-
PDF
- Split View
-
Views
-
Cite
Cite
Kohsuke Hashimoto, Hisako Igarashi, Shoji Mano, Mikio Nishimura, Teruo Shimmen, Etsuo Yokota, Peroxisomal Localization of a Myosin XI Isoform in Arabidopsis thaliana, Plant and Cell Physiology, Volume 46, Issue 5, May 2005, Pages 782–789, https://doi.org/10.1093/pcp/pci085
Close - Share Icon Share
Abstract
The genome of Arabidopsis thaliana contains 13 myosin XI isoforms. Here we prepared a specific antibody against a peptide that mimics a unique C-terminal region from the myosin XI isoform, MYA2. The resulting antibody was used to demonstrate that MYA2 in Arabidopsis protein extracts co-sedimented with actin filaments and dissociated from the filaments with ATP treatment. Immunolocalization studies showed that MYA2 co-localized predominantly with actin filaments in clustered punctuate dots in leaf epidermal cells, root hair cells and suspension-cultured cells. In a transgenic plant in which peroxisomes are labeled with green fluorescent protein, some MYA2 signals were localized on peroxisomes in an actin-dependent manner. We propose that the peroxisome is one of the cargos translocated by MYA2 on actin filaments.
Introduction
Myosins are actin-based molecular motors that are ubiquitous in eukaryotic cells. They are composed of motor domain (head region), neck and tail regions. The motor domain interacts with actin filaments in an ATP-dependent manner. On the basis of the primary structure of this domain, myosins are classified into at least 18 phylogenetic classes (Mermall et al. 1998, Yamashita et al. 2000). The C-terminal tail region is diverse in size and primary structure among each myosin class, and so is inferred to determine their physiological functions in the cells.
In plant cells, three classes of myosin genes, VIII, XI and XIII, have been identified (Reichelt and Kendrick-Jones 2000, Sellers 2000). Myosin proteins have been isolated and partially characterized from germinating lily pollen (Yokota and Shimmen 1994), Chara internodal cells (Yamamoto et al. 1994, Higashi-Fujime et al. 1995) and tobacco suspension-cultured cells (Yokota et al. 1999). Recent molecular biological studies revealed that these myosins belong to myosin XI (see review by Shimmen and Yokota 2004). Tobacco myosin XI of mol. wt 175 kDa (Yokota et al. 1999) moves processively along actin filaments with a very high velocity (Tominaga et al. 2003).
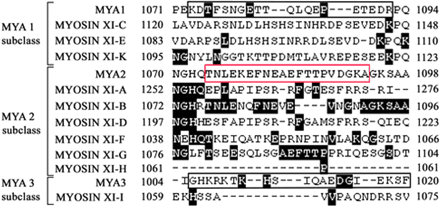
In Arabidopsis thaliana, myosin XI consists of 13 genes and is classified further into MYA1, MYA2 and MYA3 subclasses. Seven isoforms are present in the MYA2 subclass (Fig. 1). The predicted molecular weight of each heavy chain is approximately 170 kDa for MYA1 and MYA2, and approximately 140 kDa for MYA3 (Kinkema et al. 1995). Northern blot analysis showed that MYA1 and MYA2 are expressed in flower, leaf, root and stem, whereas MYA3 is present in flower and leaf (Kinkema and Schiefelbein 1994, Kinkema et al. 1995). However, the intracellular localization of myosin XI isoforms from Arabidopsis has not been established. In the present study, we focused on the MYA2 isoform and found that it is partly localized on peroxisomes in Arabidopsis cells. Furthermore, phenotypes associated with a MYA2 T-DNA disruption allele were analyzed.
Results
Specificity of the MYA2 antibody
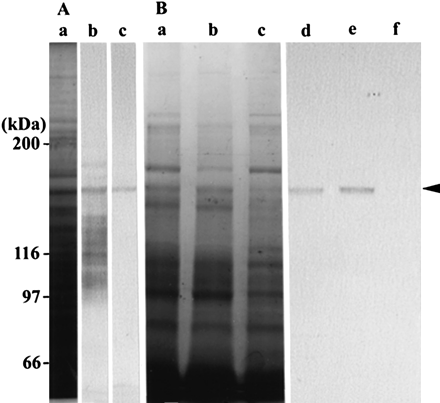
To make a specific antibody against MYA2, we compared the amino acid sequences in the diverse tail region of myosin XI isoforms, and identified regions with relatively low similarity to corresponding sequences of other isoforms (Fig. 1). The antiserum raised against this peptide cross-reacted with several polypeptides (lane b in Fig. 2A) in a crude protein sample prepared from wild-type Arabidopsis seedlings, whereas an antibody affinity purified from the serum recognized only a 170 kDa protein (lane c in Fig. 2A), as expected for MYA2 (169.9 kDa).
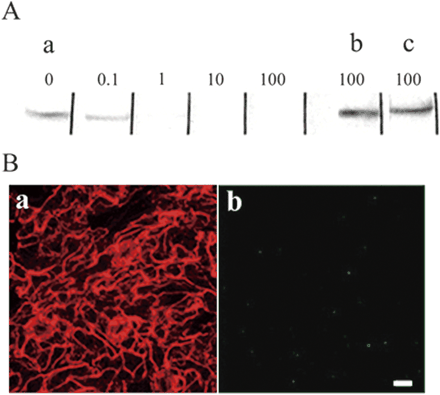
When the affinity-purified antibody was incubated with the antigen peptide before immunoblotting, the signal intensity of the 170 kDa protein was decreased as the peptide concentration was raised above 10 µg ml–1 (Fig. 3A). In contrast, the 170 kDa signal was not affected by prior incubation with the corresponding peptides from MYA1 and MYA3 isoforms (black boxes in Fig. 1), even at a concentration of 100 µg ml–1 (lanes b and c in Fig. 3A).
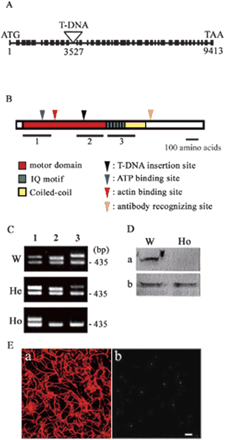
We identified an Arabidopsis line in which a T-DNA was inserted in the 11th intron of MYA2 (Fig. 4A). To examine MYA2 expression, reverse trnascription–polymerase chain reaction (RT–PCR) was performed at three regions of the MYA2 gene (Fig. 4B). In plants homozygous for the T-DNA insertion, the MYA2 transcript appeared to be truncated after the T-DNA insertion site (Fig. 4C). Accordingly, the MYA2 antibody raised against the C-terminal tail region did not detect the 170 kDa protein in extracts from homozygous mutant plants (Fig. 4D). Taken together, these results demonstrated that the purified antibody specifically recognized the MYA2 isoform.
MYA2 binds to F-actin in an ATP-dependent manner
When the crude protein extract from wild-type Arabidopsis seedlings was supplemented with polymerized F-actin, centrifuged and examined by immunoblotting with the MYA2 antibody, MYA2 protein was co-sedimented with actin filaments and recovered in the pellet fraction (lane d in Fig. 2B). Futhermore, when the pellet was incubated with a solution containing ATP, MYA2 was recovered in the supernatant fraction after centrifugation, suggesting that the putative MYA2–F-actin complex was dissociated (lane e in Fig. 2B). These results indicated that MYA2 binds to F-actin in the absence of ATP and detaches from F-actin in the presence of ATP.
Intracellular localization of MYA2
We examined the intracellular localization of MYA2 in epidermal cells of leaf (Fig. 5A–C) and stem (data not shown). In guard cells, strong MYA2 signals were observed (arrowheads in Fig. 5B). These dots were predominantly co-localized with actin filaments (yellow dots in Fig. 5C). In root hair cells and suspension-cultured cells, punctuate signals appeared to form clusters in regions where actin filaments were present at higher density (Fig. 6B, C, E, F). When the epidermal cells were treated with latrunculin B (LB), an actin-depolymerizing drug, no filamentous actin signals were observed (Fig. 7A) and small MYA2 dots were dispersed throughout the cytoplasm (Fig. 7B). When the MYA2 antibody was mixed with the MYA2 antigen peptide at 100 µg ml–1 before immunostaining, no strong signals were detected in the leaf epidermal cells (panel b, Fig. 3B). Furthermore, the MYA2 antibody did not detect significant signals in the leaf epidermal cells from the homozygous T-DNA disruption allele (panel b, Fig. 4E). Thus, the punctuate signals observed in this study represent genuine MYA2 protein localization.
MYA2 partly co-localizes on peroxisomes
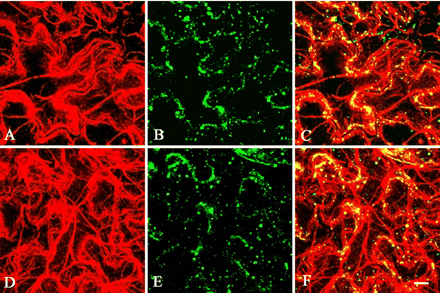
It has been reported that the movement of peroxisomes is driven by an actin–myosin system in plant cells (Jedd and Chua 2002, Mano et al. 2002, Mathur et al. 2002, Collings et al. 2003). To test whether MYA2 is a motor protein responsible for the peroxisome movement, MYA2 localization was examined in leaf epidermal cells and guard cells of transgenic Arabidopsis plants in which green fluorescent protein (GFP) was fused to the peroxisomal targeting signal peptide and stably expressed (Mano et al. 2002). Due to the methanol treatment of plant samples during the freeze-substitution procedure, the intrinsic fluorescence of GFP was not detectable under an epifluorescence microscope (data not shown). Therefore, an antibody against GFP was used to detect the GFP protein. In a crude protein extract prepared from the transgenic seedlings, the GFP antibody recognized only one protein of approximately 30 kDa, as expected for the peroxisomal targeted GFP (data not shown). Furthermore, immunostaining of epidermal (Fig. 8C) and guard cells (Fig. 8G) from leaves with the anti-GFP antibody appeared to be similar to images of GFP signals in those cells (Fig. 8A, E for epidermal and guard cells, respectively) of living transgenic plants, with respect to the size and the density. Double immunostaining using anti-MYA2 antibody (Fig. 8B, F) and anti-GFP antibody (Fig. 8C, G) showed that some MYA2 dot signals of a relatively large size were co-localized with GFP-labeled peroxisomes (yellow signals in Fig. 8D, H). However, fine punctuate MYA2 signals, especially in guard cells, did not overlap with peroxisomes (red signals in Fig. 8D, H).
Phenotypes of the mya2 mutant
In the mya2 T-DNA insertion allele, the mutant MYA2 protein, if present, should lack the C-terminal motor, neck and tail domains, and thus is considered to be non-functional. Morphology, growth and fertility were indistinguishable between wild-type and the mya2 null allele plants (data not shown). Next, we compared the distribution of peroxisomes in epidermal cells of wild-type leaves with that of the mya2 null allele by immunostaining using an antibody against a peroxisomal marker protein, catalase (Mano et al. 2002). The distribution patterns of peroxisomes were indistinguishable between wild-type and mutant epidermal cells (Fig. 9B, E). These results indicate that disruption of MYA2 does not cause gross cellular and morphological abnormalities.
Discussion
In the present study, we used an affinity-purified antibody raised against a synthetic peptide of the MYA2 isoform of Arabidopsis myosin XI to examine the function of this motor protein. Myosin binds tightly to actin filament in the absence of ATP, while it repeats binding to and dissociation from actin filament in the presence of ATP, and as a result the equilibrium shifts to dissociation (Adelstein and Eisenberg 1980, Harrington and Rodgers 1984). By co-sedimentation analysis, we revealed the ATP-dependent interaction of MYA2 with F-actin. The results are similar to other native myosin XI proteins from other plant species, such as germinating lily pollen (170 kDa myosin; Yokota and Shimmen 1994), cultured cells of Catharanthus roseus (170 kDa myosin; Yokota et al. 1995b) or tobacco cultured cells BY-2 (170 and 175 kDa myosin; Yokota et al. 1999).
Immunolocalization studies showed that most of the MYA2 punctate signals co-localized with actin filaments in leaf epidermal cells, root hair cells and suspension-cultured cells, and some of them with relatively large size overlapped with GFP-labeled peroxisomes in leaf epidermal cells and guard cells. The dispersal of MYA2 dots with LB treatment revealed that localization of MYA2 depends on binding to actin filaments. From pharmacological studies using an actin-depolymerizing drug (LB) and an inhibitor of myosin activity (2,3-butanedione monoxine), it has been reported that the actin–myosin system is involved in the movement of peroxisomes. The present study implicates MYA2 as a motor responsible for translocating peroxisomes in Arabidopsis.
On the other hand, fine dots and punctate signals stained with antibody against MYA2 were not co-localized with peroxisomes in epidermal and guard cells. It has been suggested that a subclass of myosin XI in maize is co-localized with mitochondria and plastids and is responsible for their translocation (Wang and Pesacreta 2004). In Arabidopsis, mitochondria show heterogeneity in size and shape, and plastids show spherical structures and a large size. (Logan and Leaver 2000). The size and shape of fine dots and punctate MYA2 signals, which did not overlap with peroxisomes, in the present study were obviously different from those of mitochondria and plastids, suggesting that MYA2 is not associated with these organelles in Arabidopsis. Thus, MYA2 may transport not only peroxisomes but also other small organelles or vesicles. Further study is needed to identify these small organelles and vesicles.
It was reported recently that a T-DNA insertion in the 29th intron (mya2-1 allele) or in the 30th exon (mya2-2 allele) of MYA2 causes pleiotropic defects, including dwarf growth and sterility (Holweg and Nick 2004). The mya2-2 homozygote was reported to be lethal. In contrast, our T-DNA insertion line did not show any obvious morphological and developmental abnormalities. In non-plant cells, single mutations in a myosin isoform generally show no or only mild phenotypes. For example, Dictyostelium cells lacking one member of the myosin I family show no or weak phenotypic defects, indicative of functional redundancy among myosin I isoforms (Ostap and Pollard 1996, Dai et al. 1999, Falk et al. 2003). Possibly other isoforms of myosin XI may act redundantly to complement the MYA2 defects in our study. In plants containing the mya2-1 and mya2-2 alleles, truncated MYA2 proteins containing intact motor and neck regions might be expressed. These truncated MYA2 proteins, if expressed, are unlikely to bind to cargo molecules because of the deletion of the predicted cargo-binding DIL domain (Shimmen and Yokota 2004), but may associate with actin filaments and other interacting partners. In this scenario, the MYA2 proteins in mya2-1 and mya2-2 might act as dominant-negative suppressors of other myosin XI isoforms, and is consistent with the semi-dominant phenotype reported for both alleles (Holweg and Nick 2004).
Materials and Methods
Plant material and growth condition
Arabidopsis thaliana ecotype Columbia was used as wild-type control for all analyses. Transgenic Arabidopsis expressing the GFP-tagged peroxisomal targeting signal peptide 1 was generated previously (Mano et al. 2002). The information for T-DNA insertion lines of MYA2 (At5g43900) was obtained from the Salk Genomic Institute Laboratory website (http://signal.salk.edu). One T-DNA, SALK_12789, was inserted in the 11th intron of MYA2 (Fig. 4A), and seeds were obtained from the Arabidopsis Biological Resource Center at Ohio State University (Alonso et al. 2003). The ecotype of the Salk T-DNA insertion line was Columbia. To distinguish the insertion line from the non-inserted plants and confirm the insertion in MYA2 alleles, PCR using a T-DNA-specific primer (5′-TGGTTCACGTAGTGGGCCATCG-3′) and two MYA2-specific primers, forward (5′-TCGATTCTACCCTGATGAAG-3′) and reverse (5′-TCACCAAGAATGAGCAATCG-3′), was performed using the genomic DNA obtained from 2-week-old leaves by the method of Edwards et al. (1991).
The surface-sterilized seeds were kept in water at 4°C for 4 d and plated on Murashige and Skoog media (Wako, Pure Chemical Industries, Co., Ltd, Osaka, Japan) supplemented with 3% sucrose and 0.8% agar. The plants were grown in a growth cabinet under a 16 h light–8 h dark cycle at 22°C for 2–3 weeks. For the observation of aerial portions, plants were transferred from agar plates into compost and grown under the same light conditions and at the same temperature as described above.
Arabidopsis Col-0 suspension cells (Mathur et al. 1998), kindly supplied by Dr. Csaba Koncz (Max-planck-Institut fur Zuchtungsforschung) and Dr. Masaaki Umeda (The University of Tokyo), were maintained in 80 ml of basal Murashige and Skoog medium supplemented with vitamins, 3% sucrose and 0.0002% 2,4-dichlorphenoxyacetic acid at 22°C by gentle agitation (130 rpm) in the dark. Cells from 5-day-old cultures were used for immunostaining.
Preparation of antibody against synthetic MYA2 peptide
Peptide (TNLEKEFNEAEFTTPVDGKA corresponding to amino acids 1,074–1,093 of MYA2, red box in Fig. 1) coupled with keyhole limpet hemocyanin through its cysteine residue was purchased from Qiagen Co. Ltd (Tokyo, Japan). It was mixed with Titer Max Gold (Kiel Laboratories, Inc., Gainesville, FL, USA) and injected into a male rabbit. A total of four boosts were given at 2 week intervals. The animal was bled 2 weeks after the final injection. The serum was stored frozen at –80°C until use.
To affinity-purify the antibody from the serum, the synthetic peptide was dissolved into a solution containing 0.1% SDS and 0.1 M sodium phosphate buffer (pH 7.4), and applied on a nitrocellulose membrane strip (Immobilon-PSQ, Millipore Co., Bedford, MA, USA). After air-drying overnight, the membrane was rinsed several times in a solution containing 0.15 M NaCl, 0.1% Tween-20, 10 mM Tris–HCl (pH 7.5) and then in a solution of 10% monoethanol amine and 1 M NaHCO3. After blocking with 2% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for 1 h, the membrane was incubated in the serum diluted 20-fold with 2% BSA in T-PBS (0.05% Tween-20 in PBS) for 1 h. After washing with T-PBS several times, the antibody adsorbed on the membrane was eluted by treatment with 0.1 M glycine-HCl (pH 2.5) for 7 min. After adjusting the pH immediately to 7–7.7 by the addition of 1 M Tris, the eluate was used as affinity-purified antibody for immunoblotting and immunostaining.
Depletion of immunoreactive activity of affinity-purified antibody
Peptides of MYA1 (KDTFSNGETTQLQEPETEDR corresponding to amino acids 1,073–1,092 in Fig. 1), MYA2 described above and MYA3 (GHKRKTKHSIQAEDGIEKSF corresponding to amino acids 1,005–1,024 in Fig. 1) were synthesized by Qiagen. Each peptide was dissolved in PBS at a concentration of 1 mg ml–1 as a stock solution and mixed with the affinity-purified antibody. The mixture was left to stand at 4°C overnight and then used for both immunoblotting and immunostaining.
F-actin co-sedimentation
The F-actin co-sedimentation procedure was performed according to the method described previously (Yokota and Shimmen 1994, Yokota et al. 1999). Each procedure described below was carried out at 0–4°C. Seedlings grown for 2 weeks were homogenized with a hand-operated glass–glass homogenizer in EMP solution [20 mM EGTA, 6 mM MgCl2, 2 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), 50 µg ml–1 leupeptin, 30 mM PIPES-KOH (pH 7.0)] supplemented with 1% casein and 0.25 M sucrose. After centrifugation at 10,000×g for 10 min, the supernatant was centrifuged further at 100,000×g for 30 min. F-actin (final concentration 0.1 mg ml–1) prepared from chicken breast muscle by the method of Kohama (1981) was added to the second supernatant (crude extract). This mixture was kept standing on ice for 30 min. After centrifugation at 100,000×g for 30 min, the pellet was suspended in the EMP solution supplemented with 10 mM ATP and 5 mM potassium phosphate buffer (pH 7.0). This suspension was used as the F-actin co-sedimentation fraction. After 10 min, the suspension was centrifuged at 100,000×g for 30 min. The pellet was re-suspended in the EMP solution.
Immunoblotting
After SDS–PAGE, proteins in the gel were electrophoretically transferred to a PVDF-nitrocellulose membrane sheet (polyvinylidene difluoride immobilon membrane; Millipore) according to the method of Towbin et al. (1979). The sheet was blocked with 4% skim milk in PBS for 1 h and then incubated for 1 h with the antiserum against P-135-ABP villin (Yokota et al. 1998), synthetic peptide or the affinity-purified antibody, which were diluted 1,000-, 500- or 100-fold, respectively, with PBS supplemented with 2% BSA and 0.05% Tween-20. The detection of antibodies on the sheet by anti-rabbit IgG conjugated with alkaline phosphatase (Sigma Chemical Co., St Louis, MO, USA) was carried out according to the method described previously (Yokota et al. 1995a). Band intensity was measured by densitometry (Densito-pattern analyzer model No. EPA-3000, Cosmo Bio. Co. Ltd, Tokyo, Japan).
Immunostaining
Samples prepared from 4-week-old plants were fixed in liquid propane and then substituted with methanol at –80°C. In the case of suspension-cultured cells, cells were adhered on a poly-l-lysine-coated coverslip and subjected to a rapid freeze–freeze substitution procedure. For LB treatment, plants were left to stand in water containing 20 µM LB for 12–14 h before fixation. After rehydrating with a graded methanol series in PHEM buffer [60 mM PIPES, 25 mM HEPES, 10 mM EGTA, 4 mM MgSO4 (pH 6.9)], samples were treated with 0.05% (w/v) pectolyase Y-23 (Kyowa Chemical Products Co., Ltd, Osaka, Japan) and 0.4 M mannitol in PHEM buffer for 20 min at room temperature. After rinsing in PHEM buffer three times, the samples were treated with 1% Triton X-100 in PBS for 15 min. They were washed with PBS three times and incubated for 1 h at room temperature with primary antibody, rabbit affinity-purified antibody against MYA2 peptide, mouse anti-actin antibody (clone C4, ICN Biochemicals, Inc. Aurora, OH, USA), mouse anti-GFP antibody (clone 3E6; Wako) or rabbit anti-catalase antibody (Mano et al. 2002) diluted 20-, 100-, 40- or 400-fold, respectively, with 1% BSA in PBS. After rinsing in PBS three times, samples were treated with secondary antibody conjugated with Alexa Fluor 488 or Alexa Fluor 594 (Molecular Probes, Inc., Eugene, OR, USA), diluted 100-fold with PBS supplemented with 1% BSA for 1 h at room temperature in the dark. After rinsing in PBS three times, samples were mounted on a glass slide in PBS supplemented with 50% glycerol and 1% p-phenylenediamine dihydrochloride (Sigma). Samples were observed with a Zeiss LSM 510 confocal microscope (Carl Zeiss, Thornwood, NY, USA).
RT–PCR
Total RNA was isolated from leaves of 4-week-old plants by the RNeasy Plant Kit (Qiagen, Valencia, CA, USA). The first strand cDNA was synthesized by Ready-To-Go You-Prime First-strand Beads (Amasham, Piscataway, NJ, USA). The 28 cycles of PCR were performed using MYA2-specific primers, N-terminal motor domain (forward, 5′-GAGTGGATGATATGACTAGAC-3′; reverse, 5′-GATCGAAGACTTGACAAACAC-3′), C-terminal motor domain (forward, 5′-GGTCAAGATCATGACTCGAAG-3′; reverse, 5′-GATTGTTTGGAATCGTCAGAC-3′), IQ motif and partial coiled-coil domain (forward, 5′-GAAAAGTTCGGTCATATCTCG-3′; reverse, 5′-CTCCGTTTCTTTCGACTTGAG-3′). As a control, EF1-α-specific primers (forward, 5′-GAGAGATCATCCTCCACC-3′; reverse, 5′-GGCCACAAACTAGGCATG-3′) were used together with these MYA2-specific primers.
Other methods
Crude protein samples for immunoblotting were prepared from 2-week-old plants using a trichloroacetic acid precipitation method according to Yokota and Shimmen (1994). SDS–PAGE was performed by the method of Laemmi (1970). Gels were stained with Coomassie brilliant blue (CBB).
Acknowledgments
We thank Dr. Csaba Koncz (Max-Planck-Institut für Züchtungsforschung) and Dr. Masaaki Umeda (The University of Tokyo) for kindly providing the Arabidopsis Col-0 cell suspension. We thank Professor Dr. Christopher J. Staiger (Purdue University) for his kind discussion and checking the manuscript. We also thank the National Live Stock Breeding Center Hyogo Station for the gift of chicken breast muscle.
Fig. 1 Amino acid sequences of the C-terminal tail regions of MYA1 (At1g17580), MYOSIN XI-C (At1g08730), MYOSIN XI-E (At1g54560), MYOSIN XI-K (At5g20490), MYA2 (At5g43900), MYOSIN XI-A (At1g04600), MYOSIN XI-B (At1g04160), MYOSIN XI-D (At2g33240), MYOSIN XI-F (At2g31900), MYOSIN XI-G (At2g20290), MYOSIN XI-H (At4g28710), MYA3 (At3g58160) and MYOSIN XI-I (At4g33200). Amino acids identical to the MYA2 polypeptide are shown with a gray background. A peptide of MYA2, TNLEKEFNEAEFTTPVDGKA (red box), was synthesized and used as an antigen for antibody production. Synthesized peptides for MYA1 (KDTFSNGETTQLQEPETEDR, black box) and MYA3 (GHKRKTKHSIQAEDGIEKSF, black box) were used for the depletion assay shown in Fig. 3.
Fig. 2 Characterization of antibody. (A) Crude protein sample from wild-type Arabidopsis seedlings. CBB-stained gel (a), and immunoblotting with an antiserum against MYA2 peptide (b), or with the affinity-purified antibody from the serum (c). (B) F-actin co-sedimentation fraction. CBB-stained gel (a, b and c) and immunoblotting (d, e and f) with the affinity-purified antibody against MYA2 peptide. F-actin was added to the crude extract prepared from seedlings. After centrifugation, the pellet (a and d) was suspended in a solution containing ATP, and further centrifuged. The resulting supernatant (b and e) and pellet (c and f) were electrophoresed with the first pellet (a and d) on the same gel. The arrowhead indicates the 170 kDa polypeptide recognized by the antibody. The molecular masses of standard proteins are indicated at the left in kDa.
Fig. 3 Specificity of the affinity-purified antibody against MYA2 peptide. (A) Immunoblot of crude extract with the affinity-purified antibody treated with MYA2 peptide (a) at final concentrations of 0 (0), 0.1 (0.1), 1.0 (1.0), 10 (10) and 100 µg ml–1 (100), or with either MYA1 peptide (b) or MYA3 peptide (c) at a final concentration of 100 µg ml–1. (B) Double labeling of epidermal cells of leaf with an antibody against actin (a) and the affinity-purified antibody mixed with MYA2 peptide at a final concentration of 100 µg ml–1 (b). Only very little signal for MYA2 was detected (b). Scale bar = 10 µm.
Fig. 4 T-DNA insertion into the MYA2 isoform. (A) Schematic diagram of the MYA2 gene showing the site of T-DNA insertion (triangle) positioned at 3,527 bp in the 11th intron. Black boxes and lines represent exons and introns, respectively. (B) Schematic diagram of three parts of the MYA2 gene, 1, N-terminal motor domain (561 bp); 2, C-terminal motor domain (536 bp); 3, IQ motif and a part of the coiled-coil domain (593 bp). The ATP-binding and actin-binding domain, T-DNA insertion position and the antibody recognition sites are shown with triangles. The primers used are given in Materials and Methods. (C) Analysis of RT–PCR products from the three parts (lanes 1, 2 and 3) of MYA2 shown in (B) using total RNA from leaves of 4-week-old wild-type (W), heterozygous mutant (He) and homozygous mutant (Ho) plants. In W and He plants, RT–PCR products from three parts, 1, 2 and 3 (lanes 1, 2 and 3, respectively), were expressed, whereas only product from part 1 was expressed in Ho plants. The presence of cDNA template in all reactions was confirmed by amplification of a product by the EF1-α-specific primers (product size 450 bp). (D) Immunoblotting of crude protein samples from W and Ho plants of MYA2 with the affinity-purified antibody against MYA2 peptide (a) and antiserum against P-135-ABP villin (b). In Ho plants, the 170 kDa polypeptide recognized by anti-MYA2 peptide antibody was not detected. On the other hand, a 123 kDa band recognized by antiserum against villin (Yokota et al. 2003) was found in both W and Ho plants. (E) Double immunostaining of epidermal cells in homozygous mutant leaf with anti-actin antibody (a) and anti-MYA2 peptide antibody (b). In contrast to wild-type plants (Fig. 5), only very few small dots with weak intensity were observed. Scale bar = 10 µm.
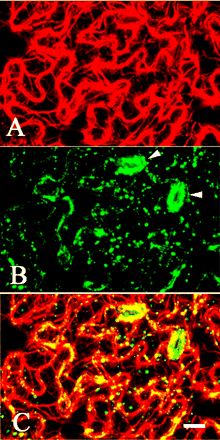
Fig. 5 Immunolocalization of actin and MYA2 in epidermal cells of leaf. Double labeling was carried out with anti-actin antibody (A) and affinity-purified antibody against MYA2 peptide (B). A merged image is shown in (C). Most of the small dots and punctate signals frequently formed lines or clusters co-localized with actin filaments. Labeling was very prominent in guard cells (arrowhead in B). Scale bar = 10 µm.
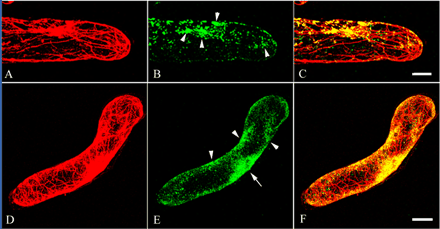
Fig. 6 Immunolocalization of actin and MYA2 in root hair cells and suspension-cultured cells. Double labeling of root hair cells (A–C) and suspension-cultured cells (D–F) was carried out with anti-actin antibody (A and D) and affinity-purified antibody against MYA2 peptide (B and E). Merged images are shown in (C) and (F). In root hair cells and suspension-cultured cells, a cluster of small dot signals was observed in some parts (arrowheads in B and E), especially around the nucleus (arrow in E). Scale bars = 10 µm.
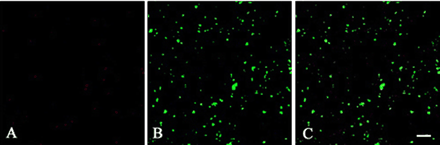
Fig. 7 Effect of LB on the distribution of MYA2 and catalase in epidermal cells of leaf. Wild-type plants were treated with LB and then double labeled for actin (A) and MYA2 (B). A merged image is shown in (C). LB treatment caused the disappearance of continuous lines or clusters of dot signals for MYA2 (B). No filamentous signal with anti-actin antibody was observed (A). Scale bar = 10 µm.
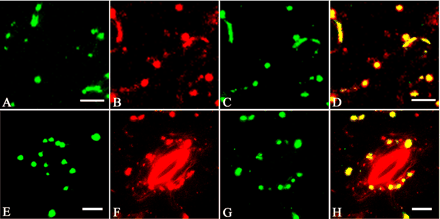
Fig. 8 Co-localization of MYA2 and peroxisomes in transgenic Arabidopsis expressing GFP-tagged peroxisomal targeting signal peptide 1. GFP signals in epidermal (A) and guard cells (E) from live transgenic leaves. Double labeling of epidermal (B, C and D) and guard cells (F, G and H) with the affinity-purified antibody against MYA2 (B and F) and the anti-GFP antibody (C and G). Merged images are shown in (D) and (H). Some large, punctate signals from anti-MYA2 antibody overlapped with those from anti-GFP antibody, whereas many small, green dots were unique. Scale bars = 10 µm (A–D) and 5 µm (E–H).
Fig. 9 Immunolocalization of catalase in wild-type and homozygous mutant plants of MYA2. Double labeling of epidermal cells from leaves of wild-type (A–C) and homozygous mutant plants (D–F) was carried out with anti-actin antibody (A and D) and the antibody against catalase (B and E). Merged images are shown in (C) and (F). Small dots and punctate signals appeared to form continuous lines or clusters, which were co-localized with actin filaments, in both wild-type and homozygous mutant plants. Scale bar = 10 µm.
Abbreviations
- BSA
bovine serum albumin
- CBB
Coomassie brilliant blue
- GFP
green fluorescent protein
- LB
latrunculin B
- PBS
phosphate-buffered saline
- RT–PCR
reverse transcription–polymerase chain reaction
References
Adelstein, R.J. and Eisenberg, E. (
Alonso, J.M., Stepanova, A.N., Leisse, T.J., Kim, C.J., Chen, H., et al. (
Collings, D.A., Harper, J.D.I. and Vaughn, K.C. (
Dai, J., Ting-Beahl, H.P., Hochmuth, R.M., Sheetz, M.P. and Titus, M.A. (
Edwards, K., Johnstone, C. and Thompson, C. (
Falk, D.L., Wessele, D., Jenkins, L., Pham, T., Kuhl, S., Titus, M.A. and Soll, D.R. (
Higashi-Fujime, S., Ishikawa, R., Kagami, O., Kurimoto, E., Kohama, K. and Hozumi, T. (
Holweg, C. and Nick, P. (
Jedd, G. and Chua, N.H. (
Kinkema, M. and Schiefelbein, J. (
Kinkema, M., Wang H. and Schiefelbein, J. (
Kohama, K. (
Laemmi, U.K. (
Logan, D.C. and Leaver, C.J. (
Mano, S., Nakamori, C., Hayashi, M., Kato, A., Kondo, M. and Nishimura, M. (
Mathur, J., Mathur, N. and Hulskamp, M. (
Mathur, J., Szabados, L., Schaefer, S., Grunenberg, B., Lossow, A., Jonas-Straube, E., Schell, J. and Koncz-Kalman, Z. (
Mermall, V., Post, P.L. and Moosker, M.S. (
Ostap, E. and Pollard, T. (
Reichelt, S. and Kendrick-Jones, J. (
Tominaga, M., Kojima, H., Yokota, E., Orii, H., Nakamori, R., Katayama, E., Anson, M., Shimmen T. and Oiwa K. (
Towbin, H., Staehelin, T. and Gordon, J. (
Wang, Z. and Pesacreta, T.C. (
Yamamoto, K., Kikuyama, M., Sutoh-Yamamoto, N. and Kamitsubo, E. (
Yamashita, R.A., Sellers, J.R. and Anderson, J.B. (
Yokota, E., McDonald, A.R., Liu, B., Shimmen, T. and Palevitz, B.A. (
Yokota, E., Mimura T., Shimmen T. (
Yokota, E. and Shimmen, T. (
Yokota, E., Takahara, K. and Shimmen T. (
Yokota, E., Vidali, L., Tominaga, M., Tahara, H., Orii, H., Morizane, Y., Hepler, P.K. and Shimmen, T. (