-
PDF
- Split View
-
Views
-
Cite
Cite
Yoshihiro Kobae, Tetsuro Sekino, Hirofumi Yoshioka, Tsuyoshi Nakagawa, Enrico Martinoia, Masayoshi Maeshima, Loss of AtPDR8, a Plasma Membrane ABC Transporter of Arabidopsis thaliana, Causes Hypersensitive Cell Death Upon Pathogen Infection, Plant and Cell Physiology, Volume 47, Issue 3, March 2006, Pages 309–318, https://doi.org/10.1093/pcp/pcj001
Close - Share Icon Share
Abstract
Plants contain a large number of ATP-binding cassette (ABC) transporters belonging to different subclasses. AtPDR8 is the only member of the pleiotropic drug resistance (PDR) ABC transporter subclass in Arabidopsis that is constitutively highly expressed. In transgenic Arabidopsis plants harboring the AtPDR8 promoter fused to β-glucuronidase (GUS), reporter expression was shown to be strong in the stomata and hydathode. In the stomata, transcripts of AtPDR8 were particularly frequent in the cells surrounding air spaces. Subcellular fractionation and immunochemical analysis showed that AtPDR8 was localized in the plasma membrane. When a knockout mutant of AtPDR8 (atpdr8) was infected with bacterial and oomycete pathogens, the plants exhibited chlorotic lesions and a hypersensitive response (HR)-like cell death. Cell death was detected in the atpdr8 mutants within 10 h of infection with the virulent bacterial pathogen, Pseudomonas syringae. As a result, the growth of P. syringae in the leaves of the atpdr8 mutant was reduced to 1% of that in the wild type. The defense response genes, PR-1, PR-2, PR-5, VPEγ, AtrbohD and AtrbohF were highly expressed when the mutant plants were grown under non-sterile conditions. The expression of the AtPDR8 gene was enhanced by infection of virulent and avirulent bacterial pathogens. Our results indicate that AtPDR8 is a key factor controlling the extent of cell death in the defense response and suggest that AtPDR8 transports some substance(s) which is closely related to the response of plants to pathogens.
Introduction
ATP-binding cassette (ABC) transporters are widely distributed in prokaryotes and eukaryotes, and form a large protein family. These transporters are involved in the membrane transport of a wide range of structurally and functionally unrelated compounds, such as glutathione conjugates, lipids, inorganic acids, peptides, secondary metabolites, toxins and drugs (Sánchez-Fernández et al. 2001, Holland et al. 2003, Martinoia et al. 2002). We have focused on the physiological function of a member of the pleiotropic drug resistance (PDR) subfamily of ABC transporters, members of which have been identified in yeast and plants, but not so far in animals. PDR-like ABC transporters were first described in yeast, where they confer resistance to a multitude of toxins and organic acids (Decottignies and Goffeau 1997, Piper et al. 1998). Many natural products synthesized in plant cells are candidate substrates for ABC transporters, and are transported across the membranes of plant tissues. PDRs are full-size ABC transporters like the multidrug-resistant protein (MDR) and multidrug resistance-associated protein (MRP) (Jasinski et al. 2003). They consist of double sets of two basic structural elements; a transmembrane domain (TMD) made up of six α-helices, and a cytosolic domain containing a nucleotide-binding domain (NBD). In contrast to MDRs and MRPs, PDRs have an inverted arrangement of the structural elements, the NBD being located at the N-terminus.
A few members of the PDR subfamily have been characterized in plants. For example, the gene for a PDR in an aquatic plant Spirodela polyrrhiza (SpTUR2) was demonstrated to be up-regulated by treatment with abscisic acid and by environmental stresses such as high salt and low temperature (Smart and Fleming 1996). Similarly, the gene for a tobacco PDR (NpPDR1) was shown to be up-regulated by sclareol, an antifungal diterpene produced in tobacco leaves (Jasinski et al. 2001). Furthermore, in cultured cells, NpPDR1 excretes sclareol. Various microbial elicitors induce another tobacco PDR (NtPDR1) (Sasabe et al. 2002), which has been demonstrated to be involved in pathogen resistance (Stukkens et al. 2005). Recently, Lee et al. (2005) demonstrated that Arabidopsis AtPDR12 contributes to lead resistance. These observations suggest the involvement of PDRs in responses to pathological and abiotic stresses, but there is little experimental evidence concerning the physiological role of PDR transporters. The actual substrate is unknown for most PDRs, exceptions being SpTUR2 and NpPDR1.
The PDR subfamily of Arabidopsis (Arabidopsis thaliana) has 15 members (Jasinski et al. 2003). AtPDR8 transcripts are the most abundant (81 expressed sequence tags) of the 15 PDRs. The corresponding protein has been annotated in proteomic studies of Arabidopsis membrane proteins of single-membrane organelles (Sazuka et al. 2004) and reported to occur in plasma membrane proteins (Alexandersson et al. 2004), as well as in chloroplasts (Kleffmann et al. 2004). In the present study, we determined its intracellular location using a specific antibody, examined its tissue specificity and characterized a knockout mutant. AtPDR8 (At1g59870) together with AtPDR1 (At3g16340) and AtPDR7 (At1g15210) forms a subgroup in the phylogenetic tree distinct from the characterized subgroups of PDRs (Jasinski et al. 2003). We found that the atpdr8 mutant showed accelerated cell death and lesions in leaves, even when infected with a virulent pathogen, and defense response genes were excessively induced. We discuss the physiological role of PDR8 in plant defense against pathogens.
Results
Immunodetection of AtPDR8 in plant tissues
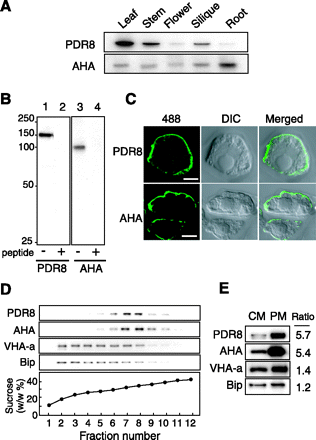
To probe for AtPDR8, we prepared an antibody against an internal sequence of AtPDR8 and subjected it to affinity purification. No other sequence identical to the antigen peptide is present among the Arabidopsis proteins. The antibody gave a band at 150 kDa with membranes from leaves, stems and siliques (Fig. 1A), but a relatively weak band in flowers and roots. This result is in agreement with the transcript levels reported in DNA microchip analysis (www.genevestigator.ethz.ch) (Zimmermann et al. 2004), indicating a correlation between transcript and protein levels. A typical plasma membrane protein, H+-ATPase (AHA), was detected in all the tissues examined. The specificity of the antibody was confirmed by competition assay (Fig. 1B). The size of the 150 kDa immunostained band was close to the calculated value (165 kDa).
Localization of AtPDR8 in plasma membranes
To determine the intracellular location of AtPDR8, we performed in situ immunolocalization using suspension-cultured cells. Using anti-PDR8 antibody and Alexa Fluor 488-labeled secondary antibody, a clear green fluorescent signal was seen surrounding the cells (Fig. 1C). This signal was similar to that observed using the antibody to AHA. The pre-immune serum did not give a signal.
Next, we subjected microsomes prepared from stems to sucrose density gradient centrifugation (Fig. 1D). AtPDR8 was recovered mainly in fractions 7 and 8 together with the AHA. In contrast, subunit a of the vacuolar H+-ATPase (VHA-a) and an endoplasmic reticulum luminal protein (BiP) were recovered in the lighter fractions 2–8. We compared the intensities of the PDR8, AHA, VHA-a and BiP bands in the crude and purified plasma membrane fraction. AtPDR8 was enriched in the plasma membrane fraction derived from crude membranes as was AHA, whereas the vacuolar membrane and endoplasmic reticulum markers were not (Fig. 1E).
AtPDR8 was predicted to be a chloroplast protein by TargetP (http://www.cbs.dtu.dk/services/TargetP/), and conflicting results have been obtained in proteomic studies; PDR8 was detected in the plasma membrane (Alexandersson et al. 2004) as well as in chloroplasts (Froehlich et al. 2003, Kleffmann et al. 2004). In the present experiment, we did not detect AtPDR8 in chloroplasts isolated from leaves (data not shown). Taking all our localization results together, we can exclude a plastid location for AtPDR8.
Expression of AtPDR8 in stomata and hydathodes
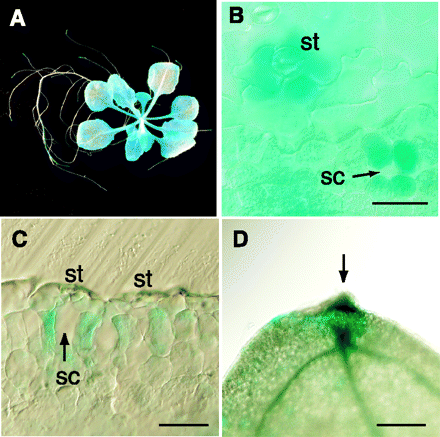
To examine the cell specificity of AtPDR8 expression, Arabidopsis was transformed with AtPDR8 promoter–GUS DNA constructs. β-Glucuronidase (GUS) staining was examined in seven independent lines of seedlings grown on sterile agar plates containing culture medium. No significant differences in expression were observed between the lines. GUS staining was observed in leaves and root tips (Fig. 2A), and was strongest around stomata after 3 h (Fig. 2B). In longitudinal sections, intense staining was restricted to the cells surrounding the air space (substomatal cavity) beneath the stomata (Fig. 2C). GUS activity was detected throughout the mesophyll tissue after staining for 8 h. The leaves of mature plants grown on non-sterile vermiculite exhibited more activity in the hydathodes at the leaf edge than in other parts (Fig. 2D). We also tested the effect of mechanical wounding of seedlings and mature leaves, and found that these treatments had no effect on GUS staining. Furthermore, aged leaves displayed the same pattern and activity as seedlings, indicating that the AtPDR8 promoter is unaffected by leaf senescence. The same observation can be made when analyzing public available microchip data (www.genevestigator.ethz.ch).
Enhanced cell death in AtPDR8 knockout mutants infected with an oomycete pathogen
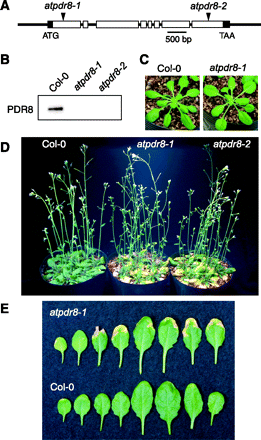
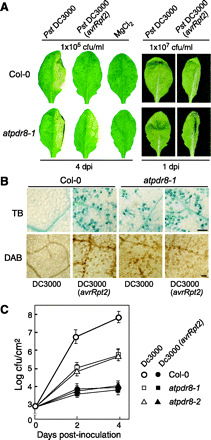
To determine the physiological role of AtPDR8, we analyzed two distinct homozygous T-DNA insertion mutants on a Columbia-0 background, atpdr8-1 (Salk_000578) and atpdr8-2 (Salk_142256) (Fig. 3A). Immunoblotting showed that neither mutant produced PDR8 protein (Fig. 3B). Both mutant plants were morphologically normal when grown under sterile conditions (Fig. 3C). On non-sterile vermiculite, however, they displayed lesions and chlorosis at their leaf margins (Fig. 3D, E); the lesions on most leaves were severe regardless of growth stage (Fig. 3E).
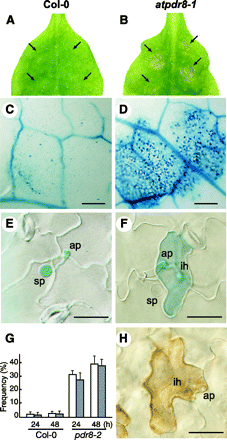
To examine the possibility that atpdr8 plants were defective in resistance to pathogens, they were inoculated with Phytophthora infestans race 0 (Yoshioka et al. 2003), which is a non-host oomycete pathogen (Huitema et al. 2003). Wild-type plants appeared healthy where droplets of P. infestans zoospores had been applied (Fig. 4A). However, atpdr8-1 mutants exhibited macroscopic cell death with brown lesions 4 d post-inoculation (d.p.i.) (Fig. 4B). We monitored cell death in leaves by trypan blue staining. A few stained epidermal cells appeared in wild-type plants (3 d.p.i.) (Fig. 4C). In contrast, the trypan blue dye was precipitated in atpdr8-1 epidermal and mesophyll cells where the zoospore droplets had been applied on the leaves (Fig. 4D). While little penetration of P. infestans hyphae into epidermal cells was observed in the wild-type plant (Fig. 4E, G), more penetration and growth of P. infestans hyphae in the atpdr8-1 mutant were observed, but they were restricted to epidermal cells, which underwent cell death on trypan blue staining (Fig. 4F, G). These infected cells stained intensely with 3,3′-diaminobenzidine (DAB), pointing to the generation of a high level of H2O2 (Fig. 4H). The frequency of cell death induced in the epidermal cells in the atpdr8 mutants was >30-fold that in wild-type plants (Fig. 4G). The results show that cells of the atpdr8 mutants could not prevent the non-host pathogen penetration and resulted in accelerated cell death.
The virulent pathogen Pst DC3000 also facilitates cell death in the atpdr8 mutant
To determine whether atpdr8 mutants are defective in resistance to bacterial pathogens, we inoculated wild type and atpdr8 mutants with virulent and avirulent strains of the bacterial pathogen Pseudomonas syringae pv. tomato (Pst) DC3000. The virulent bacterial pathogen Pst DC3000 produces disease symptoms characterized by chlorosis and water soaking of infected leaves (Katagiri et al. 2002). When such strains were transformed with the avirulence (avr) gene avrRpt2 they lost their virulence and did not produce disease symptoms upon inoculation (Dong et al. 1991). The hypersensitive response (HR) against the avirulent derivative Pst DC3000 (avrRpt2) is triggered by the resistance protein RPS2 of Arabidopsis Columbia-0 (Kunkel et al. 1993, Yu et al. 1993). Both wild-type and atpdr8 mutants inoculated with a low concentration [1×105 colony-forming units (c.f.u.) ml–1] of the virulent Pst DC3000 strain showed macroscopic lesions 4 d after inoculation (Fig. 5A), although growth of the bacteria was drastically reduced in the atpdr8 plants (see below). Furthermore, the atpdr8 plants also exhibited mild lesions when inoculated with the avirulent Pst DC3000 (avrRpt2) strain, which produced no macroscopic lesions in wild-type plants. The MgCl2 solution used as a negative control did not produce lesions.
A significant macroscopic HR was observed in both wild-type and mutant plants 1 d after inoculation with avirulent Pst DC3000 (avrRpt2) (Fig. 5A). The HR induced in wild-type and atpdr8 plants was visualized by trypan blue and DAB staining (Fig. 5B). The growth test revealed that Pst DC3000 (avrRpt2) hardly grew in the wild-type or mutant plants (Fig. 5C). This observation indicates that the atpdr8 mutation does not affect the disease resistance mediated by RPS2, which has been reported to be involved in resistance to P. syringae strains (Kunkel et al. 1993, Yu et al. 1993). Interestingly, Pst DC3000 induced significant cell death only in atpdr8 plants. The virulent strain grew well in the wild-type plants, compared with which its growth in atpdr8 mutants was reduced to 1%. The atpdr8 plants generated H2O2 and underwent significant cell death when inoculated with a high concentration (1×107 c.f.u. ml–1) of either the virulent or avirulent strain (Fig. 5B).
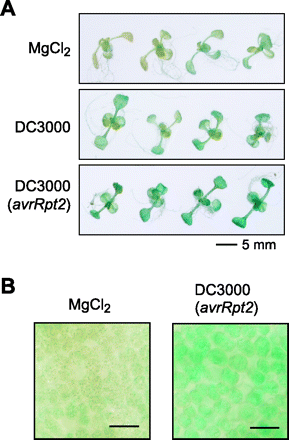
To test the effect of bacterial infection on the expression of the AtPDR8 gene, Arabidopsis containing the AtPDR8 promoter–GUS were infected with the virulent Pst DC3000 or the avirulent Pst DC3000 (avrRpt2). The plantlets were submerged in bacterial solution (1×108 c.f.u. ml–1) and the GUS staining was carried out after 12 h. Although this method is mild compared with the infiltration method using a needleless syringe, the GUS activity was enhanced by inoculation with both strains, but not by MgCl2 (Fig. 6A). The enhanced GUS activity was detected even in the leaf mesophyll cells (Fig. 6B).
Enhanced expression of PR genes in atpdr8 mutants
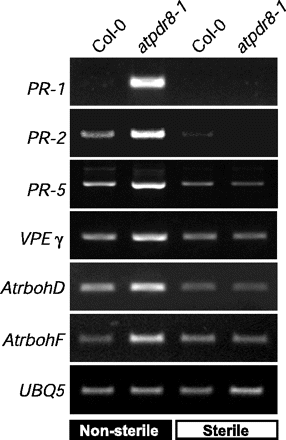
Plant defense against pathogens is associated with the expression of numerous defense genes, including pathogenesis-related (PR) and defense-related genes (Glazebrook 2001). We measured levels of the mRNA of these genes by reverse transcription–PCR (RT–PCR) with specific primers for PR-1, PR-2 and PR-5 using total RNA from wild-type and atpdr8 plants (Fig. 7). PR-1 and PR-5 have antifungal and anti-oomycete activities and PR-2 is a β-1,3-glucanase that degrades polysaccharides in mycelial walls.
Arabidopsis plants were grown under sterile conditions (in sterilized agar plates) or non-sterile conditions (in pots with unsterilized vermiculite). Under non-sterile conditions, spontaneous, moderate infection with pathogens, which exist in unsterilized vermiculite, may occur and facilitates the lesion formation in atpdr8 plants as shown in Fig. 3. Under sterile conditions, the expression of PR-2 and PR-5 was very low in wild-type and atpdr8 plants, and transcripts for PR-1 were not detectable at all (Fig. 7). When wild-type plants were grown in non-sterile conditions, transcript levels of PR-2 and PR-5 were slightly increased compared with that in sterile conditions, while the level of PR-1 was still undetectable. In contrast, in atpdr-8 plants grown in non-sterile conditions, expression of PR-1 was strong and transcript levels for PR-2 and PR-5 were markedly increased compared with wild-type plants grown under the same conditions.
We also examined the expression of VPEγ, AtrbohD, AtrbohF and UBQ5. VPEγ (vacuolar processing enzymeγ) is a caspase-like cysteine protease that regulates vacuole-mediated cell dismantling during cell death (Hatsugai et al. 2004). AtrbohD and AtrbohF encode NADPH oxidases (respiratory burst oxidases) involved in generating reactive oxygen intermediates (Torres et al. 2002). The levels of VPEγ, AtrbohD and AtrbohF mRNAs were also markedly higher under non-sterile conditions in the atpdr8 mutants than in wild-type plants (Fig. 7).
Discussion
In this study, we determined the subcellular location and tissue specificity of AtPDR8, and investigated its physiological function. AtPDR8 consists of 1,469 amino acid residues (pI = 8.08) and has 13 predicted TMDs. Immunochemical analyses revealed that AtPDR8 is located in the plasma membrane where it may export substrates synthesized in the cell, or excrete xenobiotic compounds entering the cell. AtPDR8 accumulated predominantly in leaves and was detected at a relatively high level in stems and siliques, but was insignificant in flowers and roots.
The observation that spontaneous chlorotic lesions arise in the leaves of atpdr8 mutants under non-sterile conditions led us to study the function of AtPDR8 in defense against pathogens. First, we examined the possibility that atpdr8 mutants were defective in the non-host response by inoculating an oomycete pathogen P. infestans. Infection of the non-host pathogen P. infestans in Arabidopsisatpdr8 mutants resulted in the enhancement of penetration of P. infestans deep into epidermal cells and wide-ranging cell death in the atpdr8 mutants, although the pathogen penetration and cell death were not observed in the wild-type plants (Fig. 4). The suppression of pathogen penetration at the surface of plant organs is the first line of non-host resistance (Thordal-Christensen 2003, Mysore and Ryu 2004). Three penetration resistance-defective lines, penetration (pen) mutants, were reported for Arabidopsis, which are disabled in non-host penetration resistance against barley powdery mildew, Blumeria graminis (Collins et al. 2003). The PEN1 gene encodes syntaxin that is a component of the SNARE complex. SNARE complex-mediated exocytosis was proposed to be involved in non-host resistance. The PEN2 glycosyl hydrolase localizes to peroxisomes and acts as a component of an inducible pre-invasion resistance mechanism (Lipka et al. 2005). The pen3 mutant has not been characterized. Previous studies and the present study suggest that export of secondary metabolites or phytoalexin using exocytosis and/or membrane transporters may be tightly related to non-host resistance. The atpdr8 mutants compromised penetration resistance at the leaf surface, and the epidermal cells invaded by pathogens showed enhanced cell death (Fig. 4). Interestingly, the mesophyll cells also allowed enhanced cell death that may be caused by pathogen invasion. The present results strongly suggest that the substrate for AtPDR8 may protect the penetration of P. infestans hyphae into plant tissues.
Infection of Arabidopsis plants with either a virulent or an avirulent strain of the biotroph bacterium, P. syringae, resulted in enhanced cell death accompanied by the generation of H2O2 in the atpdr8 lines. Interestingly, bacterial growth of the virulent strain was strongly suppressed in the atpdr8 mutants compared with that in wild-type plants, while the growth rate of an avirulent strain was suppressed in both the wild type and mutants (Fig. 5). Thus there may be two reasons for growth suppression of virulent bacteria; (i) reactive oxygen species generated by infected plant cells may restrict the growth; or (ii) death of infected plant cells may reduce nutrient compounds for biotroph organisms, such as P. syringae.
We also semi-quantitatively investigated the expression of several defense-related genes. Genes encoding PR-1, PR-2, PR-5, VPEγ, AtrbohD and AtrbohF were highly expressed under non-sterile conditions, but not under sterile conditions, and their mRNA levels were several times higher than in the wild type (Fig. 7). PR genes are induced under biotic and abiotic stresses (Srivastava et al. 2004) and during senescence (Quirino et al. 2000). Recently, VPEγ has been suggested to be a caspase-like cysteine protease involved in virus-induced programmed cell death (Hatsugai et al. 2004). AtrbohD and AtrbohE are enzymes required for the oxidative burst in response to pathogens (Torres et al. 2002). Although these defense-related genes were highly expressed in the atpdr8 mutants (Fig. 7) and bacterial growth was suppressed (Fig. 5), the cell death observed in the mutants did not result in the enhanced disease resistance (Fig. 3–5). It is thought that a toxic substance is produced by pathogen attack and this is accumulated in the atpdr8 mutant and triggers cell death. The present study with oomycete and bacterial pathogens suggests that AtPDR8 may export a compound from plant cells for protection from pathogen invasion and that the compound may be toxic to plant cells when accumulated at high concentrations. In the mutant, this hypothetical compound cannot be exported, triggering massive induction of PRs, VPEγ, AtrbohD and AtrbohF and causing accelerated cell death even in tissues infected by a virulent pathogen. In addition, the high concentration of reactive oxygen species generated by the induced enzymes (AtrbohD and AtrbohF) may injure the cells. A second possibility is that the compound accumulated in the mutants is harmful, and damages the plants’ own cells.
The nature of the transported substrate is key to understanding the physiological function of AtPDR8. Very little is known about the potential substrates of plant PDRs (Martinoia et al. 2002, Jasinski et al. 2003). A tobacco PDR (NpPDR1) is thought to excrete sclareol, produced in the leaf epidermis as an antifungal diterpene (Jasinski et al. 2001, Stukkens et al. 2005). In leaves and cell cultures, accumulation of NpPDR1 was strongly enhanced by sclareol and the cells acquired the ability to excrete a sclareol analog. Transcripts of AtPDR12 (At1g15520) are low under normal conditions, but increase in response to fungal infection and sclareol treatment (Campbell et al. 2003). A PDR in S. polyrrhiza was induced by treatment with the sesquiterpene, ABA (Smart and Fleming 1996). This S. polyrrhiza PDR is also able to transport sclareol (van den Brüle et al. 2002).
Seo et al. (2003) recently reported that a diterpene functioned as an endogenous signal activating defense responses to viral infection and wounding in tobacco. The diterpene at submicromolar levels activated defense-related mitogen-activated protein kinases, such as wound-induced protein kinase (WIPK) and salicylic acid-induced protein kinase (SIPK), and induced several defense-related genes. It has also been reported that transient expression of a constitutively active mutant kinase StMEK1DD, which acts upstream of WIPK and SIPK, induces NbrbohB, and causes oxidative burst-mediated cell death in tobacco (Yoshioka et al. 2003). Plant terpenoids are thus candidate substrates for AtPDR8. Recent research has demonstrated that Arabidopsis produces a range of these compounds (von Roepenack-Lahaye et al. 2004) and possesses genes for diterpene synthase (Tholl et al. 2005). AtPDR8 was highly expressed in cells surrounding the substomatal cavity and the hydathode (Fig. 2), from which pathogens can enter plant tissue (Hugouvieux et al. 1998). Thus, AtPDR8 may export terpenes or related compounds to block pathogen invasion. Interestingly, Niinemets et al. (2004) reported a high concentration of some volatile terpenoids in the leaf boundary layer when the stomata were closed.
Recently, Stukkens et al. (2005) provided evidence for a critical role for PDR transporters in the defense response. Transgenic tobacco plants in which NpPDR1 expression was prevented by RNA interference showed reduced resistance to the fungus Botrytis cinerea. This phenotype is in contrast to that of atpdr8 mutants which suppressed the growth of the bacterial virulent strain. In addition, the expression profile of NpPDR1 is different from that of AtPDR8. AtPDR8 was highly, constitutively accumulated in the leaf cells, while NpPDR1 was expressed in glandular trichomes and the protein level was low without pathogen inoculation. In addition, the mRNA level of AtPDR12, the nearest equivalent to NpPDR1 in Arabidopsis, was low under normal conditions, but increased in response to fungal infection (Campbell et al. 2003). Moreover, the advanced analysis tool ATTED-2 (A. thaliana trans-factor and cis-element prediction database) (Obayashi et al. 2004) revealed that expression of AtPDR8 and AtPDR12 is distinctly regulated by biotic or abiotic stresses. These data suggest that the phytopathological role of AtPDR8 is different from that of NpPDR1 or AtPDR12.
The DNA array analyses revealed that expression of AtPDR8 was enhanced by avirulent Pst DC3000 (Glombitza et al. 2004) or flg22 peptide that is a conserved region of flagellin (Zipfel et al. 2004). This was confirmed by the present experiments. In transgenic plants expressing an AtPDR8 promoter–GUS construct, GUS expression was enhanced by inoculation of Pst DC3000 or Pst DC3000 (avrRpt2) (Fig. 6). Probably, inoculation of the wild-type plant with virulent and avirulent strains induces the production of a toxic substrate for AtPDR8 and expression of the AtPDR8 gene. However, the enhancement of transcription was not reflected in the protein level of AtPDR8. In the present experiments, the protein level was not affected significantly by inoculation with virulent Pst DC3000, avirulent Pst DC3000 (avrRpt2) or non-specific elicitor flg22 (data not shown). The protein accumulation is generally regulated by the rates of biosynthesis and degradation of proteins. Bacterial inoculation may stimulate both synthesis and degradation of AtPDR8. Here, we note that the constant level of AtPDR8 in plant tissue may be related to the quick response to pathogen infection and that the enhancement of AtPDR8 gene expression by pathogen inoculation may be essential for maintenance of theAtPDR8 level.
In our experiments, systemic acquired resistance (SAR) could be induced in the atpdr8 mutant plants. We applied the avirulent strain to leaves and tested for induction of PR-1, a marker of SAR, in distant leaves. PR-1 was strongly up-regulated in the mutant plants just as in the wild-type plants (data not shown). Therefore, we consider that PDR8 transports a compound that is closely related to the primary defense response to pathogens but not to SAR.
The present study clearly demonstrates the central involvement of AtPDR8, an ABC transporter, in non-host resistance of plants to pathogens. AtPDR8 may excrete toxic compound to protect the plants from invasion and infection by pathogens, and this substance may be harmful to plant cells if accumulated in the tissues. Transport of terpenes by AtPDR8 should be examined in vitro. Further study of the actual substrate of AtPDR8 and the functional regulation of the protein should provide information on its physiological role.
Materials and Methods
Plant materials
T-DNA PDR8 insertion lines (atpdr8-1 and atpdr8-2; registration no. Salk_000578 and Salk_142256) of Arabidopsis (ecotype, Columbia-0) were obtained from the Arabidopsis Biological Resource Center. Information concerning the insertion mutants was obtained from The Salk Institute Genomic Analysis Laboratory website (http://signal.salk.edu). A homozygous transgenic line was selected by PCR using the T-DNA left border primer 5′-GCGTGGACCGCTTGCTGCAACT-3′ and a PDR8-specific primer, 5′-TGAAGATATCTTCTCATCTGGTTC-3′ (atpdr8-1) or 5′-TGATTGGTACAGTCTTCTGGC-3′ (atpdr8-2), and the T-DNA insertion sites were confirmed. Suspension-cultured cells (T87) of Arabidopsis (Col-0) were supplied by RIKEN Bio Resource Center and cultured as described previously (Kobae et al. 2004).
Membrane preparation
Plant tissues were homogenized in a medium containing 50 mM Tris–HCl, pH 8.0, 250 mM sorbitol, 10 mM EDTA, 4 mM dithiothreitol, 0.1% 2-mercaptoethanol and 100 µM p-(amidinophenyl)methanesulfonyl fluoride hydrochloride. The homogenate was filtered and centrifuged at 10,000×g for 10 min. The supernatant was centrifuged at 100,000×g for 30 min and the resulting pellet was suspended in the homogenizing medium. The suspension was layered on a linear sucrose density gradient [11 ml, 15–45% (w/w)], centrifuged at 77,000×g for 17 h and collected in 0.9 ml fractions. Plasma membranes were purified from 4-week-old rosette leaves (60 g) with an aqueous two-phase partitioning system (Alexandersson et al. 2004).
Antibody preparation, immunoblotting and immunocytochemistry
A peptide corresponding to an internal sequence of AtPDR8 (positions 810–823; NEDADQGKD.P.M.RRS+C; Cys, additional residue for linkage with the carrier protein) was synthesized. This peptide was linked to keyhole limpet hemocyanin and injected into two rabbits. The peptide and antibodies were prepared by Operon Biotechnology (Tokyo, Japan). The AtPDR8-specific IgG was purified using Sepharose resin linked with the corresponding antigen peptide. The peptide-specific antibodies against AHA, VHA-a and BiP were described previously (Kobae et al. 2004). The anti-AHA antibodies react with at least AHA1, AHA2 and AHA3. SDS–PAGE and immunoblotting were carried out as described previously (Kobae et al. 2004).
Suspension-cultured cells (T87) were fixed in 3.5% (w/v) paraformaldehyde, treated with 0.1% (w/v) pectolyase Y-23 (Kikkoman, Tokyo, Japan) and permeabilized with 0.3% Triton X-100. The fixed cells were incubated with affinity-purified anti-PDR8 or anti-AHA antibodies and visualized with Alexa Fluor 488-conjugated goat anti-rabbit antibody (Molecular Probes, Eugene, OR, USA). Fluorescence was viewed with a confocal laser scanning microscope (model FV500, Olympus, Tokyo, Japan).
Pathogen strains and pathology tests
A culture of Pst DC3000 was incubated overnight in the presence of kanamycin (25 mg l–1) and rifampicin (100 mg l–1) (Katagiri et al. 2002), washed once in 10 mM MgCl2, and suspended at a concentration of 1×105 c.f.u. ml–1 for in planta growth assay, and at 1×107 c.f.u. ml–1 for HR tests. An avirulent strain, Pst DC3000 (avrRpt2), was kindly provided by Dr. Ken Shirasu (John Innes Centre, Norwich, UK). Bacterial suspensions were infiltrated onto the surfaces of abaxial leaves using needleless syringes, and bacterial growth was monitored as described (Katagiri et al. 2002). Phytophthora infestans race 0 was maintained on tubers of susceptible potatos and a suspension of the zoospores was prepared (Yoshioka et al. 1999). Samples (5 µl) of a suspension containing 1,000 zoospores were applied to the adaxial sides of leaves. After incubation under high humidity at 20°C, HR and H2O2 were detected by staining with trypan blue (Shirasu et al. 1999) and with DAB (Yoshioka et al. 2003), respectively.
Expression of pathogenesis-related genes
Total RNA was prepared from leaves of 5-week-old wild-type and atpdr8 plants and used as template for RT–PCR. Primers for the RT–PCR analysis were PR-1 (At2g14610) forward (5′-GTAGGTGCTCTTGTTCTTCCC-3′) and reverse (5′-CACATAATTCCCACGAGGATC-3′). Primers for PR-2 (At3g57260), PR-5 (At1g75040) and ubiquitin 5 (At3g62250), designed by Glazebrook et al. (1996), were used. Primers for VPEγ (At4g32940) were (forward, 5′-GAAAACGACGACGATTCTAACTCCG-3′; reverse, 5′-GTCTCAGTCTGTAAATTGTGCATAC-3′); AtrbohD (At5g47910) (forward, 5′-CATGAACTTGGGATTCTACGAGG-3′; reverse, 5′-AGACCTTTGAGTGCGTGGATGG-3′); and AtrbohF (At1g64060) (forward, 5′-GACGATGACACAATCGTTCTTCGT-3′; reverse, 5′-TTGAGCGAAATCGGAGCGATAGAT-3′).
AtPDR8 promoter–GUS expression analysis
Information on the AtPDR8 gene was taken from the databases (gene ID, At1g59870; protein accession number, AAD39329; NCBI reference number, NP_176196). A genomic sequence (–1735 to +270 from the first ATG) was determined by PCR using primers 5′-CACCTAAGACTATGTGGGAACAGCGAGAACTTCAGTTGTG-3′ and 5′-ACCTCCTTGCTCATGAGCTGGTTACCGTAAACATC-3′. The amplified fragment was treated as the AtPDR8 promoter, ligated into GATEWAY entry vector pENTR/D-TOPO by a TOPO cloning reaction (Invitrogen, Carlsbad, CA, USA) and introduced upstream of the promoter-less GUS gene on binary vector pGWB203, using the GATEWAY system (Invitrogen), in order to produce a translational fusion with GUS. The construct was introduced into Agrobacterium tumefaciens strain GV3101::PM90 and plants were transformed by the floral dip method (Clough and Bent 1998). Transformed plants of T1 or T2 were selected in medium containing hygromycin (15 mg l–1) and kanamycin (35 mg l–1) and stained for GUS using 5-bromo-4-chloro-3-indolyl-β-D-glucuronide (X-Gluc) (Abel and Theologis 1998).
In some experiments, 2-week-old plantlets expressing AtPDR8 promoter–GUS were submerged in a solution containing the virulent pathogen Pst DC3000 or the avirulent strain Pst DC3000 (avrRpt2). Bacterial pathogens were suspended at a final concentration of 1×108 c.f.u. ml–1. After 12 h, the plantlets were stained with X-Gluc. As a control experiment, plantlets were treated with 10 mM MgCl2.
Acknowledgments
We thank Ken Shirasu for providing Pst DC3000 (avrRpt2), and Yoichi Nakanishi and Yuko Ohashi for valuable discussions. We are grateful to the Salk Institute Genomic Analysis Laboratory for providing the Arabidopsis mutants. This work was supported by Grants-in-Aid for Scientific Research 16380068, 16085204 and 13CE2005 from the Ministry of Education, Sports, Culture, Science and Technology of Japan to M.M. E.M would like to thank the NCCR project ‘plant survival’ for support.
Fig. 1 (A) Levels of AtPDR8 in different tissues. Crude membranes (5 µg) prepared from the indicated organs were subjected to SDS–PAGE and immunoblotted with anti-AtPDR8 antibody. The antibody to plasma membrane H+-ATPase (AHA) served as the control. (B) Competition assay. Immunoblotting was carried out with microsomes (5 µg) from Arabidopsis T87 cells with the anti-AtPDR8 (lanes 1 and 2) or anti-AHA antibody (lanes 3 and 4). The corresponding authentic peptide was added to the antibody solution prior to immunostaining (lanes 2 and 4). (C) Immunolocalization of AtPDR8 in T87 cells with anti-AtPDR8 or anti-AHA. Alexa Fluor 488-conjugated antibody was used as secondary antibody. Alexa Fluor 488 fluorescence (left), Nomarski (middle) and merged images (right). Bar = 3 µm. (D) Sucrose density gradient centrifugation of crude membranes prepared from stems. Proteins were detected with antibodies against AtPDR8, AHA, VHA-a and BiP. (E) Dot blot analysis of crude membranes (CM) and plasma membranes (PM) prepared from shoots. Protein samples (5 µg) were immunoblotted with the indicated antibodies, and the intensities of the immunostained bands were determined densitometrically. Ratios of the signal intensities of the PM and CM are shown.
Fig. 2 Representative AtPDR8 promoter–GUS expression patterns. (A) X-Gluc staining (for 3 h) for AtPDR8 promoter–GUS expression in a 15-day-old seedling grown on sterile medium. (B) The abaxial side of a leaf. Stoma (st) and the substomatal cavity (sc) beneath the stoma are indicated. Bar = 50 µm. (C) Light micrographs of a longitudinal section of an X-Gluc-stained leaf. Bar = 40 µm. (D) GUS expression in the hydathode (arrow) of a leaf from a 5-week-old plant grown under sterile conditions. Bar = 200 µm.
Fig. 3 Mutants of atpdr8. (A) Insertion positions of T-DNA in AtPDR8. Filled boxes and open boxes represent the 5′- and 3′-untranslated regions, and exons of the gene, respectively. Insertions were at positions +263 and +4542 for atpdr8-1 and atpdr8-2. (B) Immunoblot analysis of crude membranes from wild-type and mutant plants (atpdr8-1 and atpdr8-2) with anti-AtPDR8 antibody. (C) Photographs of wild-type (Col-0) and mutant (atpdr8-1) plants grown for 25 d in vermiculite under sterile conditions. (D) Photograph of Col-0 and mutant (atpdr8-1 and atpdr8-2) plants grown for 40 d on non-sterile vermiculite. (E) Leaves of Col-0 and atpdr8-1 plants grown for 30 d on non-sterile vermiculite. Younger leaves are on the right.
Fig. 4 Arabidopsis atpdr8 plants inoculated with Phytophthora infestans. (A) Photographs of P. infestrans inoculation on the leaf of a Col-0 plant, 4 d post-inoculation (d.p.i.). Arrows indicate the inoculated points. (B) Photographs of P. infestans inoculation on the leaf of atpdr8, 4 d.p.i. (C) Trypan blue staining of an inoculated leaf of Col-0, 3 d.p.i. Bar = 0.3 mm. (D) Trypan blue staining of an inoculated leaf of an atpdr8-1 plant, 3 d.p.i. Bar = 0.3 mm. (E) Trypan blue staining of an inoculated leaf of a Col-0 plant 24 h post-inoculation (h.p.i.). Bar = 20 µm. (F) Trypan blue staining of an inoculated leaf of atpdr8-1 16 h.p.i. Bar = 20 µm. (G) The frequency of penetration of P. infestrans into Arabidopsis cells (white bars) and cell death in plant tissue (shaded bars) at pathogen–leaf interaction sites (n = 30) was determined 24 and 48 h after inoculation. Penetration of infective hyphae was viewed by microscopy. Cell death was monitored by trypan blue staining. (H) DAB staining of an inoculated leaf of atpdr8-1, 16 h.p.i. sp, spore; ap, appressorium; ih, infection hyphae. Bar = 20 µm. All plants were 5 weeks old.
Fig. 5 The atpdr8 mutants exhibit enhanced HR-like cell death. (A) Disease phenotype of the wild type (Col-0) and atpdr8-1 mutant. The right halves of leaves of 4-week-old plants were infiltrated with the virulent pathogen Pst DC3000, the avirulent strain Pst DC3000 (avrRpt2) or 10 mM MgCl2. Bacterial concentrations were 1×105 or 1×107 c.f.u. ml–1. Leaves were photographed 4 d.p.i. (1×105 c.f.u. ml–1) or 1 d.p.i. (1×107 c.f.u. ml–1). (B) HR-like cell death. Five-week-old Col-0 and atpdr8-1 leaves were infiltrated with Pst DC3000 or Pst DC3000 (avrRpt2) (1×107 c.f.u. ml–1), and dead cells and H2O2 production were detected by staining with trypan blue (dark blue) and DAB (dark brown), respectively. Photographs were taken 10 h.p.i. Bar = 100 µm. (C) Bacterial growth of Pst DC3000 and Pst DC3000 (avrRpt2) (1×105 c.f.u. ml–1), inoculated into Col-0, atpdr8-1 and atpdr8-2. These experiments were repeated three times with similar results.
Fig. 6 Pathogen infection enhanced the expression of the AtPDR8 gene. (A) Two-week-old plantlets expressing AtPDR8 promoter–GUS were infected with the virulent pathogen Pst DC3000 or the avirulent strain Pst DC3000 (avrRpt2). After 12 h, the plantlets were stained for GUS under the same conditions. As a control experiment, plantlets were treated with 10 mM MgCl2. (B) Light micrographs of leaves treated with 10 mM MgCl2 (left panel) or the avirulent strain Pst DC3000 (avrRpt2) (right panel). The leaf mesophyll cells were focused and photographed. Bars, 50 µm.
Fig. 7 Expression of defense genes in atpdr8 mutants. Levels of transcripts of defense genes, PR-1, PR-2, PR-5, VPEγ, AtrbohD and AtrbohF in wild-type (Col-0) and mutant (atpdr8-1) plants grown for 4 weeks on non-sterile vermiculite or sterile MS medium. cDNAs were synthesized from total RNA prepared from rosette leaves and subjected to RT–PCR. Expression of UBQ5 was monitored as a constitutive control.
Abbreviations
- ABC
ATP-binding cassette
- AHA
plasma membrane H+-ATPase
- DAB
3,3′-diaminobenzidine
- d.p.i.
days post-inoculation
- GUS
β-glucuronidase
- HR
hypersensitive response
- MDR
multidrug-resistant protein
- MRP
multidrug resistance-associated protein
- NBD
nucleotide-binding domain
- PDR
pleiotropic drug resistance
- PR
pathogenesis related
- RT–PCR
reverse transcription–PCR
- SAR
systemic aquired resistance
- TMD
transmembrane domain
- VHA-a
subunit a of the vacuolar H+-ATPase
- VPE
vacuolar processing enzyme
- X-Gluc
5-bromo-4-chloro-3-indolyl-β-D-glucuronide
References
Abel, S. and Theologis, A. (
Alexandersson, E., Saalbach, G., Larsson, C. and Kjellbom, P. (
Campbell, E.J., Schenk, P.M., Kazan, K., Penninckx, I.A., Anderson, J.P., Maclean, D.J., Cammue, B.P., Ebert, P.R. and Manners, J.M. (
Clough, S.J. and Bent, A.F. (
Collins, N.C., Thordal-Christensen, H., Lipka, V., Bau, S., Kombrink, E., et al. (
Decottignies, A. and Goffeau, A. (
Dong, X., Mindrinos, M., Davis, K.R. and Ausubel, F.M. (
Froehlich, J.E., Wilkerson, C.G., Ray, W.K., McAndrew, R.S., Osteryoung, K.W., Gage, D.A. and Phinney, B.S. (
Glazebrook, J. (
Glazebrook, J., Rogers, E.E. and Ausubel, F.M. (
Glombitza, S., Dubuis, P.H., Thulke, O., Welzl, G., Bovet, L., et al. (
Hatsugai, N., Kuroyanagi, M., Yamada, K., Meshi, T., Tsuda, S., Kondo, M., Nishimura, M. and Hara-Nishimura, I. (
Holland, I.B., Cole, S.P.C., Kuchler, K. and Higgins, C.F. (
Hugouvieux, V., Barber, C.E. and Daniels, M.J. (
Huitema, E., Vleeshouwers, V.G.A.A. Francis, D.M. and Kamoun, S. (
Jasinski, M., Ducos, E., Martinoia, E. and Boutry, M. (
Jasinski, M., Stukkens, Y., Degand, H., Purnelle, B., Marchand-Brynaert, J. and Boutry, M. (
Katagiri, F., Thilmony, R. and He, S.Y. (
Kleffmann, T., Russenberger, D., von Zychlinski, A., Christopher, W., Sjolander, K., et al. (
Kobae, Y., Uemura, T., Sato, M.H., Ohnishi, M., Mimura, T., Nakagawa, T. and Maeshima, M. (
Kunkel, B.N., Bent, A.F, Dahlbeck, D., Innes, R.W. and Staskawicz, B.J. (
Lee, M., Lee, K., Lee, J., Noh, E.W. and Lee, Y. (
Lipka, V., Dittgen, J., Bednarek, P., Bhat, R., Wiermer, M., et al. (
Martinoia, E., Klein, M., Geisler, M., Bovet, L., Forestier, C., Kolukisaoglu, U., Muller-Rober, B. and Schulz, B. (
Mysore, K.S. and Ryu, C.M. (
Niinemets, U., Loreto, F. and Reichstein M. (
Obayashi, T., Okegawa, T., Sasaki-Sekimoto, Y., Shimada, H., Masuda, T., et al. (
Piper, P. Mahe, Y., Thompson, S., Pandjaitan, R., Holyoak, C., Egner, R., Muhlbauer, M., Coote, P. and Kuchler, K. (
Quirino, B.F., Noh, Y.S., Himelblau, E. and Amasino, R.M. (
Sánchez-Fernández, R., Davies, T.G., Coleman, J.O. and Rea, P.A. (
Sasabe, M., Toyoda, K., Shiraishi, T., Inagaki, Y. and Ichinose, Y. (
Sazuka, T., Keta, S., Shiratake, K., Yamaki, S. and Shibata, D. (
Shirasu, K., Lahaye, L., Tan, M.-W., Zhou, F., Azevedo, C. and Schulze-Lefert, P. (
Seo, S., Seto, H., Koshino, H., Yoshida, S. and Ohashi, Y. (
Smart, C.C. and Fleming, A.J. (
Srivastava, S., Fristensky, B. and Kav, N.N. (
Stukkens, Y., Bultreys, A., Grec, S., Trombik, T., Vanham, D. and Boutry, M. (
Tholl, D., Chen, F., Petri, J., Gershenzon, J. and Pichersky, E. (
Thordal-Christensen, H. (
Torres, M.A., Dangl, J.L. and Jones, J.D.G. (
van den Brüle, S., Muller, A., Fleming. A.J. and Smart, C.C. (
von Roepenack-Lahaye, E., Degenkolb, T., Zerjeski, M., Franz, M., Roth, U., Wessjohann, L., Schmidt, J., Scheel, D. and Clemens, S. (
Yoshioka, H., Numata, N., Nakajima, K., Katou, S., Kawakita, K., Rowland, O., Jones, J.D. and Doke, N. (
Yoshioka, H., Yamada, N. and Doke, N. (
Yu, G.L., Katagiri, F. and Ausubel, F.M. (
Zimmermann, P., Hirsch-Hoffmann, M., Hennig, L. and Gruissem, W. (