, 0.5 µM CuSO4, 0.5 µM (NH4)6Mo7O24, and 0.5 mM NH4NO3. For the induction study, 5-week-old plants were transferred to –N solution for 1 week (other nutrients remained as before), then re-induced with 0.5 mM Ca(NO3)2 for from 0 to 72 h. The pH of the nutrient solution was maintained with CaCO3 at around 6.2. The walk-in environment chamber was maintained under the following conditions: light/dark, 8/16 h; 25/20°C; RH = 70%. Light was provided from fluorescent tubes (VITA-LITE, 150 E m–2 s–1). All flux determinations and plant harvesting for RNA extraction were undertaken at 4 h after the light period began, and all pretreatments were appropriately staggered to meet this requirement. All experiments were repeated at least twice. Physiological data were means of those experiments, and some data such as gel pictures were representatives of the experiments.
RNA isolation and relative quantitative RT-PCR
RNA was isolated using TRIzol Reagent (Life Technology, Grand Island, NY, U.S.A.) according to the manufacturer’s method followed by additional chloroform isolation and sodium acetate precipitation steps. Total RNA concentrations were determined by UV spectrophotometry. RT-PCR was carried out using OneStep RT-PCR Kit (Qiagen Inc., Mississauga, ON, U.S.A.) with QuantumRNA 18S Internal Standards (Ambion, Inc., Austin, TX, U.S.A.) under the following conditions: 50°C for 30 min; 95°C for 15 min; 19–37 cycles (Table 2) of 94°C for 30 s, 65°C for 30 s, 72°C for 1 min; 72°C for 10 min. Total RNA, 25–250 ng (Table 2), were added to the reaction mixture contained 400 µM dNTP, 0.6 µM of gene specific primers, 0.4 µM of the QuantumRNA 18S primers, 0.2 µg of hexamers, as well as buffer and enzymes according to the manufacturer’s protocol, and RNase-free water was added to make a final volume of 12.5 µl. The RT-PCR products were electrophoresed on 1.3% agarose gels and stained with SYBRGold (Molecular Probes, Inc., Eugene, OR, U.S.A.) for 30 min. The signals of the targeted gene products on the stained gels were captured and densitometry analyzed with an AlphaImager™ 1200 (Alpha Innotech Corp., San Leandro, CA, U.S.A.). For each gene, the optimization of RT-PCR was strictly carried out as followed. (1) Preliminary RT-PCR trials to determine the specificities of the gene specific primers and the presence of the gene expression (e.g. product size and its sequence, and approximate expression levels among the samples). (2) Determination of the PCR cycle number which gave linear range of gene amplification, choosing one sample in which the target gene was expected to be the most abundant. After the RT step, aliquots were taken every two cycles in the PCR step. Signal intensities were then plotted versus cycle numbers on a log scale. A cycle number in the linear range was chosen for the subsequent experiments (Table 2). (3) The amount of the 18S rRNA internal standard primers were determined according to the manufacturer’s protocol in order to amplify both the target gene and the 18S at a similar level.
13NO3– influx experiments
Nitrate influx using 13NO3– was measured as described before (Zhuo et al. 1999). The basic components of the solution for pretreatment, influx, and washing were the same as the growth media. Prior to measuring 13NO3– influx, plants were pretreated with the solution containing either 100 µM or 5 mM of 14NO3– for 5 min, then transferred to the appropriate influx solution which contained either 100 µM or 5 mM NO3– labeled with 13NO3– for 10 min. After the influx period, the plants were washed with “cold” solution (same as pretreatment solution) for 3 min to desorb 13NO3–, after which radioactivities of plant samples were determined using a γ-counter (MINAXI γ Auto-Gamma 5000 series, Packard Instruments, Meriden, CT, U.S.A.).
Bioinformatics
DNA and predicted amino acid sequences were obtained from previous work or online databases. AtNRT2.1 and 2.2 were isolated by our group (Zhuo et al. 1999), and the sequence of AtNRT2.1 was used for homology searches, which were carried out on the BLAST server (http://www.ncbi.nlm.nih.gov/blast). CLUSTAL W or X was used for an initial alignment analysis (Thompson et al. 1994, Thompson et al. 1997). Sequences were also searched for domains with ProSite (Hofmann et al. 1999). Other bioinformatic programs including Bioedit (Hall 1999), GeneDoc (Nicholas and Nicholas 1997), Sequence Assistant (http://www2s.biglobe.ne.jp/~haruta/), AnnHyb (http://annhyb.free.fr), and Altemis (http://www.sanger.ac.uk/Software/altemis) were also employed to support the analysis.
Acknowledgments
This work has been supported by the NSERC. We thank the TRIUMF for provision of 13NO3–; Dr. J. Schjoerring for sharing his data; and Dr. M.Y. Siddiqi for excellent feedback and discussion.
Present address: Section of Cell and Developmental Biology, Division of Biology, University of California at San Diego, 9500 Gilman Drive, La Jolla, CA 92093, U.S.A.
Present address: Agrigenomics, Edmonton, Alberta, T6G 2E1, Canada.
Corresponding author: E-mail, aglass@interchange.ubc.ca; Fax, +1-604-822-6089.
Fig. 1 Alignments of the two AtNRT families. (A) and (B) Amino acid sequence alignments of AtNRT2 (A) and AtNRT1 (B) families. Sequences were initially aligned with CLUSTAL W and finely adjusted using GeneDoc (Nicholas and Nicholas 1997). Amino acid residues in a black background indicate that >50% of members (i.e. ≥4 of seven sequences, and ≥3 out of four sequences in AtNRT2 and AtNRT1, respectively) were identical. Twelve predicted TMS regions of AtNRT2.1 and AtNRT1.1, determined by the HMMTOP program (Tusnady and Simon 1998), are indicated by numbered lines above the sequences. TMS locations of other members were close to those of AtNRT2.1, and AtNRT1.1 except for TMS1 of AtNRT1.2 (see Results). Potential protein kinase C and casein kinase 2 phosphorylation, and N-glycosylation sites, specified in red, blue, and green, respectively, were searched with the ProSite database (http://www.expasy.ch/prosite/). The threonine/serine residues in purple, indicated with an asterisk, are recognition sites for both PKC and CK2. Conserved sequence regions, MFS, NNP, C1 and C2 in (A), PTR1 and 2 in (B), are indicated by thick lines under the sequences.
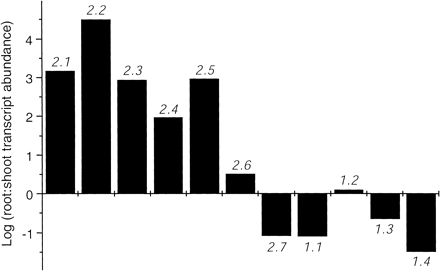
Fig. 2 Estimation of relative expression levels of AtNRT genes in shoots and roots. Ratios of transcript abundances were calculated by the amount of RNA and the numbers of PCR cycles required for RT-PCR (Table 2). Ratio (Root/Shoot) = (Aroot × 2Nroot)–1 / (Ashoot × 2Nroot)–1, where A is amount of RNA and N is the number of PCR cycles. Y-axis is expressed on a logarithmic scale.
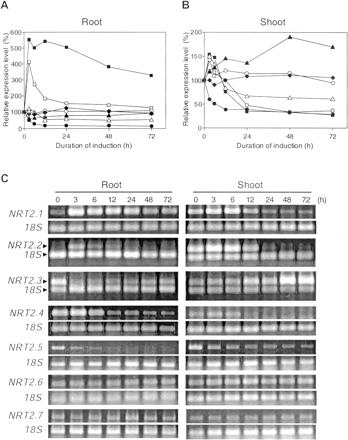
Fig. 3 Expression patterns of AtNRT2 genes in response to nitrate provision. RT-PCR products were obtained from 6-week-old Arabidopsis plants, which were grown hydroponically for 5 weeks in media containing 0.5 mM NH4NO3. Plants were N deprived for 1 week (0 h), and then re-supplied with 0.5 mM Ca(NO3)2 for 3–72 h. Relative expression values in root (A), and shoot (B) are the ratios of the gene specific amplicon to the 18S amplicon. The values shown are means of three RT-PCR replicates. Symbols: AtNRT2.1 (closed squares), AtNRT2.2 (open squares), AtNRT2.3 (closed triangles), AtNRT2.4 (open triangles), AtNRT2.5 (closed circles), AtNRT2.6 (open circles), and AtNRT2.7 (closed diamonds). (C) Representative images of RT-PCR products in 1.3% agarose gels stained with SYBR Gold.
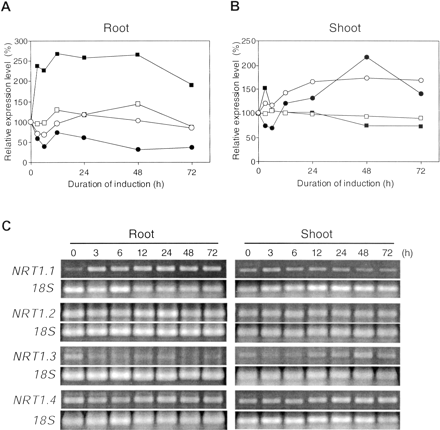
Fig. 4 Expression patterns of AtNRT1 genes in response to nitrate provision. Relative expression values of AtNRT1.1 (closed squares), AtNRT1.2 (open squares), AtNRT1.3 (closed circles), and AtNRT1.4 (open circles) in root (A), and shoot (B) were obtained by the same manner as described for Fig. 3. The values shown are means of three RT-PCR replicates. Representative gel images are shown in (C).
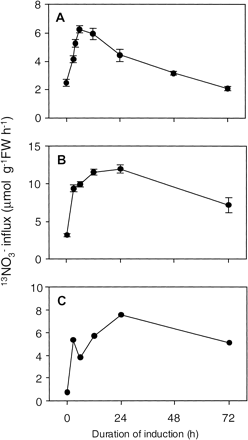
Fig. 5 13NO3– influx into Arabidopsis roots. (A) High-affinity nitrate influx measured with 100 µM NO3–. Six-week-old plants were N deprived for 7 d before being transferred to 1 mM NO3– solution for 0–72 h. (B) Low-affinity nitrate influx measured with 5 mM NO3–. Plants were grown and prepared same as (A). (C) LATS activity at 5 mM NO3–. To estimate LATS-mediated nitrate influx, mean values at 100 µM subtracted from those at 5 mM. The values are the means of eight replicates, and vertical bars indicate SE.
NRT (nitrate transporter) families in Arabidopsis
| Gene (Alternative name) | AGI gene code a | Accession no. | Amino acids | Identity b (%) | |
| Gene | Protein ID | ||||
| NRT2 family | |||||
| AtNRT2.1(ACH1) | At1g08090 | AF019748 | AAC35883 | 530 | 100 |
| AtNRT2.2(ACH2) | At1g08100 | AF019749 | AAC35884 | 522 | 87 |
| AtNRT2.3 | At5g69780 | AB015472 | BAB10099 | 539 | 68 |
| AtNRT2.4 | At5g60770 | AB015472 | BAB10098 | 527 | 82 |
| AtNRT2.5 | At1g12940 | AC012187 | AAF78499 | 502 | 56 |
| AtNRT2.6 | At3g45060 | AL353992 | CAB89321 | 542 | 68 |
| AtNRT2.7 | At5g14570 | AL163792 | CAB87624 | 493 | 44 |
| NRT1 family | |||||
| AtNRT1.1(CHL1) | At1g12110 | Q05085 | AAA32770 | 590 | 100 |
| AtNRT1.2(NTL1) | At1g69850 | AF073361 | AAC28086 | 585 | 34 |
| AtNRT1.3(NTP3) | At3g21670 | AB019232 | BAB02362 | 590 | 47 |
| AtNRT1.4(NTP2) | At2g26690 | AC003105 | AAB95302 | 586 | 51 |
| Gene (Alternative name) | AGI gene code a | Accession no. | Amino acids | Identity b (%) | |
| Gene | Protein ID | ||||
| NRT2 family | |||||
| AtNRT2.1(ACH1) | At1g08090 | AF019748 | AAC35883 | 530 | 100 |
| AtNRT2.2(ACH2) | At1g08100 | AF019749 | AAC35884 | 522 | 87 |
| AtNRT2.3 | At5g69780 | AB015472 | BAB10099 | 539 | 68 |
| AtNRT2.4 | At5g60770 | AB015472 | BAB10098 | 527 | 82 |
| AtNRT2.5 | At1g12940 | AC012187 | AAF78499 | 502 | 56 |
| AtNRT2.6 | At3g45060 | AL353992 | CAB89321 | 542 | 68 |
| AtNRT2.7 | At5g14570 | AL163792 | CAB87624 | 493 | 44 |
| NRT1 family | |||||
| AtNRT1.1(CHL1) | At1g12110 | Q05085 | AAA32770 | 590 | 100 |
| AtNRT1.2(NTL1) | At1g69850 | AF073361 | AAC28086 | 585 | 34 |
| AtNRT1.3(NTP3) | At3g21670 | AB019232 | BAB02362 | 590 | 47 |
| AtNRT1.4(NTP2) | At2g26690 | AC003105 | AAB95302 | 586 | 51 |
a Obtained from MATDB (http://mips.gsf.de/proj/thal/db/index.html).
b Percentage identity at the amino acid level.
NRT (nitrate transporter) families in Arabidopsis
| Gene (Alternative name) | AGI gene code a | Accession no. | Amino acids | Identity b (%) | |
| Gene | Protein ID | ||||
| NRT2 family | |||||
| AtNRT2.1(ACH1) | At1g08090 | AF019748 | AAC35883 | 530 | 100 |
| AtNRT2.2(ACH2) | At1g08100 | AF019749 | AAC35884 | 522 | 87 |
| AtNRT2.3 | At5g69780 | AB015472 | BAB10099 | 539 | 68 |
| AtNRT2.4 | At5g60770 | AB015472 | BAB10098 | 527 | 82 |
| AtNRT2.5 | At1g12940 | AC012187 | AAF78499 | 502 | 56 |
| AtNRT2.6 | At3g45060 | AL353992 | CAB89321 | 542 | 68 |
| AtNRT2.7 | At5g14570 | AL163792 | CAB87624 | 493 | 44 |
| NRT1 family | |||||
| AtNRT1.1(CHL1) | At1g12110 | Q05085 | AAA32770 | 590 | 100 |
| AtNRT1.2(NTL1) | At1g69850 | AF073361 | AAC28086 | 585 | 34 |
| AtNRT1.3(NTP3) | At3g21670 | AB019232 | BAB02362 | 590 | 47 |
| AtNRT1.4(NTP2) | At2g26690 | AC003105 | AAB95302 | 586 | 51 |
| Gene (Alternative name) | AGI gene code a | Accession no. | Amino acids | Identity b (%) | |
| Gene | Protein ID | ||||
| NRT2 family | |||||
| AtNRT2.1(ACH1) | At1g08090 | AF019748 | AAC35883 | 530 | 100 |
| AtNRT2.2(ACH2) | At1g08100 | AF019749 | AAC35884 | 522 | 87 |
| AtNRT2.3 | At5g69780 | AB015472 | BAB10099 | 539 | 68 |
| AtNRT2.4 | At5g60770 | AB015472 | BAB10098 | 527 | 82 |
| AtNRT2.5 | At1g12940 | AC012187 | AAF78499 | 502 | 56 |
| AtNRT2.6 | At3g45060 | AL353992 | CAB89321 | 542 | 68 |
| AtNRT2.7 | At5g14570 | AL163792 | CAB87624 | 493 | 44 |
| NRT1 family | |||||
| AtNRT1.1(CHL1) | At1g12110 | Q05085 | AAA32770 | 590 | 100 |
| AtNRT1.2(NTL1) | At1g69850 | AF073361 | AAC28086 | 585 | 34 |
| AtNRT1.3(NTP3) | At3g21670 | AB019232 | BAB02362 | 590 | 47 |
| AtNRT1.4(NTP2) | At2g26690 | AC003105 | AAB95302 | 586 | 51 |
a Obtained from MATDB (http://mips.gsf.de/proj/thal/db/index.html).
b Percentage identity at the amino acid level.
Gene specific primers and conditionsa of RT-PCR
| Gene | Product size (bp) | Shoot | Root | |||||
| RNA b (ng) | Cycle number | RNA b (ng) | Cycle number | |||||
| AtNRT2.1 | Forward | 5′CCGACAAGACGGCCAAGTTCGACCT | 770 | 250 | 28 | 25 | 21 | |
| Reverse | 5′TCCGTAGAGAAGAACGAAGATCCAAG | |||||||
| AtNRT2.2 | Forward | 5′CAGGTGGAAACAGAGCTGCCATGG | 401 | 250 | 33 | 25 | 21 | |
| Reverse | 5′GGACCATAGATACAACGGCAGTGACGAG | |||||||
| AtNRT2.3 | Forward | 5′TTCTCCAAGGTTTTCTGGTTTGCTG | 366 | 250 | 37 | 250 | 27 | |
| Reverse | 5′TGTACCGATTGAGAAGAGCATCATTGCTAG | |||||||
| AtNRT2.4 | Forward | 5′GCTGTGCTTTCCTCGTCATGCTCTCT | 520 | 250 | 33 | 250 | 27 | |
| Reverse | 5′GCGATGACGTTATCGGTTGTGAGCTCT | |||||||
| AtNRT2.5 | Forward | 5′GTTGATTCGTAATATGGGAGCCACCAA | 691 | 250 | 28 | 125 | 30 | |
| Reverse | 5′ACCCGTCTCTCTCGTGTATGTCGATCC | |||||||
| AtNRT2.6 | Forward | 5′TTCTCCAAGGTCTTTTGGTTCGCT | 505 | 250 | 32 | 250 | 30 | |
| Reverse | 5′ACCTCGAGGAAGAGAAGAAAAGAAGTTGAGTAACT | |||||||
| AtNRT2.7 | Forward | 5′ACTCGAACCAGAACAAACTCACAATTC | 503 | 250 | 26 | 250 | 19 | |
| Reverse | 5′TCCTAACGATCGCGTGGAGACG | |||||||
| AtNRT1.1 | Forward | 5′CATGATTCTTTGTATTGAGGCCGTGGAGA | 809 | 250 | 21 | 250 | 26 | |
| Reverse | 5′ATGAACGGAATTGTTCAGTGTGTGGCA | |||||||
| AtNRT1.2 | Forward | 5′CATCGAGTTTTTGGGATTGATCATACTCACAAT | 463 | 250 | 23 | 25 | 26 | |
| Reverse | 5′AACCGAAGCCGCAAGAAGAACCTTC | |||||||
| AtNRT1.3 | Forward | 5′GGTTTAATTTTAGGGAGCGAGCTATCAGAGAGAA | 609 | 250 | 21 | 25 | 26 | |
| Reverse | 5′TAAAACAATGGCTGCAACCACCATAGTCG | |||||||
| AtNRT1.4 | Forward | 5′CACTTGTTGGACAAGGCGGCCATA | 621 | 250 | 21 | 250 | 26 | |
| Reverse | 5′TGATAAAGTGGTCAAGAAAGATCCAGTGCTCATA | |||||||
| Gene | Product size (bp) | Shoot | Root | |||||
| RNA b (ng) | Cycle number | RNA b (ng) | Cycle number | |||||
| AtNRT2.1 | Forward | 5′CCGACAAGACGGCCAAGTTCGACCT | 770 | 250 | 28 | 25 | 21 | |
| Reverse | 5′TCCGTAGAGAAGAACGAAGATCCAAG | |||||||
| AtNRT2.2 | Forward | 5′CAGGTGGAAACAGAGCTGCCATGG | 401 | 250 | 33 | 25 | 21 | |
| Reverse | 5′GGACCATAGATACAACGGCAGTGACGAG | |||||||
| AtNRT2.3 | Forward | 5′TTCTCCAAGGTTTTCTGGTTTGCTG | 366 | 250 | 37 | 250 | 27 | |
| Reverse | 5′TGTACCGATTGAGAAGAGCATCATTGCTAG | |||||||
| AtNRT2.4 | Forward | 5′GCTGTGCTTTCCTCGTCATGCTCTCT | 520 | 250 | 33 | 250 | 27 | |
| Reverse | 5′GCGATGACGTTATCGGTTGTGAGCTCT | |||||||
| AtNRT2.5 | Forward | 5′GTTGATTCGTAATATGGGAGCCACCAA | 691 | 250 | 28 | 125 | 30 | |
| Reverse | 5′ACCCGTCTCTCTCGTGTATGTCGATCC | |||||||
| AtNRT2.6 | Forward | 5′TTCTCCAAGGTCTTTTGGTTCGCT | 505 | 250 | 32 | 250 | 30 | |
| Reverse | 5′ACCTCGAGGAAGAGAAGAAAAGAAGTTGAGTAACT | |||||||
| AtNRT2.7 | Forward | 5′ACTCGAACCAGAACAAACTCACAATTC | 503 | 250 | 26 | 250 | 19 | |
| Reverse | 5′TCCTAACGATCGCGTGGAGACG | |||||||
| AtNRT1.1 | Forward | 5′CATGATTCTTTGTATTGAGGCCGTGGAGA | 809 | 250 | 21 | 250 | 26 | |
| Reverse | 5′ATGAACGGAATTGTTCAGTGTGTGGCA | |||||||
| AtNRT1.2 | Forward | 5′CATCGAGTTTTTGGGATTGATCATACTCACAAT | 463 | 250 | 23 | 25 | 26 | |
| Reverse | 5′AACCGAAGCCGCAAGAAGAACCTTC | |||||||
| AtNRT1.3 | Forward | 5′GGTTTAATTTTAGGGAGCGAGCTATCAGAGAGAA | 609 | 250 | 21 | 25 | 26 | |
| Reverse | 5′TAAAACAATGGCTGCAACCACCATAGTCG | |||||||
| AtNRT1.4 | Forward | 5′CACTTGTTGGACAAGGCGGCCATA | 621 | 250 | 21 | 250 | 26 | |
| Reverse | 5′TGATAAAGTGGTCAAGAAAGATCCAGTGCTCATA | |||||||
a Detail conditions are described in Materials and Methods.
b Amount of total RNA used in single RT-PCR (see Materials and Methods).
Gene specific primers and conditionsa of RT-PCR
| Gene | Product size (bp) | Shoot | Root | |||||
| RNA b (ng) | Cycle number | RNA b (ng) | Cycle number | |||||
| AtNRT2.1 | Forward | 5′CCGACAAGACGGCCAAGTTCGACCT | 770 | 250 | 28 | 25 | 21 | |
| Reverse | 5′TCCGTAGAGAAGAACGAAGATCCAAG | |||||||
| AtNRT2.2 | Forward | 5′CAGGTGGAAACAGAGCTGCCATGG | 401 | 250 | 33 | 25 | 21 | |
| Reverse | 5′GGACCATAGATACAACGGCAGTGACGAG | |||||||
| AtNRT2.3 | Forward | 5′TTCTCCAAGGTTTTCTGGTTTGCTG | 366 | 250 | 37 | 250 | 27 | |
| Reverse | 5′TGTACCGATTGAGAAGAGCATCATTGCTAG | |||||||
| AtNRT2.4 | Forward | 5′GCTGTGCTTTCCTCGTCATGCTCTCT | 520 | 250 | 33 | 250 | 27 | |
| Reverse | 5′GCGATGACGTTATCGGTTGTGAGCTCT | |||||||
| AtNRT2.5 | Forward | 5′GTTGATTCGTAATATGGGAGCCACCAA | 691 | 250 | 28 | 125 | 30 | |
| Reverse | 5′ACCCGTCTCTCTCGTGTATGTCGATCC | |||||||
| AtNRT2.6 | Forward | 5′TTCTCCAAGGTCTTTTGGTTCGCT | 505 | 250 | 32 | 250 | 30 | |
| Reverse | 5′ACCTCGAGGAAGAGAAGAAAAGAAGTTGAGTAACT | |||||||
| AtNRT2.7 | Forward | 5′ACTCGAACCAGAACAAACTCACAATTC | 503 | 250 | 26 | 250 | 19 | |
| Reverse | 5′TCCTAACGATCGCGTGGAGACG | |||||||
| AtNRT1.1 | Forward | 5′CATGATTCTTTGTATTGAGGCCGTGGAGA | 809 | 250 | 21 | 250 | 26 | |
| Reverse | 5′ATGAACGGAATTGTTCAGTGTGTGGCA | |||||||
| AtNRT1.2 | Forward | 5′CATCGAGTTTTTGGGATTGATCATACTCACAAT | 463 | 250 | 23 | 25 | 26 | |
| Reverse | 5′AACCGAAGCCGCAAGAAGAACCTTC | |||||||
| AtNRT1.3 | Forward | 5′GGTTTAATTTTAGGGAGCGAGCTATCAGAGAGAA | 609 | 250 | 21 | 25 | 26 | |
| Reverse | 5′TAAAACAATGGCTGCAACCACCATAGTCG | |||||||
| AtNRT1.4 | Forward | 5′CACTTGTTGGACAAGGCGGCCATA | 621 | 250 | 21 | 250 | 26 | |
| Reverse | 5′TGATAAAGTGGTCAAGAAAGATCCAGTGCTCATA | |||||||
| Gene | Product size (bp) | Shoot | Root | |||||
| RNA b (ng) | Cycle number | RNA b (ng) | Cycle number | |||||
| AtNRT2.1 | Forward | 5′CCGACAAGACGGCCAAGTTCGACCT | 770 | 250 | 28 | 25 | 21 | |
| Reverse | 5′TCCGTAGAGAAGAACGAAGATCCAAG | |||||||
| AtNRT2.2 | Forward | 5′CAGGTGGAAACAGAGCTGCCATGG | 401 | 250 | 33 | 25 | 21 | |
| Reverse | 5′GGACCATAGATACAACGGCAGTGACGAG | |||||||
| AtNRT2.3 | Forward | 5′TTCTCCAAGGTTTTCTGGTTTGCTG | 366 | 250 | 37 | 250 | 27 | |
| Reverse | 5′TGTACCGATTGAGAAGAGCATCATTGCTAG | |||||||
| AtNRT2.4 | Forward | 5′GCTGTGCTTTCCTCGTCATGCTCTCT | 520 | 250 | 33 | 250 | 27 | |
| Reverse | 5′GCGATGACGTTATCGGTTGTGAGCTCT | |||||||
| AtNRT2.5 | Forward | 5′GTTGATTCGTAATATGGGAGCCACCAA | 691 | 250 | 28 | 125 | 30 | |
| Reverse | 5′ACCCGTCTCTCTCGTGTATGTCGATCC | |||||||
| AtNRT2.6 | Forward | 5′TTCTCCAAGGTCTTTTGGTTCGCT | 505 | 250 | 32 | 250 | 30 | |
| Reverse | 5′ACCTCGAGGAAGAGAAGAAAAGAAGTTGAGTAACT | |||||||
| AtNRT2.7 | Forward | 5′ACTCGAACCAGAACAAACTCACAATTC | 503 | 250 | 26 | 250 | 19 | |
| Reverse | 5′TCCTAACGATCGCGTGGAGACG | |||||||
| AtNRT1.1 | Forward | 5′CATGATTCTTTGTATTGAGGCCGTGGAGA | 809 | 250 | 21 | 250 | 26 | |
| Reverse | 5′ATGAACGGAATTGTTCAGTGTGTGGCA | |||||||
| AtNRT1.2 | Forward | 5′CATCGAGTTTTTGGGATTGATCATACTCACAAT | 463 | 250 | 23 | 25 | 26 | |
| Reverse | 5′AACCGAAGCCGCAAGAAGAACCTTC | |||||||
| AtNRT1.3 | Forward | 5′GGTTTAATTTTAGGGAGCGAGCTATCAGAGAGAA | 609 | 250 | 21 | 25 | 26 | |
| Reverse | 5′TAAAACAATGGCTGCAACCACCATAGTCG | |||||||
| AtNRT1.4 | Forward | 5′CACTTGTTGGACAAGGCGGCCATA | 621 | 250 | 21 | 250 | 26 | |
| Reverse | 5′TGATAAAGTGGTCAAGAAAGATCCAGTGCTCATA | |||||||
a Detail conditions are described in Materials and Methods.
b Amount of total RNA used in single RT-PCR (see Materials and Methods).
Correlation coefficients (r) for the relationships between AtNRT gene expression levels and two nitrate transport systems
| Gene | ra | |
| HATS | LATS | |
| AtNRT2 family | ||
| NRT2.1 | +0.74 b | +0.78 |
| NRT2.2 | +0.46 | –0.2 |
| NRT2.3 | –0.78 b | –0.41 |
| NRT2.4 | +0.1 | +0.5 |
| NRT2.5 | –0.33 | –0.85 b |
| NRT2.6 | –0.47 | +0.44 |
| NRT2.7 | –0.17 | +0.42 |
| AtNRT1 family | ||
| NRT1.1 | +0.56 | +0.88 b |
| NRT1.2 | +0.2 | +0.4 |
| NRT1.3 | –0.1 | –0.52 |
| NRT1.4 | –0.25 | +0.25 |
| Gene | ra | |
| HATS | LATS | |
| AtNRT2 family | ||
| NRT2.1 | +0.74 b | +0.78 |
| NRT2.2 | +0.46 | –0.2 |
| NRT2.3 | –0.78 b | –0.41 |
| NRT2.4 | +0.1 | +0.5 |
| NRT2.5 | –0.33 | –0.85 b |
| NRT2.6 | –0.47 | +0.44 |
| NRT2.7 | –0.17 | +0.42 |
| AtNRT1 family | ||
| NRT1.1 | +0.56 | +0.88 b |
| NRT1.2 | +0.2 | +0.4 |
| NRT1.3 | –0.1 | –0.52 |
| NRT1.4 | –0.25 | +0.25 |
Correlation coefficients (r) for the relationships between AtNRT gene expression levels and two nitrate transport systems
| Gene | ra | |
| HATS | LATS | |
| AtNRT2 family | ||
| NRT2.1 | +0.74 b | +0.78 |
| NRT2.2 | +0.46 | –0.2 |
| NRT2.3 | –0.78 b | –0.41 |
| NRT2.4 | +0.1 | +0.5 |
| NRT2.5 | –0.33 | –0.85 b |
| NRT2.6 | –0.47 | +0.44 |
| NRT2.7 | –0.17 | +0.42 |
| AtNRT1 family | ||
| NRT1.1 | +0.56 | +0.88 b |
| NRT1.2 | +0.2 | +0.4 |
| NRT1.3 | –0.1 | –0.52 |
| NRT1.4 | –0.25 | +0.25 |
| Gene | ra | |
| HATS | LATS | |
| AtNRT2 family | ||
| NRT2.1 | +0.74 b | +0.78 |
| NRT2.2 | +0.46 | –0.2 |
| NRT2.3 | –0.78 b | –0.41 |
| NRT2.4 | +0.1 | +0.5 |
| NRT2.5 | –0.33 | –0.85 b |
| NRT2.6 | –0.47 | +0.44 |
| NRT2.7 | –0.17 | +0.42 |
| AtNRT1 family | ||
| NRT1.1 | +0.56 | +0.88 b |
| NRT1.2 | +0.2 | +0.4 |
| NRT1.3 | –0.1 | –0.52 |
| NRT1.4 | –0.25 | +0.25 |
Abbreviations
- CK2
casein kinase 2
- HATS
high-affinity transporter system
- LATS
low-affinity transporter system
- MFS
major facilitator superfamily
- NNP
nitrate/nitrite porter
- NRT
nitrate transporter
- PKC
protein kinase C
- RT-PCR
reverse transcription-PCR
- TMS
trans-membrane-spanners.
References
Amarasinghe, B.H.R.R, de Bruxelles, G.L., Braddon, M., Onyeocha, I., Forde, B.G. and Udvardi, M.K. (
Arabidopsis Genome Initiative, The (
Aslam, M., Travis, R.L. and Huffaker, R.C. (
Cerezo, M., Tillard, P., Filleur, S., Munos, S., Daniel-Vedele, F. and Gojon, A. (
Claros, M.G. and von Heijne, G. (
Crawford, N.M. and Glass, A.D.M. (
Doddema, H. and Telkamp, G.P. (
Filleur, S. and Daniel-Vedele, F. (
Filleur, S., Dorbe, M.F., Cerezo, M., Orsel, M., Granier, F., Gojon, A. and Daniel-Vedele, F. (
Forde, B.G. (
Galvan, A., Quesada, A. and Fernandez, E. (
Gansel, X., Munos, S., Tillard, P. and Gojon, A. (
Gibeaut, D.M., Hulett, J., Cramer, G.R. and Seemann, J.R. (
Glass, A.D.M. (
Glass, A.D.M., Brito, D.T., Kaiser, B.N., Kronzucker, H.J., Kumar, A., Okamoto, M., Rawat, S.R., Siddiqi, M.Y., Silim, S.M., Vidmar, J.J. and Zhuo, D. (
Glass, A.D.M., Shaff, J.E. and Kochian, L.V. (
Glass, A.D.M. and Siddiqi, M.Y. (
Guo, F.Q., Wang, R., Chen, M. and Crawford, N.M. (
Hall, T.A. (
Hanisch ten Cate, C.H. and Breteler, H. (
Hatzfeld, Y. and Saito, K. (
Hofmann, K., Bucher, P., Falquet, L. and Bairoch, A. (
Hofmann, K. and Stoffel, W. (
Huang, N.C., Chiang, C.S., Crawford, N.M. and Tsay, Y.F. (
Huang, N.C., Liu, K.H., Lo, H.J. and Tsay, Y.F. (
Jones, D.T., Taylor, W.R. and Thornton, J.M. (
King, B.J., Siddiqi, M.Y., Ruth, T.J., Warner, R.L. and Glass, A.D.M. (
Krapp, A. and Stitt, M. (
Krapp, A., Fraisier, V., Scheible, W.R., Quesada, A., Gojon, A., Stitt, M., Caboche, M. and Daniel-Vedele, F. (
Kronzucker, H., Glass, A.D.M. and Siddiqi, M. (
Lejay, L., Tillard, P., Lepetit, M., Olive, F.D., Filleur, S., Daniel-Vedele, F. and Gojon, A. (
Liu, K.H., Huang, C.Y. and Tsay, Y.F. (
Nakai, K. and Kanehisa, M. (
Nicholas, K.B. and Nicholas, H.B. Jr. (
Nielsen, H., Brunak, S. and von Heijne, G. (
Ono, F., Frommer, W.B. and von Wiren, N. (
Orsel, M., Krapp, A. and Daniel-Vedele, F. (
Pao, S.S., Paulsen, I.T. and Saier, M.H. (
Perez, M.D., Gonzalez, C., Avila, J., Brito, N. and Siverio, J.M. (
Quesada, A., Krapp, A., Trueman, L.J., Daniel-Vedele, F., Fernandez, E., Forde, B.G. and Caboche, M. (
Rowe, J.J., Ubbink-Kok, T., Molenaar, D., Konings, W.N. and Driessen, A.J. (
Siddiqi, M.Y. and Glass, A.D.M. (
Siddiqi, M.Y., Glass, A.D.M., Ruth, T.J. and Fernando, M. (
Siddiqi, M.Y., Glass, A.D.M., Ruth, T.J. and Rufty, T.W. Jr. (
Sonnhammer, E.L.L., von Heijne, G. and Krogh, A. (
Steiner, H., Naider, F. and Becker, J.M. (
Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmougin, F. and Higgins, D.G. (
Thompson, J.D., Higgins, D.G. and Gibson, T.J. (
Touraine, B. and Glass, A.D.M. (
Trueman, L.J., Richardson, A. and Forde, B.G. (
Tsay, Y.F., Schroeder, J., I, Feldmann, K.A. and Crawford, N.M. (
Tusnady, G.E. and Simon, I. (
Unkles, S.E., Hawker, K.L., Grieve, C., Campbell, E., I, Montague, P. and Kinghorn, J.R. (
Unkles, S.E., Zhuo, D., Siddiqi, M.Y., Kinghorn, J.R. and Glass, A.D.M. (
Vidmar, J.J., Zhuo, D.G., Siddiqi, M.Y. and Glass, A.D.M. (
Wang, R. and Crawford, N.M. (
Wang, R., Liu, D. and Crawford, N.M. (