-
PDF
- Split View
-
Views
-
Cite
Cite
Yosuke Fukamatsu, Naoto Yabe, Kohji Hasunuma, Arabidopsis NDK1 is a Component of ROS Signaling by Interacting with Three Catalases, Plant and Cell Physiology, Volume 44, Issue 10, 15 October 2003, Pages 982–989, https://doi.org/10.1093/pcp/pcg140
Close - Share Icon Share
Abstract
Plants sense various environmental stimuli and have specific signaling pathways to respond to these cues. We focused on light responsive components and found that NDKs were phosphorylated specifically after red light irradiation in Pisumsativum [Tanaka et al. (1998)J. Photochem. Photobiol. B 45: 113] and after blue light irradiation in Neurospora crassa [Oda and Hasunuma (1997)Mol. Gen. Genet. 256: 593, Ogura et al. (2001)J. Biol. Chem. 276: 21228]. We performed yeast two-hybrid screening using AtNDK1, the counterpart of NDK-P1 (PisumsativumNDK1) in Arabidopsis, as bait, and isolated catalase3 (AtCat3). Interactions between AtNDK1-AtCAT1 and AtNDK1-AtCAT2 were also detected with the two-hybrid system. Non-denaturing two-dimensional gel electrophoresis of crude extracts from plants revealed that catalase and NDK activities co-migrated in the same area of the gel. Transgenic plants expressing AtNDK1 under control of the CaMV 35S promoter exhibited tolerance to paraquat and high ability to eliminate exogenous H2O2. These results indicate that AtNDK1 has a role in ROS response.
(Received July 14, 2003; Accepted August 18, 2003)
Introduction
Plants perceive and respond to environmental stimuli such as light, temperature, humidity and nutritional state of the soil. We focused on light responsive components and found that phosphorylation of an 18-kDa protein increased after red light irradiation in Pisum sativum (Tanaka et al. 1998) and a 15-kDa protein after blue light irradiation in Neurospora crassa (Oda and Hasunuma 1997, Ogura et al. 2001). Purification and partial amino acid sequencing analysis revealed that both proteins were nucleoside diphosphate kinase (NDK; EC 2.7.4.6.) and designated as NDK-P1 and NDK-1, respectively (Tanaka et al. 1998, Ogura et al. 1999). In Neurospora, P72H substitution in ndk-1 resulted in loss of light response for perithecial polarity (Ogura et al. 2001). In Arabidopsis thaliana, information from total genomic sequence suggested that NDK gene family consists of five members including putative genes. Among them, AtNDK1 is the most homologous to NDK-P1 in its amino acid sequence and supposed to be located in the cytosol. Therefore it could be the counterpart of NDK-P1 in Arabidopsis, playing a role in photo-signaling.
NDK has NDP kinase activity, catalyzing the transfer of a phosphate group from a nucleoside triphosphate to a nucleoside diphosphate (Park et al. 1973). NDK can produce all kinds of NTP except for ATP and has been considered to be an essential housekeeping enzyme for the maintenance of intracellular NTP levels. The sequences of NDK genes from various organisms have revealed that most of these proteins consist of approximately 150 amino acids and the amino acid sequence is extensively conserved from bacteria to humans. The crystal structure has made it clear that NDK functions as a tetramer in prokaryotes, Escherichia coli (Almaula et al. 1995) and a hexamer in eukaryote, Dictyostelium discoideum (Dumas et al. 1992) and in humans (Chiadmi et al. 1993).
Traces of information on the NDKs from various organisms gave some hint as to the biological functions of NDKs in vivo. In humans, NDK was identified as a tumor metastasis suppresser nm23 (Steeg et al. 1988), transcriptional activator of c-myc (Agou et al. 1999, Postel et al. 1993) and supplier of GTP to G-protein (Kimura and Johnson 1983). The Drosophila melanogaster developmental mutant, awd (abnormal wing disc) phenotype, was caused by a mutation in the NDK gene (Biggs et al. 1990). Various partners interacting with NDK have been isolated. In humans, nm23-H1 interacted with glyceraldehyde-3-phosphate dehydrogenase and activated a protein phosphotransferase function (Engel et al. 1998). Co-purification of NDK with DnaK, HSP70 in E. coli, was reported (Barthel and Walker 1999). In plants, ectopic expression of ArabidopsisAtNDK2 rescued the UV-sensitive phenotype of a gcn4 mutant in yeast (Zimmermann et al. 1999). AtNDK2 interacted with a red-light-activated form of PHYB in vitro and worked as a component of the light signal transduction with its alternation in Km of NDK activity (Choi et al. 1999). Recently, it was reported that AtNDK2 interacted with Arabidopsis mitogen-activated protein kinase homologues, AtMPK3 and 6, which were activated by reactive oxygen species (ROS) (Ichimura et al. 2000, Kovtun et al. 2000, Yuasa et al. 2001), and AtNDK2 enhanced the protein phosphorylation activity of AtMPK3 (Moon et al. 2003). Under heat stress, pea mitochondrial NDK interacted with a heat shock protein, suggesting a role for NDK in heat stress response (Galvis et al. 2001).
NDKs thus play diverse roles in various biological responses by interacting with the other factors. Therefore, isolation of the protein interacting with NDKs would provide an insight into the function of AtNDK1 in intracellular signaling. We employed a yeast two-hybrid system using the C-termini of AtNDK1 as bait. C-termini of AtNDK1 have un-conserved domain among the AtNDK family and Kpn-loop domain for protein–protein interaction. This system is suitable for detecting stable and transient protein–protein interaction in semi vivo conditions (Fields and Song 1989). In this paper, we report the interactions between AtNDK1 and AtCAT1, 2 and 3 confirmed this using the two-hybrid system and non-denaturing two-dimensional gel electrophoresis. In addition, we present the phenotypes of transgenic plants expressing AtNDK1 under control of the CaMV 35S promoter (AtNDK1-EX) under ROS stress.
Results
Catalase3 as a final candidate interacting with AtNDK1 by yeast two-hybrid screening
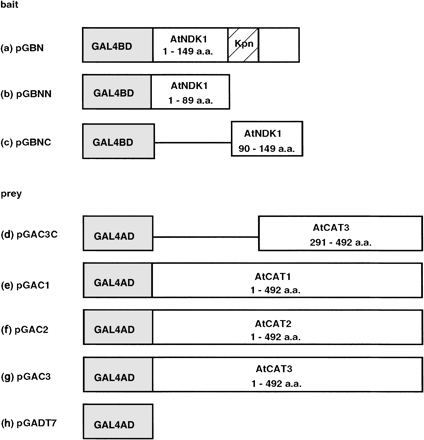
To isolate components interacting with AtNDK1, we carried out a yeast two-hybrid screening. First, we tried to use pGBN and pGBNN as bait for screening (Fig. 1a, b). However, yeast AH109 carrying these vectors showed high levels of β-galactosidase activity (data not shown). Then, we employed pGBNC containing the C-terminal un-conserved domain of the AtNDK family and the Kpn-loop predicted for a protein–protein interaction domain as bait (Fig. 1c). Two-hybrid screening was performed by co-transformation of AH109 carrying pGBNC with a 2×106 cDNA library in pACT2, the vector containing GAL4-AD gene fused with partial cDNA. As a result, 124 candidate clones were selected as positive. Among them, four candidates had significant open reading frames. One of them, the C-terminal domain (291–492 aa) of Catalase3 (AtCAT3), which responds to light (Zhong et al. 1997), was chosen (Fig. 1d).
Interaction of AtNDK1 with the AtCAT family in yeast two-hybrid system
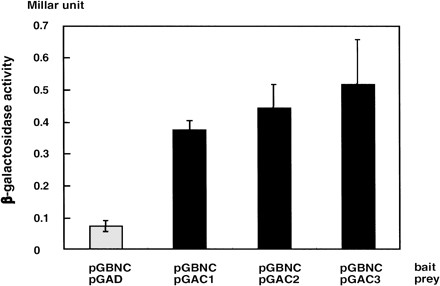
We examined whether the interaction between AtNDK1 and AtCAT3 was specific or not, because the cDNA fragment of AtCAT3 in candidates contained a domain highly conserved in the AtCAT family. Thus interactions between the C-terminal domain of AtNDK1 and each full length of AtCAT1, 2 and 3 were examined in the two-hybrid system (Fig. 1e, f, g). β-galactosidase activities of AH109 carrying pGBNC and pGAC1, 2 and 3 indicated the interactions of C-terminal domain of AtNDK1 with AtCAT1, 2 and 3 (Fig. 2).
In vivo interaction of AtNDK1 with AtCATs on non-denaturing two-dimensional gel
Non-denaturing two-dimensional gel electrophoresis was carried out for the detection of interaction between AtNDK1 and AtCATs in vivo. First, isoelectric-focusing (IEF) gels without urea and SDS for keeping protein complexes was used as the first dimensional gel. The amounts of each sample applied to IEF gels were equalized as 10 µg. Non-denaturing 7.5% polyacrylamide gel was used as the second dimensional gel. AtCAT1, 2 and 3 formed three spots (Fig. 3a(i)). The positions of catalase spots picked up were determined as described in “Materials and Methods”. A spot not containing catalase activity was selected at random. All spots corresponding to AtCAT1, 2 and 3 (Orendi et al. 2001) contained NDK activity (Fig. 3a(iii)). When crude extract from AtNDK1-EX plants was applied, NDK activity in the area with catalase activity was higher than that from the wild type (Fig. 3b(iii)). The results suggested that NDK activities of the AtCAT spots were dependent on the AtNDK1 among the AtNDK family. NDK activity of the spots not containing catalase activity would be caused by AtNDK homo-complex or AtNDK-other factor complex. At least, AtNDK1 and catalase co-migration was confirmed. Molecular weights of monomeric AtNDK1 and AtCAT were around 16 kDa and 56 kDa, respectively. In eukaryotes, NDK consisted of hexamers (Dumas et al. 1992) and CAT of tetramers (Fita and Rossmann 1985), therefore the approximate molecular weights of each complex would be about 96 kDa and 224 kDa. Though non-denaturing gel electrophoresis does not reflect the exact molecular weight, AtNDK1 and AtCAT homo-complex should not exist in the same area, as there is a distinct difference in molecular weights between them.
Tolerance to paraquat of wild-type and AtNDK1-EX plants
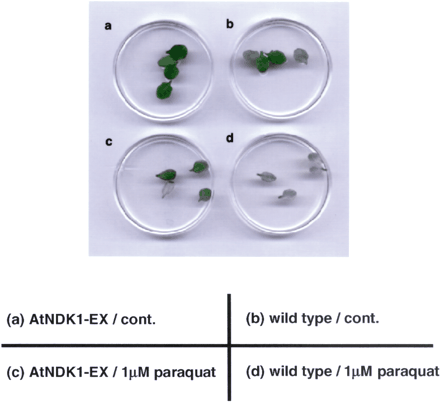
Catalase-deficient tobacco showed a significant decrease in ROS stress tolerance compared with the wild-type plants (Willekens et al. 1997). To investigate whether there were some differences in the catalase-related phenotype between wild-type and AtNDK1-EX plants, leaves from 16-day-old wild-type and AtNDK1-EX plants were incubated on 1/10 MS medium containing 1 µM paraquat and 0.025% Tween 20 for 40 h. Paraquat is a superoxide-releasing reagent in chloroplasts and superoxide is dismutated to oxygen and H2O2 by super oxide dismutase (Alscher et al. 2002). Accordingly, the degree of bleaching would reflect the catalase activity. Leaves from AtNDK1-EX showed higher tolerance to paraquat bleaching than those from the wild type (Fig. 4c, d). A few of leaves treated with 0.025% Tween 20 were relatively pale green in AtNDK1-EX and the wild type (Fig. 4a, b). Tween 20, a detergent for solubilization of membrane, might cause this phenomenon. Independent three lines of T3 AtNDK1-EX transgenic plants were examined. Although the three lines showed similar phenotype, one line exhibiting relatively markedly phenotype in the paraquat bleaching assay, is shown in Fig. 4.
Elimination of exogenous H2O2 by plant
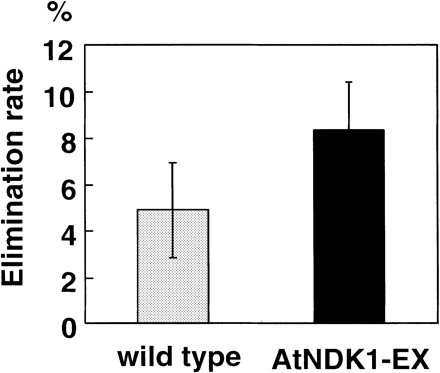
To investigate the ability of plants to decompose H2O2, we calculated the elimination rate of exogenous H2O2 using a whole plant. The H2O2 concentration was measured by UV spectrometer at OD240. The H2O2 elimination rate of AtNDK1-EX was almost 2-fold higher than that of wild type after 9 h incubation (Fig. 5). Thus AtNDK1-EX plants had higher ability to eliminate exogenous H2O2 than the wild type.
Catalase activities in wild-type and AtNDK1-EX plants treated with H2O2
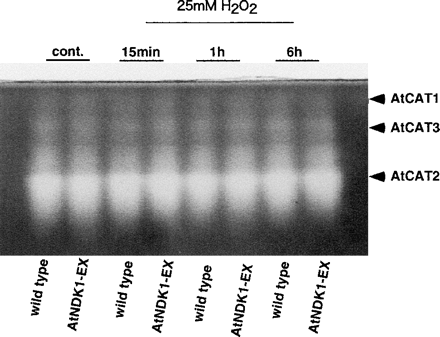
From the fact that AtNDK1-EX plants had higher tolerance to ROS stress than the wild type (Fig. 4, 5), we expected AtNDK1-EX plants to have higher catalase activity than the wild type. Intact plants were incubated in 25 mM H2O2 on a 5.5 cm diameter Petri dish from 15 min to 6 h and catalase activity staining was performed. However, there was no significant difference in catalase activity between AtNDK1-EX and the wild type (Fig. 6).
Discussion
We isolated C-termini of catalase3 by yeast two-hybrid screening with C-termini of AtNDK1 as bait (Fig. 1c, d) and showed that AtNDK1 equally interacted with all the AtCAT family in this system (Fig. 2). Furthermore, we presented evidence for the interaction between AtNDK1 and AtCAT1, 2 and 3 in vivo by non-denaturing two-dimensional gel electrophoresis. In this experiment, major catalase activities were detected as three spots. All these three spots contained higher levels of NDK activity than No. 6, 7 and 8 spots without catalase activity (Fig. 3a(iii)). High NDK activity of No. 4 and 5 spots would be caused by AtNDK homo-complex or AtNDK-other protein complex (Fig. 3a(iii)). When the crude extract from AtNDK1-EX plants was subjected to this two-dimensional gel electrophoresis, the NDK activity of each spot with catalase activity was higher than that in the wild type (Fig. 3b(iii)). This result suggests that the NDK activity contained in the gels of AtCAT spots was dependent on the amount of AtNDK1 among the AtNDK family. Co-migration of AtNDK1 and AtCAT in the gel indicated AtNDK1–AtCAT interaction in vivo. These two experiments, in yeast nucleus and plants, show that AtNDK1 interacts with all the AtCAT family in vivo.
The tetrameric catalase (EC 1.11.1.6) converts 2H2O2 into O2 + 2H2O. AtCAT form a small gene family consisting of AtCAT1, 2 and 3 in Arabidopsis (Chevalier et al. 1992, Newman et al. 1994, Frugoil et al. 1996). The subcellular localization of catalase in plants is thought to occur in peroxisomes, glyoxysomes and cytosol (Bray et al. 2000) or only in peroxisomes (Mittler 2002). The expression of AtCAT2 and 3 was regulated by the circadian clock and light (Zhong et al. 1994). The direct regulation of catalase is known as described below. Calmodulin interacted with and activated AtCAT3 in the presence of calcium (Yang and Poovaiah 2002). Mammalian non-receptor tyrosine kinases, c-Abl and Arg, phosphorylated and activated catalase in H2O2-mediated apoptosis signaling (Cao et al. 2003). Therefore AtCAT activity would be regulated by other components. Tobacco catalase-deficient mutants accumulated oxidized glutathione and decreased ascorbate, and had less tolerance to oxidative stress induced by high light, paraquat, H2O2, salt and ozone (Willekens et al. 1997, Dat et al. 2001). From these reports, catalase has an important role for ROS stress responses in plant. AtNDK1–AtCAT interaction may provide an elaborated machinery to control the activity of AtCAT and affect the tolerance to ROS stress. We performed paraquat-bleaching experiments using leaves from 16-day-old wild-type and the AtNDK1-EX plants. The AtNDK1-EX plants had higher bleaching tolerance than the wild type (Fig. 4). To investigate what caused this increased tolerance to ROS, the rate of elimination of exogenous H2O2 by the AtNDK1-EX and wild-type intact plants was determined as described by Willekens et al. (1997). The AtNDK1-EX plant eliminated H2O2 at a higher rate than the wild type (Fig. 5). These two experiments suggested that the intracellular abundance of AtNDK1 affected the tolerance to ROS stress via its highly activated catalase. Though, to distinguish whether catalase activity of the AtNDK1-EX plants was higher than that of wild type in the presence of H2O2, catalase activities were measured in plants treated with H2O2. However, the difference in catalase activity of the crude extracts from the AtNDK1-EX and wild-type plants was not distinguishable in this assay (Fig. 6). A similar result was reported in Helicobacter pylori, that the catalase-associated protein deficient mutant (kapA) was sensitive to H2O2 stress, but catalase activity was not altered (Harris et al. 2002). AtNDK1 may play a chaperon-like role of AtCAT or inhibit AtCAT from translocating to peroxisomes. Cottonseed catalase has a peroxisomal targeting signal in its C-terminus (Mullen et al. 1997). The C-terminal domain of Catalase3 isolated by the two-hybrid screening contained peroxisome transit peptide described in their report. In Saccharomyces cerevisiae, transcription of cytosolic catalase T is induced by various stresses (Marchler et al. 1993). AtNDK1 might cover the transit peptide of AtCAT. “Cytosolic AtCAT” would be effective for ROS stress without increasing the catalase activity.
In ROS signaling of Arabidopsis, AtMPK3, 4 and 6 were investigated in detail (Ichimura et al. 2000, Kovtun et al. 2000, Yuasa et al. 2001). AtNDK2, reported as a phytochrome-interacting protein, having its Km regulated by phytochrome in a Pr-Pfr reversible manner (Choi et al. 1999), was also shown to interact with AtMPK3 and AtMPK6 (Moon et al. 2003). It was demonstrated that AtNDK2 activated AtMPK3 in vitro suggesting that AtNDK2 plays a role in the AtMPK3-mediated ROS signaling pathway.
Although, the mechanism by which AtNDK1–AtCAT interactions affect the ROS responses in plants remains unsettled, the present study partially revealed the function of NDK in ROS responses and provides a clue to solving the ROS signaling in plants.
Materials and Methods
Two-hybrid screening
A GAL4-based two-hybrid system (Fields and Song 1989), Matchmaker System3 (Clontech, CA, U.S.A.), was used as recommended by the manufacturer. pGBNC, which is a two-hybrid vector containing the C-terminal domain of AtNDK1 fused with GAL4 binding domain in pGBKT7 (Fig. 1c), was transformed into a yeast strain AH109 (MATα, trp1-901, leu2-3, 112, ura3-52, his3-200, gal4Δ, gal80Δ,LYS2::GAL1UAS-GAL1TATA-HIS3, GAL2UAS-GAL2TATA-ADE2, URA3::MEL1UAS-MEL1TATA-lacZ). In the first screening, Arabidopsis cDNA library fused with GAL4 activating domain in pACT2, provided by the Arabidopsis Biological Resource Center (CD4-10), was co-transformed into AH109 cells carrying pGBNC on plate with minimal synthetic dropout (SD) medium containing adenine. A total of 2×106 yeast transformants was screened and 1×103 clones were selected as His+ colonies. The second screening was performed on SD plates containing α-galactosidase without amino acids and 124 clones were selected. After sequence analysis, four candidates having a long open reading frame and catalase3, which is known to respond to light, were selected (Zhong et al. 1997).
Each full-length cDNA of AtCAT1, 2 and 3, fused in-frame with GAL4 activating domain in the plasmid pGADT7, to give pGAC1, pGAC2 and pGAC3, respectively (Fig. 1e, f, g), was co-transformed in AH109 carrying pGBNC for a re-transformation assay.
Growth conditions of plants
Seeds were surface-sterilized by HClO, sowed on MS (Murashige and Skoog 1962) solid media [1× MS salt mix (Sigma, MO, U.S.A.), 1× Gamborg B5 vitamin (Gamborg et al. 1976), 2.5% sucrose, 0.2% GELRITE (Sigma)] and incubated at 4°C in darkness for 3 d to synchronize germination and grown under 16 h light/8 h dark photoperiod (about 80 µmol m–2 s–1) at 21°C.
Quantification of catalase activity
First, plants frozen in liquid nitrogen were homogenized on ice in 0.4 ml of extraction buffer [100 mM Tris-HCl, pH 8.0, 20% glycerol, 10 mM DTT (dithiothreitol), 10 mg ml–1 leupeptin, 0.5 mM PMSF (phenylmethylsulfonylfluoride) and 10 mg ml–1 pepstatin A] with a pestle and mortar and then centrifuged at 15,000 rpm for 10 min at 4°C to recover the supernatant. A protein assay kit (Bio-Rad, CA, U.S.A.) was used to determine the protein concentration in the supernatant. Active staining for the activity of catalase was based on Orendi et al. (2001) with slight modification. The protein extracts were separated on a 7.5% non-denaturing polyacrylamide gel (0.4 M Tris-HCl, pH 8.8) with a 4.8% stacking gel (0.125 M Tris-HCl, pH 6.8) using electrophoresis buffer (200 mM glycine, 25 mM Tris-HCl, pH 8.5). The gels were soaked in 5% methanol for 5 min, washed in water for 10 min, soaked in 6 mM H2O2 and washed in water for 1 min. Then the gels were incubated with developing buffer (1% FeCl3, 1% K3[Fe(CN)6]) for 5 min. The reaction was stopped by soaking gels in stop buffer (5% methanol, 10% acetic acid).
NDK assay
NDK activity was determined as reported by Munoz-Dorado et al. (1990). Crude extracts or separated gel slices were incubated in 20 ml of reaction buffer (250 mM HEPES-NaOH, pH 8.0, 250 mM NaCl, 50 mM MgCl, 5 mM DTT, 1 mM CDP, 90 pmol 110 Mbq pmol–1 [γ-32P]ATP) at room temperature for 1 h. Then 1 ml of reaction mixture was mixed in 10 ml of 10 mM EDTA to stop the reaction. Next, 1 ml from the stopped mixture was separated with PEI cellulose F TLC plastic sheets (Merck, Darmstadt, Germany) using 0.75 M KH2PO4 as a developer. Radio-labeled spots corresponding to [γ-32P]CTP and [γ-32P]ATP on TLC were visualized using X-ray film, X-Omat AR (Kodak, NY, U.S.A.).
Plant transformation
A. thaliana (Columbia) was transformed by Agrobacterium-mediated transformation using the vacuum method (Bechtold and Pelletier 1998). Cauliflower mosaic virus 35S promoter-AtNDK1 was ligated into HindIII and SacI sites of the pBI-H1 vector after removing the GUS gene by digestion of HindIII and SacI sites. The transgenic plant was designated AtNDK1-EX.
Non-denaturing two-dimensional gel electrophoresis
IEF gel [7% acrylamide, 1.6% Ampholine, pH 6–8, 4.4% Ampholine, pH 3.5–10 (Amersham Pharmacia, Uppsala, Sweden)] was used. Non-denaturing electrophoresis was performed using 7.5% polyacrylamide gels (0.4 M Tris-HCl, pH 8.8). Protein extraction buffer (50 mM Tris-HCl, pH 8.0, 20% glycerol 10 mM DTT, 10 mg ml–1 leupeptin, 0.5 mM PMSF, 10 mg ml–1 pepstatinA) was used. Ten µg protein from the crude extract was loaded on two IEF gels. These gels were washed with 50 ml of electrophoresis buffer (200 mM glycine, 25 mM Tris-HCl, pH 8.5) for 2 h and placed on the top of the non-denaturing gel, side-by-side. After electrophoresis, catalase activity staining was performed on half of the gel. The other half of the gel was placed on the stained gel and the spots corresponding to catalase activity were picked up. NDK activity in the gel fragments was determined as described for the NDK assay.
Paraquat (methyl-viologen) bleaching assay
This assay was based on the method reported by Yuasa et al. (2001). First, 16-day-old leaves from wild-type and AtNDK1-EX plants were floated on liquid 1/10 MS media containing 1 mM paraquat (methyl-viologen, Sigma) and 0.025% Tween 20 at 21°C, under continuous light (about 100 mmol m–2 s–1), for 40 h. Samples were applied 12 h after the beginning of the photoperiod. Then the level of bleaching was determined by photographing the leaves.
Measurement of H2O2 elimination by whole plants
The single 16-day-old whole plant grown under 16 h light/8 h dark photoperiod was transferred into 3 ml of distilled water containing 7 mM H2O2 in a 5 cm-diameter Petri dish at 3 h after the beginning of the photoperiod. After 9 h incubation under continuous light (about 80 µmol m–2 s–1), H2O2 consumption by the plant was measured as the change in OD240 of the medium (Willekens et al. 1997).
Quantification of catalase activity of plants treated with H2O2
16-day-old plants were treated with 1/10 MS liquid medium containing 25 mM H2O2. After 15 min, 1 h and 6 h incubation under continuous light (about 80 µmol m–2 s–1), plants were frozen in liquid nitrogen. As a control experiment, plants treated with 1/10 MS liquid medium without H2O2 for 15 min. Catalase activity was detected as described in “Quantification of catalase activity”. Quantities of the proteins in crude extract applied to each lane were equalized as 2.0 µg.
Acknowledgments
We would like to thank Dr K. Nakamura for providing pBI-101Hm vector and Dr T. Yuasa for giving us detailed protocol for paraquat assay. We are grateful to Dr K. Satoh for critical reading of this manuscript. We appreciate Y. Nakamura for establishing transgenic Arabidopsis plants.
Present address: Naoto Yabe, Department of Biological Sciences, Graduate School of Science, University of Tokyo, 7-3-1 Hongo, Tokyo, 113-0033 Japan.
Corresponding author: E-mail, kohji@yokohama-cu.ac.jp; Fax, +81-45-820-1901.
Fig. 1 Construction of vectors used in yeast two-hybrid analysis. Bait construction: (a) pGBN, full-length cDNA of AtNDK1 (149 aa) fused with GAL4 DNA binding domain (GAL4BD) in pGBKT7. “Kpn” (hatched box) indicates Kpn-loop. (b) pGBNN, N-terminal domain of AtNDK1 (1–89 aa) fused with GAL4BD in pGBKT7. (c) pGBNC, C-terminal domain of AtNDK1 (90–149 aa) fused with GAL4BD in pGBKT7. Prey construction; (d) pGAC3C; C-terminal domain (291–492 aa) of catalase3 in pACT2. (e) pGAC1, full-length cDNA of catalase1 (492 aa) fused with GAL4 activation domain (GAL4AD) in pGADT7. (f) pGAC2, full-length cDNA of catalase2 (492 aa) fused with GAL4AD in pGADT7. (g) pGAC3, full-length cDNA of catalase3 (492 aa) fused with GAL4AD in pGADT7. (h) pGADT7, GAL4AD vector without cDNA insert. The reduction of scales of the boxes does not reflect exact lengths.
Fig. 2 Interaction between AtNDK1 and AtCAT1, 2 and 3 in yeast two-hybrid system. β-galactosidedase activity of yeast AH109 carrying pGBNC and pGAD was shown as a negative control. The vectors on the upper are the baits and on the bottom are the preys. β-galactosidase activity was quantified as Millar units (103 × OD240 × reaction time–1 × OD600–1). The values present the averages of three independent experiments with standard errors.
Fig. 3 Co-migration of catalase activity and NDK activity in the non-denaturing two-dimensional gel electrophoresis. Crude extracts from (a) wild-type and (b) AtNDK1-EX plants were subjected to non-denaturing two-dimensional gel electrophoresis, which consisted of IEF gel electrophoresis as the first dimension and non-denaturing gel electrophoresis as the second. Quantities of the protein in crude extract applied to each IEF gel were equalized as 10 µg. (i) Catalase in-gel activity staining is present. (ii) The spots containing catalase activity (No. 1–3) and other spots (No. 4–8) were picked up from the copy gels. (iii) NDK activities of each spots. [γ-32P]ATP and -CDP were used as substrates of NDK activity. No. 1, 2 and 3 lanes underlined correspond to the spots containing catalase2, 3 and 1 activity, respectively.
Fig. 4 Paraquat bleaching experiment using leaves from wild-type and AtNDK1-EX plants. Leaves from AtNDK1-EX and the wild-type plants were floated on 1/10 MS medium containing 0.025% Tween 20 with 1 µM paraquat (c) and (d) or without paraquat (a) and (b) for 40 h under continuous light (80 µmol m–2 s–1) at 21°C.
Fig. 5 Elimination of exogenous H2O2 by wild-type and AtNDK1-EX plants. Decreased H2O2 concentrations after 9 h incubation of wild-type and AtNDK1-EX plants in 7 mM H2O2 under continuous light (80 µmol m–2 s–1) at 21°C are shown. Values present the averages of the decreased rate of fifteen independent plants with standard errors.
Fig. 6 Catalase activity of wild-type and AtNDK1-EX plants treated with 25 mM H2O2 for 15 min to 6 h. A whole plant was incubated with 5 ml of 25 mM H2O2 solution under continuous light (80 µmol m–2 s–1) at 21°C. As a control, a whole plant incubated without H2O2 under light for 15 min was processed. Identification of AtCAT was based on Orendi et al. (2001). Quantities of the protein in crude extract applied to each lane were equalized as 2.0 µg.
Abbreviations
- aa
amino acid residues
- CaMV
cauliflower mosaic virus
- ROS
reactive oxygen species
- AtNDK1-EX
ectopic expressor of AtNDK1.
References
Agou, F., Raveh, S., Mesnildrey, S. and Veron, M. (
Alscher, R.G., Erturk, N. and Heath, L.S. (
Almaula, N., Lu, Q., Delgado, J., Belkin, S. and Inouye, M. (
Barthel, T. and Walker, G. (
Bechtold, N. and Pelletier, G. (
Biggs, J., Herspeger, E., Steeg, P.S., Liotta, L.A. and Shearm, A. (
Bray, E.A., Bailey-Serres, J. and Weretilnyk, E. (
Cao, C., Leng, Y. and Kufe, D. (
Chevalier, C., Yamaguchi, J. and McCourt, P. (
Chiadmi, M., Morera, S., Lascu, I., Dumas, C., LeBras, G., Veron, M. and Janin, J. (
Choi, G., Yi, H., Lee, J., Kwon, Y.K., Soh, M.S., Shin, B., Luka, Z., Hahn, T.R. and Song, P.S. (
Dat, J., Inze, D. and Van, B.F. (
Dumas, C., Lascu, I., Morera, S., Glaser, P., Fourme, R., Wallet, V., Lacombe, M.L., Veron, M. and Janin, J. (
Engel, M., Seifert, M., Theisinger, B., Seyfert, U. and Welter, C. (
Fields, S. and Song, O. (
Frugoil, J.A, Zhong, H.H., Nuccio, M.L., McCourt, P., McPeek, M.A., Thomas, T.L. and McClung, C.R. (
Galvis, M., Marttila, S., Hakansson, G., Forsberg, J. and Knorpp, C. (
Gamborg, O.L., Murashige, T., Thorpe, T.A. and Vasil, I.K. (
Harris, A., Hinds, F., Beckhouse, A., Kolesnikow, T. and Hazell, S. (
Ichimura, K., Mizoguchi, T., Yoshida, R., Yuasa, T. and Shinozaki, K. (
Kimura, N. and Johnson, G.S. (
Kovtun, Y., Chiu, W.L., Tena, G. and Sheen, J. (
Marchler, G., Schuller, C., Adam, G. and Ruis, H. (
Mittler, R. (
Moon, H., Lee, B., Choi, G., Shin, D., Prasad, D., Lee, O., Kwak, S., Kim, D., Nam, J., Cho, M., Lim, C. and Yun, D. (
Mullen, R.T., Lee, M.S. and Trelease, R.N. (
Munoz-Dorado, J., Inouye, S. and Inouye, M. (
Murashige, T. and Skoog, F. (
Newman, T., de Bruijn, F., Green, P., Keegstra, K., Kende, H., McIntosh, L., Ohlrogge, J., Raikhel, N., Somerville, S., Thomashow, M., Retzel, E. and Somervilles, C. (
Oda, K. and Hasunuma, K. (
Ogura, Y., Yoshida, Y., Ichimura, K., Yabe, N. and Hasunuma, K. (
Ogura, Y., Yoshida, Y., Yabe, N. and Hasunuma, K. (
Orendi, G., Zimmermann, P., Baar, C. and Zentgraf, U. (
Park, R.E., Jr. and Agarwal, R.P. (
Postel, E.H., Berberich, S.J., Flint, S.J. and Ferrone, C.A. (
Steeg, P.S., Beviracqua, G., Pozzatti, R., Liotta, L.A. and Sobel, M.E. (
Tanaka, N., Ogura, T., Noguchi, T., Hamada, T., Hirano, H., Yabe, N. and Hasunuma, K. (
Willekens, H., Chamnongpol, S., Davey, M., Schraudner, M., Langebartels, C., Van Montagu, M., Inze, D. and Van Camp, W. (
Yang, T. and Poovaiah, B. (
Yuasa, T., Ichimura, K., Mizoguchi, T. and Shinozaki, K. (
Zhong, H., Young, J., Pease, E., Hangarter, R. and McClung, C. (
Zhong, H., Resnick, A., Straume, M. and McClung, C. (



![Fig. 3 Co-migration of catalase activity and NDK activity in the non-denaturing two-dimensional gel electrophoresis. Crude extracts from (a) wild-type and (b) AtNDK1-EX plants were subjected to non-denaturing two-dimensional gel electrophoresis, which consisted of IEF gel electrophoresis as the first dimension and non-denaturing gel electrophoresis as the second. Quantities of the protein in crude extract applied to each IEF gel were equalized as 10 µg. (i) Catalase in-gel activity staining is present. (ii) The spots containing catalase activity (No. 1–3) and other spots (No. 4–8) were picked up from the copy gels. (iii) NDK activities of each spots. [γ-32P]ATP and -CDP were used as substrates of NDK activity. No. 1, 2 and 3 lanes underlined correspond to the spots containing catalase2, 3 and 1 activity, respectively.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/pcp/44/10/10.1093_pcp_pcg140/2/m_pcg14003.gif?Expires=1716396967&Signature=L7WIQfpehbwbSXVG56Y8oL~MZ1cPl4TP-2WhX41dayMjG74K5tVS2y0hKKLvDRYf8I2hQicB~-p8QUQXUdUmlZ-6q-Y4PbHIMxdwdjCEvQh4iZezf4-TOgUHR-9xTq7UVvA2yiJrbYy0QhQTu0QLq-lxG1niy4OIqjFOEVcphVk1FXz5ukeqIASn2zyi5ojzZsfn8FgHX~x8zIQDezQFCOvtotWfrgSylkpJZJSkJgiKt-Yr8uf6Tsz7yXFkPksaiW8kPIJMOHClbrrGilf0ZN-MyYyzyPaP27V7vlcscybiJtm95wpcKRIwRu~YKFRlf3CvjCmDX4C1pqi3vMYUsA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)