-
PDF
- Split View
-
Views
-
Cite
Cite
R. Hesselstrand, A. Scheja, G. Q. Shen, A. Wiik, A. Åkesson, The association of antinuclear antibodies with organ involvement and survival in systemic sclerosis, Rheumatology, Volume 42, Issue 4, April 2003, Pages 534–540, https://doi.org/10.1093/rheumatology/keg170
Close - Share Icon Share
Abstract
Objectives. To study the frequency and specificity of antinuclear antibodies (ANA) and their association with internal organ involvement and survival in systemic sclerosis (SSc).
Methods. Sera from 276 SSc patients were analysed by an indirect immunofluorescence (IIF) technique with HEp‐2 cells as a substrate to categorize centromeric (ACA), nucleolar, speckled and homogeneous nuclear IIF patterns. Specific ANA were determined as follows: anti‐DNA topoisomerase I (anti‐topo I) by double immunodiffusion, anti‐U1 RNP by passive haemagglutination, anti‐RNA polymerase I, II and III (anti‐RNAP) and anti‐histone (AHA) antibodies by enzyme immunoassays. During the follow‐up of 7.0±4.5 (mean±s.d.) yr the occurrence of clinical manifestations and internal organ involvement was registered.
Results. ANA were present in 84% of the patients. The most common patterns of the IIF were speckled (41%), homogeneous (25%), nucleolar (24%) and centromeric (18%). A nucleolar pattern was associated with pulmonary fibrosis (P < 0.01) and cardiomegaly (P < 0.05). ACA were related to organic vasculopathy (P < 0.05) and renal involvement (P < 0.01), but not to pulmonary fibrosis (P < 0.01). Anti‐topo I were present in 9.4%, anti‐U1 RNP in 21%, anti‐RNAP in 22% and AHA in 16% of the patients. Pulmonary involvement was more common in patients with anti‐topo I (P < 0.05), whereas AHA‐positive patients were characterized by cardiac (P < 0.05), pulmonary (P < 0.05) and renal (P < 0.05) involvement. A nucleolar IIF pattern and AHA were both associated with a decreased survival [relative risk of death 1.71 (P < 0.05) and 2.36 (P < 0.01), respectively].
Conclusions. AHA and a nucleolar HEp‐2 cell pattern may indicate critical organ involvement and predict a reduced survival in SSc patients.
Systemic sclerosis (SSc, scleroderma) is characterized by microvascular injury and abnormalities of the immune system, leading to fibrosis of the skin and internal organs [1]. The prognosis is highly variable and SSc is associated with an increased mortality [2–4]. Death rates are dependent on the degree of skin involvement [4–6], sex [3, 4, 6, 7], age at diagnosis [2, 6], and involvement of the kidneys [2, 5, 7], heart [2, 5, 7] or lungs [2, 5, 7].
SSc is also characterized by the occurrence of antinuclear autoantibodies (ANA). Anti‐centromere antibodies (ACA) are almost exclusively found in limited cutaneous SSc (lSSc) [8–10]. Patients with ACA have a lower frequency of lung [9–11], heart [9] and kidney involvement [9, 10]. The cause of death in this subgroup is often isolated pulmonary hypertension (PHT) [9]. Anti‐DNA topoisomerase I antibodies (anti‐topo I, anti‐Scl‐70) are associated with more extensive skin involvement, as in diffuse cutaneous SSc (dSSc) [8, 9] and with pulmonary fibrosis [10–12]. In patients with anti‐topo I the cause of death is often related to pulmonary [13], cardiac or renal involvement [9]. Anti‐RNA polymerase antibodies (anti‐RNAP) are also associated with dSSc [10, 12, 13]. Patients with anti‐RNAP have more cardiac [13] and renal [10, 12, 13] involvement, but less pulmonary fibrosis [13]. The presence of anti‐U1 RNP antibodies is associated with vasospastic manifestations and, sometimes, isolated PHT [13, 14].
There is a need for prognostic markers in SSc, and analysis of ANA has been reported to be useful in predicting the long‐term outcome. Kuwana et al. [13] reported the 10‐yr survival rates to be 93% in patients with ACA, 72% in patients with anti‐U1 RNP, 66% in patients with anti‐topo I, but only 30% in those with anti‐RNAP I antibodies.
Anti‐histone antibodies (AHA) are commonly observed in drug‐induced lupus erythematosus and in systemic lupus erythematosus [15]. In earlier studies by Sato et al. [16] and Hasegawa et al. [17] the frequency of AHA in SSc was 29% and 27%, respectively, and AHA were found to be associated with severe pulmonary fibrosis [16]. AHA are reported to be heterogeneous with regard to specificity for individual histone components and histone complexes [17]. IgM antibodies to histone H1 were related to mild clinical features, whereas IgG antibodies to inner core molecules of native histones such as histone H2B or complexes including H2B were associated with severe clinical features.
The purpose of this study was to determine the frequency and the specificity of ANA, and their association to internal organ involvement and survival in SSc patients.
Methods and patients
Patients
The study population comprised 276 consecutive patients, referred from hospitals throughout Sweden during the 16‐yr period 1983–1998. All patients but one were Caucasians and all fulfilled the ACR criteria for SSc [18]. The disease was classified as dSSc if truncal scleroderma was present, or lSSc if truncal scleroderma was absent [1]. The disease onset was defined as the beginning of skin involvement. The time of entry into the study (i.e. the beginning of the follow‐up) was the time of the first SSc‐related visit to the department. In the majority of cases, this was also the time of diagnosis. The occurrence of organ involvement was assessed both at the initial visit and on a regular basis during follow‐up. All patients were re‐evaluated after 1 yr. Thereafter only patients with immunosuppressive medication were investigated yearly, whereas patients with dSSc were examined every 2 yr and patients with lSSc were examined every 3 yr.
Clinical features
Skin involvement was determined by palpation by a modified Rodnan score [19] with rating on a 4‐point scale (0–3), where 0=normal skin, 1=thickened skin, 2=thickened skin unable to pinch and 3=thickened immobile skin. The sum of scores from all sites gave a total skin score, with a theoretical span from 0 to 72.
The patients were investigated by the measurement of finger blood pressure with finger cooling. Organic vascular changes were considered to be present if finger systolic pressure was less than 80% of simultaneous arm blood pressure in the contralateral arm at a finger temperature of 30°C or more. Cold‐induced vasospasm was considered to be present when the finger systolic pressure was less than 65% of the simultaneous arm blood pressure at a finger temperature of 10°C [20]. This investigation was only undertaken at the initial evaluation.
Glomerular filtration rate (GFR) was determined by [51Cr]‐EDTA clearance and expressed as the age‐adjusted percentage of mean values for healthy subjects [19]. Vital lung capacity (VC) was measured with a water‐filled spirometer. Static elastic recoil pressure was recorded during flow interruptions covering most of the vital capacity. Static lung compliance (Cst) was measured over the pressure interval 5–15 cmH2O. Measurement of the transfer factor for carbon monoxide (DLCO) was made with the single breath method [19].
Chest radiography with standard posteroanterior and lateral radiographs was undertaken [19]. The systolic pulmonary artery pressure (PAPsyst) was determined by Doppler echocardiography [19]. PHT was defined as PAPsyst >30 mmHg [20]. Cardiac function was assessed from radiological examination of the chest and a 12‐lead ECG [19]. Scleroderma renal crisis (SRC) was defined as progressive renal insufficiency and accelerated hypertension and diagnosed by renal biopsy.
Serological tests
Serum samples from the initial visit were used to analyse antibodies, which was only done once in this study. ANA were studied by the indirect immunofluorescence (IIF) technique at a serum dilution of 1:160 using the human HEp‐2 cell line as a substrate (ImmunoConcepts, Sacramento, USA) and an IgG‐specific fluorescein isothiocyanate (FITC) conjugate (DAKO, Glostrup, Denmark) at a dilution of 1:40. Fluorescence patterns were termed as described by Humbel [21] and strengths of reactivity given as strong, intermediate or weak positive fluorescence. Anti‐topo I were revealed by double immunodiffusion against a thymus–spleen extract (auto‐ID, ImmunoConcepts) and reference sera [11]. If immunodiffusion results were doubtful, serum was run additionally on Western blot using HeLa cell nuclear extract as antigen and a positive band at 100 kDa used as confirmation. Anti‐U1 RNP and anti‐Sm antibodies were demonstrated by the passive haemagglutination technique [22].
Anti‐dsDNA antibodies were revealed by the enzyme‐linked immunosorbent assay (ELISA) technique using the SynELISA kit from Pharmacia, Freiburg, Germany, according to the manufacturer's instructions. Positive sera (≥55 U/ml) were then studied on Crithidia luciliae slides (ImmunoConcepts) at dilution 1:10 using an IgG‐specific FITC conjugate (DAKO). Only sera that gave positive results in both tests were called anti‐dsDNA positive. AHA of the IgG class were sought using an anti‐histone kit (QuantaLite, histone, INOVA, San Diego, USA) following the manufacturer's instructions. IgG antibodies to the RNA polymerases I, II and III were demonstrated by an ELISA technique, using all three proteins coated on the ELISA plates at a concentration of 2 mg/ml RNA polymerase I and II and 1 mg/ml RNA polymerase III, as described earlier [23]. Anti‐SSA (Ro) and anti‐SSB (La) were demonstrated by use of kits from Shields Diagnostics (Diastat anti‐Ro and Diastat anti‐La, Shields Diagnostics, Dundee, UK) following the manufacturer's instructions. Finally, anti‐mitochondrial antibodies were demonstrated by the ELISA technique, as described earlier [24].
Statistical analyses
Differences between groups were calculated using the χ2 test if five or more cases were expected in each cell, or with Fisher's exact probability test if less than five cases were expected in any cell. Survival was calculated using the Kaplan–Meier method and differences in survival were analysed with a proportional hazards model using the Breslow test. Multivariate analysis was performed using the Cox proportional hazards regression model. The components entered into the multivariate model are age, gender and presence of certain antibodies.
Results
Of the 276 patients, 68 (25%) had dSSc and 208 (75%) had lSSc; 204 (74%) patients were females. During the follow‐up 68 patients died (Table 1). A total of 232 (84%) patients were ANA positive. The most common IIF patterns were speckled (41%), homogeneous (25%), nucleolar (24%) and centromeric (18%), whereas nuclear dots (1.1%) and mitotic spindle apparatus (0.7%) patterns were rare (Table 2).
Analyses of specific antibodies in serum showed 1.4% anti‐dsDNA, 9.4% anti‐topo I, 15.9% AHA, 21.4% anti‐U1 RNP, 8.3% anti‐SSA, 2.2% anti‐SSB, 1.1% anti‐mitochondrial, 1.1% anti‐Sm and 21.7% anti‐RNAP antibodies (Table 3).
Anti‐topo I positivity was not seen in ACA‐positive patients, but was related to nucleolar and homogeneous IIF patterns. AHA positivity was related to a homogeneous pattern and anti‐U1 RNP to a speckled pattern (Table 4).
Of the 276 patients 26 had more than one of the four antibodies ACA, anti‐topo I, anti‐U1 RNP and anti‐RNAP. The most common combination was anti‐U1 RNP and anti‐RNAP. Of the 44 ANA‐negative patients, seven were positive for anti‐RNAP, but none had any other antibodies.
There was a difference in organ involvement between patients with different ANA (Tables 2 and 3). The presence of ACA indicated a female preponderance, limited skin involvement (lSSc), organic vasculopathy, low frequency of pulmonary fibrosis, but high frequency of renal involvement (Table 2). In contrast, patients with anti‐topo I were more often men with dSSc and pulmonary fibrosis (Table 3). Patients with anti‐U1 RNP were younger at disease onset (P < 0.001) and had more vasospasm, whereas patients with anti‐RNAP had more overall pulmonary involvement (Table 3). Only patients with AHA demonstrated an increased risk of pulmonary, cardiac and renal involvement (Table 3).
There was a tendency, although not significant, that ACA was associated with isolated PHT and anti‐topo I with PHT associated with pulmonary fibrosis (Tables 2 and 3).
Six patients (three lSSc, three dSSc) developed SRC. They were all ANA positive; two had a nucleolar, two had a speckled and two had both a nucleolar and a speckled pattern on the IIF. Two patients were anti‐RNAP positive, whereas none was anti‐topo I positive.
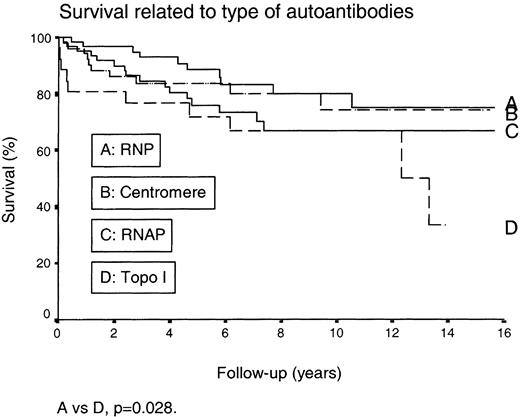
The overall 5‐, 10‐ and 15‐yr survival rates were 84, 70 and 63%, respectively. When corrected for age and sex, the relative risk of death was increased in patients with a nucleolar IIF pattern (P=0.035) and in AHA‐positive patients (P=0.0035) (Table 5). When different subsets were compared, patients with anti‐U1 RNP had more favourable prognoses than patients with anti‐topo I, but this difference was not significant when corrected for sex and age (Fig. 1).
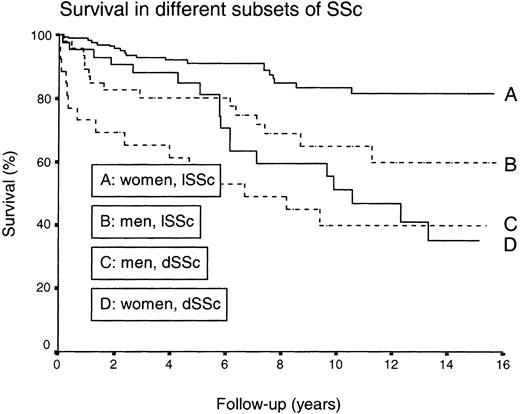
The survival rates were reduced in male SSc patients (P < 0.0001) and in the dSSc (P < 0.0001) subset compared with females and the lSSc subgroup (Fig. 2). When corrected for sex and age, patients with VC <80% (P=0.0097), Cst <60% (P=0.0019), isolated PHT (P=0.0004), PHT associated with pulmonary fibrosis (P=0.035), cardiomegaly (P=0.027) or GFR <80% (P=0.0005) had a reduced survival (Table 6).
Mainly due to progressive pulmonary involvement, 78 patients were treated with cyclophosphamide. This treatment had been given especially to patients with a nucleolar (P=0.021) or a homogeneous IIF (P < 0.001) pattern or if the patient was anti‐topo I (P=0.002) or AHA (P=0.002) positive or ACA (P=0.001) negative.
Demographic characteristics of 276 SSc patients
| lSSc | dSSc | Total | |
| Included (male/female) | 46/162 | 26/42 | 72/204 |
| Deceased (male/female) | 15/20 | 16/17 | 31/37 |
| Age at entry (yr) [mean (s.d.)] | 48.3 (13.6) | 52.0 (15.6) | 49.2 (14.2) |
| Follow‐up (yr) [mean (s.d.)] | 7.1 (4.3) | 6.6 (4.8) | 7.0 (4.5) |
| lSSc | dSSc | Total | |
| Included (male/female) | 46/162 | 26/42 | 72/204 |
| Deceased (male/female) | 15/20 | 16/17 | 31/37 |
| Age at entry (yr) [mean (s.d.)] | 48.3 (13.6) | 52.0 (15.6) | 49.2 (14.2) |
| Follow‐up (yr) [mean (s.d.)] | 7.1 (4.3) | 6.6 (4.8) | 7.0 (4.5) |
Demographic characteristics of 276 SSc patients
| lSSc | dSSc | Total | |
| Included (male/female) | 46/162 | 26/42 | 72/204 |
| Deceased (male/female) | 15/20 | 16/17 | 31/37 |
| Age at entry (yr) [mean (s.d.)] | 48.3 (13.6) | 52.0 (15.6) | 49.2 (14.2) |
| Follow‐up (yr) [mean (s.d.)] | 7.1 (4.3) | 6.6 (4.8) | 7.0 (4.5) |
| lSSc | dSSc | Total | |
| Included (male/female) | 46/162 | 26/42 | 72/204 |
| Deceased (male/female) | 15/20 | 16/17 | 31/37 |
| Age at entry (yr) [mean (s.d.)] | 48.3 (13.6) | 52.0 (15.6) | 49.2 (14.2) |
| Follow‐up (yr) [mean (s.d.)] | 7.1 (4.3) | 6.6 (4.8) | 7.0 (4.5) |
Distribution of disease subsets, and frequencies of organ involvement in 276 Swedish SSc patients, in relation to ANA pattern
| ANA pattern | ||||||||
| Centromeric | Nucleolar | Homogeneous | Speckled | |||||
| +ve | −ve | +ve | −ve | +ve | −ve | +ve | −ve | |
| n=51 | n=225 | n=66 | n=210 | n=69 | n=207 | n=114 | n=162 | |
| Max.±s.d. skin score | 9.6±6.6*** | 15.0±11.0 | 16.4±11.3 | 13.3±10.2 | 18.0±12.3*** | 2.7±9.1 | 13.1±9.8 | 14.7±10.9 |
| Age at entry (yr) | 48.8 | 49.8 | 50.8 | 49.2 | 51.0 | 49.1 | 47.6 | 51.0 |
| Females (%) | 86.3* | 70.7 | 66.7 | 75.7 | 62.3* | 77.3 | 77.2 | 71.0 |
| lSSc (%) | 94.1*** | 70.7 | 68.2 | 77.1 | 62.3** | 79.2 | 77.2 | 73.5 |
| Organ involvement (%) | ||||||||
| Organic vasculopathy | 84.2* | 65.8 | 66.2 | 69.7 | 68.4 | 68.9 | 70.0 | 67.9 |
| Vasospasm | 68.4 | 80.6 | 74.6 | 80.0 | 82.5 | 77.4 | 81.0 | 76.9 |
| Pulmonary fibrosis | 16.3** | 40.3 | 51.6** | 31.1 | 51.5** | 30.9 | 35.4 | 36.3 |
| VC <80% | 18.4* | 35.9 | 38.5 | 30.9 | 47.8** | 27.8 | 36.3 | 30.2 |
| DLCO <80% | 90.9 | 79.3 | 80.4 | 81.8 | 86.0 | 80.0 | 84.8 | 79.0 |
| Cst <60% | 30.4 | 31.4 | 36.2 | 29.2 | 40.9 | 27.6 | 32.8 | 30.1 |
| Isolated PHT | 30.3 | 17.5 | 23.5 | 19.5 | 25.6 | 18.5 | 18.3 | 21.8 |
| PHT associated with PF | 9.1 | 21.9 | 26.5 | 16.8 | 30.8* | 14.8 | 20.0 | 18.4 |
| Abnormal ECG | 66.0 | 44.8 | 52.3 | 58.6 | 61.2 | 55.8 | 55.8 | 58.1 |
| Cardiomegaly | 10.0 | 20.2 | 27.7* | 15.4 | 31.3** | 14.1 | 19.5 | 17.5 |
| GFR <80% | 40.0** | 21.0 | 24.6 | 24.5 | 27.2 | 23.5 | 19.6 | 28.0 |
| ANA pattern | ||||||||
| Centromeric | Nucleolar | Homogeneous | Speckled | |||||
| +ve | −ve | +ve | −ve | +ve | −ve | +ve | −ve | |
| n=51 | n=225 | n=66 | n=210 | n=69 | n=207 | n=114 | n=162 | |
| Max.±s.d. skin score | 9.6±6.6*** | 15.0±11.0 | 16.4±11.3 | 13.3±10.2 | 18.0±12.3*** | 2.7±9.1 | 13.1±9.8 | 14.7±10.9 |
| Age at entry (yr) | 48.8 | 49.8 | 50.8 | 49.2 | 51.0 | 49.1 | 47.6 | 51.0 |
| Females (%) | 86.3* | 70.7 | 66.7 | 75.7 | 62.3* | 77.3 | 77.2 | 71.0 |
| lSSc (%) | 94.1*** | 70.7 | 68.2 | 77.1 | 62.3** | 79.2 | 77.2 | 73.5 |
| Organ involvement (%) | ||||||||
| Organic vasculopathy | 84.2* | 65.8 | 66.2 | 69.7 | 68.4 | 68.9 | 70.0 | 67.9 |
| Vasospasm | 68.4 | 80.6 | 74.6 | 80.0 | 82.5 | 77.4 | 81.0 | 76.9 |
| Pulmonary fibrosis | 16.3** | 40.3 | 51.6** | 31.1 | 51.5** | 30.9 | 35.4 | 36.3 |
| VC <80% | 18.4* | 35.9 | 38.5 | 30.9 | 47.8** | 27.8 | 36.3 | 30.2 |
| DLCO <80% | 90.9 | 79.3 | 80.4 | 81.8 | 86.0 | 80.0 | 84.8 | 79.0 |
| Cst <60% | 30.4 | 31.4 | 36.2 | 29.2 | 40.9 | 27.6 | 32.8 | 30.1 |
| Isolated PHT | 30.3 | 17.5 | 23.5 | 19.5 | 25.6 | 18.5 | 18.3 | 21.8 |
| PHT associated with PF | 9.1 | 21.9 | 26.5 | 16.8 | 30.8* | 14.8 | 20.0 | 18.4 |
| Abnormal ECG | 66.0 | 44.8 | 52.3 | 58.6 | 61.2 | 55.8 | 55.8 | 58.1 |
| Cardiomegaly | 10.0 | 20.2 | 27.7* | 15.4 | 31.3** | 14.1 | 19.5 | 17.5 |
| GFR <80% | 40.0** | 21.0 | 24.6 | 24.5 | 27.2 | 23.5 | 19.6 | 28.0 |
PF, pulmonary fibrosis.
*P < 0.05; **P < 0.01; ***P < 0.001 vs antibody‐negative patients.
Distribution of disease subsets, and frequencies of organ involvement in 276 Swedish SSc patients, in relation to ANA pattern
| ANA pattern | ||||||||
| Centromeric | Nucleolar | Homogeneous | Speckled | |||||
| +ve | −ve | +ve | −ve | +ve | −ve | +ve | −ve | |
| n=51 | n=225 | n=66 | n=210 | n=69 | n=207 | n=114 | n=162 | |
| Max.±s.d. skin score | 9.6±6.6*** | 15.0±11.0 | 16.4±11.3 | 13.3±10.2 | 18.0±12.3*** | 2.7±9.1 | 13.1±9.8 | 14.7±10.9 |
| Age at entry (yr) | 48.8 | 49.8 | 50.8 | 49.2 | 51.0 | 49.1 | 47.6 | 51.0 |
| Females (%) | 86.3* | 70.7 | 66.7 | 75.7 | 62.3* | 77.3 | 77.2 | 71.0 |
| lSSc (%) | 94.1*** | 70.7 | 68.2 | 77.1 | 62.3** | 79.2 | 77.2 | 73.5 |
| Organ involvement (%) | ||||||||
| Organic vasculopathy | 84.2* | 65.8 | 66.2 | 69.7 | 68.4 | 68.9 | 70.0 | 67.9 |
| Vasospasm | 68.4 | 80.6 | 74.6 | 80.0 | 82.5 | 77.4 | 81.0 | 76.9 |
| Pulmonary fibrosis | 16.3** | 40.3 | 51.6** | 31.1 | 51.5** | 30.9 | 35.4 | 36.3 |
| VC <80% | 18.4* | 35.9 | 38.5 | 30.9 | 47.8** | 27.8 | 36.3 | 30.2 |
| DLCO <80% | 90.9 | 79.3 | 80.4 | 81.8 | 86.0 | 80.0 | 84.8 | 79.0 |
| Cst <60% | 30.4 | 31.4 | 36.2 | 29.2 | 40.9 | 27.6 | 32.8 | 30.1 |
| Isolated PHT | 30.3 | 17.5 | 23.5 | 19.5 | 25.6 | 18.5 | 18.3 | 21.8 |
| PHT associated with PF | 9.1 | 21.9 | 26.5 | 16.8 | 30.8* | 14.8 | 20.0 | 18.4 |
| Abnormal ECG | 66.0 | 44.8 | 52.3 | 58.6 | 61.2 | 55.8 | 55.8 | 58.1 |
| Cardiomegaly | 10.0 | 20.2 | 27.7* | 15.4 | 31.3** | 14.1 | 19.5 | 17.5 |
| GFR <80% | 40.0** | 21.0 | 24.6 | 24.5 | 27.2 | 23.5 | 19.6 | 28.0 |
| ANA pattern | ||||||||
| Centromeric | Nucleolar | Homogeneous | Speckled | |||||
| +ve | −ve | +ve | −ve | +ve | −ve | +ve | −ve | |
| n=51 | n=225 | n=66 | n=210 | n=69 | n=207 | n=114 | n=162 | |
| Max.±s.d. skin score | 9.6±6.6*** | 15.0±11.0 | 16.4±11.3 | 13.3±10.2 | 18.0±12.3*** | 2.7±9.1 | 13.1±9.8 | 14.7±10.9 |
| Age at entry (yr) | 48.8 | 49.8 | 50.8 | 49.2 | 51.0 | 49.1 | 47.6 | 51.0 |
| Females (%) | 86.3* | 70.7 | 66.7 | 75.7 | 62.3* | 77.3 | 77.2 | 71.0 |
| lSSc (%) | 94.1*** | 70.7 | 68.2 | 77.1 | 62.3** | 79.2 | 77.2 | 73.5 |
| Organ involvement (%) | ||||||||
| Organic vasculopathy | 84.2* | 65.8 | 66.2 | 69.7 | 68.4 | 68.9 | 70.0 | 67.9 |
| Vasospasm | 68.4 | 80.6 | 74.6 | 80.0 | 82.5 | 77.4 | 81.0 | 76.9 |
| Pulmonary fibrosis | 16.3** | 40.3 | 51.6** | 31.1 | 51.5** | 30.9 | 35.4 | 36.3 |
| VC <80% | 18.4* | 35.9 | 38.5 | 30.9 | 47.8** | 27.8 | 36.3 | 30.2 |
| DLCO <80% | 90.9 | 79.3 | 80.4 | 81.8 | 86.0 | 80.0 | 84.8 | 79.0 |
| Cst <60% | 30.4 | 31.4 | 36.2 | 29.2 | 40.9 | 27.6 | 32.8 | 30.1 |
| Isolated PHT | 30.3 | 17.5 | 23.5 | 19.5 | 25.6 | 18.5 | 18.3 | 21.8 |
| PHT associated with PF | 9.1 | 21.9 | 26.5 | 16.8 | 30.8* | 14.8 | 20.0 | 18.4 |
| Abnormal ECG | 66.0 | 44.8 | 52.3 | 58.6 | 61.2 | 55.8 | 55.8 | 58.1 |
| Cardiomegaly | 10.0 | 20.2 | 27.7* | 15.4 | 31.3** | 14.1 | 19.5 | 17.5 |
| GFR <80% | 40.0** | 21.0 | 24.6 | 24.5 | 27.2 | 23.5 | 19.6 | 28.0 |
PF, pulmonary fibrosis.
*P < 0.05; **P < 0.01; ***P < 0.001 vs antibody‐negative patients.
Distribution of disease subsets, and frequencies of organ involvement in 276 SSc patients, in relation to antinuclear antibodies
| Anti‐topo I | Anti‐histone | Anti‐U1 RNP | Anti‐RNAP | |||||
| +ve | −ve | +ve | −ve | +ve | −ve | +ve | −ve | |
| n=26 | n=250 | n=44 | n=232 | n=59 | n=217 | n=60 | n=216 | |
| Max.±s.d. skin score | 25.9±12.6*** | 12.6±9.50 | 15.5±10.2 | 13.7±10.5 | 11.8±10.3 | 14.6±10.5 | 14.7±10.4 | 13.8±10.5 |
| Age at entry (yr) | 48.8 | 49.7 | 48.9 | 49.7 | 45.1** | 50.9 | 49.8 | 49.5 |
| Females (%) | 53.8* | 75.6 | 79.5 | 72.4 | 74.6 | 73.3 | 75.0 | 73.1 |
| lSSc (%) | 42.3*** | 78.4 | 65.9 | 76.7 | 83.1 | 72.8 | 76.7 | 74.5 |
| Organ involvement (%) | ||||||||
| Organic vasculopathy | 77.3 | 67.9 | 72.2 | 68.2 | 73.5 | 67.6 | 55.8* | 73.3 |
| Vasospasm | 81.8 | 78.3 | 75.0 | 79.3 | 89.8* | 75.7 | 76.9 | 79.1 |
| Pulmonary fibrosis | 58.3* | 33.7 | 45.2 | 34.2 | 31.0 | 37.3 | 41.4 | 34.4 |
| VC <80% | 64.0*** | 29.6 | 47.6* | 30.0 | 35.6 | 31.9 | 33.9 | 32.4 |
| DLCO <80% | 91.3 | 80.4 | 90.6 | 80.0 | 86.3 | 80.1 | 90.2 | 79.0 |
| Cst <60% | 41.2 | 30.1 | 50.0* | 27.6 | 30.6 | 31.5 | 39.4 | 29.1 |
| Isolated PHT | 17.6 | 20.8 | 25.0 | 19.7 | 19.4 | 20.7 | 25.0 | 18.9 |
| PHT associated with PF | 29.4 | 17.7 | 25.0 | 18.1 | 29.0 | 16.4 | 22.2 | 18.0 |
| Abnormal ECG | 48.0 | 58.1 | 74.4* | 53.9 | 48.3 | 59.5 | 66.1 | 54.7 |
| Cardiomegaly | 28.0 | 17.3 | 27.9 | 16.5 | 17.2 | 18.6 | 22.0 | 17.3 |
| GFR <80% | 33.3 | 23.7 | 38.1* | 22.0 | 22.8 | 25.0 | 27.1 | 23.8 |
| Anti‐topo I | Anti‐histone | Anti‐U1 RNP | Anti‐RNAP | |||||
| +ve | −ve | +ve | −ve | +ve | −ve | +ve | −ve | |
| n=26 | n=250 | n=44 | n=232 | n=59 | n=217 | n=60 | n=216 | |
| Max.±s.d. skin score | 25.9±12.6*** | 12.6±9.50 | 15.5±10.2 | 13.7±10.5 | 11.8±10.3 | 14.6±10.5 | 14.7±10.4 | 13.8±10.5 |
| Age at entry (yr) | 48.8 | 49.7 | 48.9 | 49.7 | 45.1** | 50.9 | 49.8 | 49.5 |
| Females (%) | 53.8* | 75.6 | 79.5 | 72.4 | 74.6 | 73.3 | 75.0 | 73.1 |
| lSSc (%) | 42.3*** | 78.4 | 65.9 | 76.7 | 83.1 | 72.8 | 76.7 | 74.5 |
| Organ involvement (%) | ||||||||
| Organic vasculopathy | 77.3 | 67.9 | 72.2 | 68.2 | 73.5 | 67.6 | 55.8* | 73.3 |
| Vasospasm | 81.8 | 78.3 | 75.0 | 79.3 | 89.8* | 75.7 | 76.9 | 79.1 |
| Pulmonary fibrosis | 58.3* | 33.7 | 45.2 | 34.2 | 31.0 | 37.3 | 41.4 | 34.4 |
| VC <80% | 64.0*** | 29.6 | 47.6* | 30.0 | 35.6 | 31.9 | 33.9 | 32.4 |
| DLCO <80% | 91.3 | 80.4 | 90.6 | 80.0 | 86.3 | 80.1 | 90.2 | 79.0 |
| Cst <60% | 41.2 | 30.1 | 50.0* | 27.6 | 30.6 | 31.5 | 39.4 | 29.1 |
| Isolated PHT | 17.6 | 20.8 | 25.0 | 19.7 | 19.4 | 20.7 | 25.0 | 18.9 |
| PHT associated with PF | 29.4 | 17.7 | 25.0 | 18.1 | 29.0 | 16.4 | 22.2 | 18.0 |
| Abnormal ECG | 48.0 | 58.1 | 74.4* | 53.9 | 48.3 | 59.5 | 66.1 | 54.7 |
| Cardiomegaly | 28.0 | 17.3 | 27.9 | 16.5 | 17.2 | 18.6 | 22.0 | 17.3 |
| GFR <80% | 33.3 | 23.7 | 38.1* | 22.0 | 22.8 | 25.0 | 27.1 | 23.8 |
*P < 0.05; **P < 0.01; ***P < 0.001 vs antibody‐negative patients.
Distribution of disease subsets, and frequencies of organ involvement in 276 SSc patients, in relation to antinuclear antibodies
| Anti‐topo I | Anti‐histone | Anti‐U1 RNP | Anti‐RNAP | |||||
| +ve | −ve | +ve | −ve | +ve | −ve | +ve | −ve | |
| n=26 | n=250 | n=44 | n=232 | n=59 | n=217 | n=60 | n=216 | |
| Max.±s.d. skin score | 25.9±12.6*** | 12.6±9.50 | 15.5±10.2 | 13.7±10.5 | 11.8±10.3 | 14.6±10.5 | 14.7±10.4 | 13.8±10.5 |
| Age at entry (yr) | 48.8 | 49.7 | 48.9 | 49.7 | 45.1** | 50.9 | 49.8 | 49.5 |
| Females (%) | 53.8* | 75.6 | 79.5 | 72.4 | 74.6 | 73.3 | 75.0 | 73.1 |
| lSSc (%) | 42.3*** | 78.4 | 65.9 | 76.7 | 83.1 | 72.8 | 76.7 | 74.5 |
| Organ involvement (%) | ||||||||
| Organic vasculopathy | 77.3 | 67.9 | 72.2 | 68.2 | 73.5 | 67.6 | 55.8* | 73.3 |
| Vasospasm | 81.8 | 78.3 | 75.0 | 79.3 | 89.8* | 75.7 | 76.9 | 79.1 |
| Pulmonary fibrosis | 58.3* | 33.7 | 45.2 | 34.2 | 31.0 | 37.3 | 41.4 | 34.4 |
| VC <80% | 64.0*** | 29.6 | 47.6* | 30.0 | 35.6 | 31.9 | 33.9 | 32.4 |
| DLCO <80% | 91.3 | 80.4 | 90.6 | 80.0 | 86.3 | 80.1 | 90.2 | 79.0 |
| Cst <60% | 41.2 | 30.1 | 50.0* | 27.6 | 30.6 | 31.5 | 39.4 | 29.1 |
| Isolated PHT | 17.6 | 20.8 | 25.0 | 19.7 | 19.4 | 20.7 | 25.0 | 18.9 |
| PHT associated with PF | 29.4 | 17.7 | 25.0 | 18.1 | 29.0 | 16.4 | 22.2 | 18.0 |
| Abnormal ECG | 48.0 | 58.1 | 74.4* | 53.9 | 48.3 | 59.5 | 66.1 | 54.7 |
| Cardiomegaly | 28.0 | 17.3 | 27.9 | 16.5 | 17.2 | 18.6 | 22.0 | 17.3 |
| GFR <80% | 33.3 | 23.7 | 38.1* | 22.0 | 22.8 | 25.0 | 27.1 | 23.8 |
| Anti‐topo I | Anti‐histone | Anti‐U1 RNP | Anti‐RNAP | |||||
| +ve | −ve | +ve | −ve | +ve | −ve | +ve | −ve | |
| n=26 | n=250 | n=44 | n=232 | n=59 | n=217 | n=60 | n=216 | |
| Max.±s.d. skin score | 25.9±12.6*** | 12.6±9.50 | 15.5±10.2 | 13.7±10.5 | 11.8±10.3 | 14.6±10.5 | 14.7±10.4 | 13.8±10.5 |
| Age at entry (yr) | 48.8 | 49.7 | 48.9 | 49.7 | 45.1** | 50.9 | 49.8 | 49.5 |
| Females (%) | 53.8* | 75.6 | 79.5 | 72.4 | 74.6 | 73.3 | 75.0 | 73.1 |
| lSSc (%) | 42.3*** | 78.4 | 65.9 | 76.7 | 83.1 | 72.8 | 76.7 | 74.5 |
| Organ involvement (%) | ||||||||
| Organic vasculopathy | 77.3 | 67.9 | 72.2 | 68.2 | 73.5 | 67.6 | 55.8* | 73.3 |
| Vasospasm | 81.8 | 78.3 | 75.0 | 79.3 | 89.8* | 75.7 | 76.9 | 79.1 |
| Pulmonary fibrosis | 58.3* | 33.7 | 45.2 | 34.2 | 31.0 | 37.3 | 41.4 | 34.4 |
| VC <80% | 64.0*** | 29.6 | 47.6* | 30.0 | 35.6 | 31.9 | 33.9 | 32.4 |
| DLCO <80% | 91.3 | 80.4 | 90.6 | 80.0 | 86.3 | 80.1 | 90.2 | 79.0 |
| Cst <60% | 41.2 | 30.1 | 50.0* | 27.6 | 30.6 | 31.5 | 39.4 | 29.1 |
| Isolated PHT | 17.6 | 20.8 | 25.0 | 19.7 | 19.4 | 20.7 | 25.0 | 18.9 |
| PHT associated with PF | 29.4 | 17.7 | 25.0 | 18.1 | 29.0 | 16.4 | 22.2 | 18.0 |
| Abnormal ECG | 48.0 | 58.1 | 74.4* | 53.9 | 48.3 | 59.5 | 66.1 | 54.7 |
| Cardiomegaly | 28.0 | 17.3 | 27.9 | 16.5 | 17.2 | 18.6 | 22.0 | 17.3 |
| GFR <80% | 33.3 | 23.7 | 38.1* | 22.0 | 22.8 | 25.0 | 27.1 | 23.8 |
*P < 0.05; **P < 0.01; ***P < 0.001 vs antibody‐negative patients.
Relationship between HEp‐2 IIF pattern and autoantibodies
| Indirect immunofluorescence pattern | ||||
| Centromeric (n=51) | Nucleolar (n=66) | Homogeneous (n=69) | Speckled (n=114) | |
| Topoisomerase I (n=26) | 0, − ** | 19, + *** | 23, + *** | 3, − *** |
| Histone (n=44) | 10 | 12 | 20, + *** | 17 |
| U1 RNP (n=59) | 4, − ** | 5, − ** | 8, − * | 50, + *** |
| Indirect immunofluorescence pattern | ||||
| Centromeric (n=51) | Nucleolar (n=66) | Homogeneous (n=69) | Speckled (n=114) | |
| Topoisomerase I (n=26) | 0, − ** | 19, + *** | 23, + *** | 3, − *** |
| Histone (n=44) | 10 | 12 | 20, + *** | 17 |
| U1 RNP (n=59) | 4, − ** | 5, − ** | 8, − * | 50, + *** |
−, negative association between indirect immunofluorescence (IIF) pattern and autoantibody; +, positive association between IIF pattern and autoantibody.
*P < 0.05; **P < 0.01; ***P < 0.001.
Relationship between HEp‐2 IIF pattern and autoantibodies
| Indirect immunofluorescence pattern | ||||
| Centromeric (n=51) | Nucleolar (n=66) | Homogeneous (n=69) | Speckled (n=114) | |
| Topoisomerase I (n=26) | 0, − ** | 19, + *** | 23, + *** | 3, − *** |
| Histone (n=44) | 10 | 12 | 20, + *** | 17 |
| U1 RNP (n=59) | 4, − ** | 5, − ** | 8, − * | 50, + *** |
| Indirect immunofluorescence pattern | ||||
| Centromeric (n=51) | Nucleolar (n=66) | Homogeneous (n=69) | Speckled (n=114) | |
| Topoisomerase I (n=26) | 0, − ** | 19, + *** | 23, + *** | 3, − *** |
| Histone (n=44) | 10 | 12 | 20, + *** | 17 |
| U1 RNP (n=59) | 4, − ** | 5, − ** | 8, − * | 50, + *** |
−, negative association between indirect immunofluorescence (IIF) pattern and autoantibody; +, positive association between IIF pattern and autoantibody.
*P < 0.05; **P < 0.01; ***P < 0.001.
Cox regression analyses of mortality with respect to autoantibodies in 276 SSc patients
| HR | 95% CI | HRa | 95% CI | |
| ANA | 1.22 | 0.61–2.46 | 1.69 | 0.83–3.45 |
| Centromere | 0.89 | 0.45–1.74 | 1.12 | 0.57–2.22 |
| Nucleolar | 1.74* | 1.06–2.86 | 1.71* | 1.04–2.82 |
| Homogeneous | 1.80* | 1.10–2.94 | 1.56 | 0.95–2.58 |
| Speckled | 0.83 | 0.51–1.35 | 1.05 | 0.64–1.73 |
| Topoisomerase I | 1.75 | 0.89–3.42 | 1.54 | 0.78–3.05 |
| Histone | 1.82* | 1.04–3.19 | 2.36** | 1.33–4.19 |
| U1 RNP | 0.60 | 0.31–1.18 | 0.82 | 0.42–1.61 |
| RNA polymerase | 1.19 | 0.68–2.09 | 1.49 | 0.84–2.66 |
| HR | 95% CI | HRa | 95% CI | |
| ANA | 1.22 | 0.61–2.46 | 1.69 | 0.83–3.45 |
| Centromere | 0.89 | 0.45–1.74 | 1.12 | 0.57–2.22 |
| Nucleolar | 1.74* | 1.06–2.86 | 1.71* | 1.04–2.82 |
| Homogeneous | 1.80* | 1.10–2.94 | 1.56 | 0.95–2.58 |
| Speckled | 0.83 | 0.51–1.35 | 1.05 | 0.64–1.73 |
| Topoisomerase I | 1.75 | 0.89–3.42 | 1.54 | 0.78–3.05 |
| Histone | 1.82* | 1.04–3.19 | 2.36** | 1.33–4.19 |
| U1 RNP | 0.60 | 0.31–1.18 | 0.82 | 0.42–1.61 |
| RNA polymerase | 1.19 | 0.68–2.09 | 1.49 | 0.84–2.66 |
Coefficients derived from separate Cox regression models for each antibody.
HR, hazard ratio, relative risk of death if autoantibody is present.
aCovariates corrected for age and sex.
*P < 0.05; **P < 0.01.
Cox regression analyses of mortality with respect to autoantibodies in 276 SSc patients
| HR | 95% CI | HRa | 95% CI | |
| ANA | 1.22 | 0.61–2.46 | 1.69 | 0.83–3.45 |
| Centromere | 0.89 | 0.45–1.74 | 1.12 | 0.57–2.22 |
| Nucleolar | 1.74* | 1.06–2.86 | 1.71* | 1.04–2.82 |
| Homogeneous | 1.80* | 1.10–2.94 | 1.56 | 0.95–2.58 |
| Speckled | 0.83 | 0.51–1.35 | 1.05 | 0.64–1.73 |
| Topoisomerase I | 1.75 | 0.89–3.42 | 1.54 | 0.78–3.05 |
| Histone | 1.82* | 1.04–3.19 | 2.36** | 1.33–4.19 |
| U1 RNP | 0.60 | 0.31–1.18 | 0.82 | 0.42–1.61 |
| RNA polymerase | 1.19 | 0.68–2.09 | 1.49 | 0.84–2.66 |
| HR | 95% CI | HRa | 95% CI | |
| ANA | 1.22 | 0.61–2.46 | 1.69 | 0.83–3.45 |
| Centromere | 0.89 | 0.45–1.74 | 1.12 | 0.57–2.22 |
| Nucleolar | 1.74* | 1.06–2.86 | 1.71* | 1.04–2.82 |
| Homogeneous | 1.80* | 1.10–2.94 | 1.56 | 0.95–2.58 |
| Speckled | 0.83 | 0.51–1.35 | 1.05 | 0.64–1.73 |
| Topoisomerase I | 1.75 | 0.89–3.42 | 1.54 | 0.78–3.05 |
| Histone | 1.82* | 1.04–3.19 | 2.36** | 1.33–4.19 |
| U1 RNP | 0.60 | 0.31–1.18 | 0.82 | 0.42–1.61 |
| RNA polymerase | 1.19 | 0.68–2.09 | 1.49 | 0.84–2.66 |
Coefficients derived from separate Cox regression models for each antibody.
HR, hazard ratio, relative risk of death if autoantibody is present.
aCovariates corrected for age and sex.
*P < 0.05; **P < 0.01.
Comparison between survival in SSc patients with anti‐U1 RNA, anti‐centromere, anti‐RNA polymerase or anti‐DNA topoisomerase I antibodies. A vs D: P=0.028.
Comparison between survival in SSc patients segregated by gender and skin involvement. A vs B: P < 0.01; A vs C: P < 0.001; A vs D: P < 0.01; B vs C: P < 0.05; B vs D: ns; C vs D: P < 0.05.
Cox regression analyses of mortality with respect to organ involvement in 276 SSc patients
| HR | 95% CI | HRa | 95% CI | |
| Organic vasculopathy | 1.24 | 0.70–2.19 | 1.41 | 0.79–2.50 |
| Vasospasm | 1.10 | 0.54–2.25 | 1.03 | 0.50–2.11 |
| Pulmonary fibrosis | 2.10** | 1.29–3.45 | 1.22 | 0.73–2.06 |
| VC <80% | 2.48*** | 1.52–4.04 | 1.93** | 1.17–3.18 |
| DLCO <80% | 1.57 | 0.61–4.02 | 1.94 | 0.75–4.99 |
| Cst <60% | 2.71*** | 1.54–4.78 | 2.47** | 1.40–4.37 |
| Pulmonary hypertension | ||||
| Isolated PHT | 4.10** | 1.64–10.23 | 5.03*** | 1.98–12.81 |
| PHT associated with PF | 2.48 | 0.93–6.66 | 3.68* | 1.31–10.40 |
| ECG abnormal | 1.73* | 1.02–2.93 | 1.11 | 0.64–1.91 |
| Cardiomegaly | 2.31** | 1.38–3.86 | 1.81* | 1.07–3.06 |
| GFR <80% | 3.56*** | 2.17–5.84 | 2.49*** | 1.49–4.16 |
| HR | 95% CI | HRa | 95% CI | |
| Organic vasculopathy | 1.24 | 0.70–2.19 | 1.41 | 0.79–2.50 |
| Vasospasm | 1.10 | 0.54–2.25 | 1.03 | 0.50–2.11 |
| Pulmonary fibrosis | 2.10** | 1.29–3.45 | 1.22 | 0.73–2.06 |
| VC <80% | 2.48*** | 1.52–4.04 | 1.93** | 1.17–3.18 |
| DLCO <80% | 1.57 | 0.61–4.02 | 1.94 | 0.75–4.99 |
| Cst <60% | 2.71*** | 1.54–4.78 | 2.47** | 1.40–4.37 |
| Pulmonary hypertension | ||||
| Isolated PHT | 4.10** | 1.64–10.23 | 5.03*** | 1.98–12.81 |
| PHT associated with PF | 2.48 | 0.93–6.66 | 3.68* | 1.31–10.40 |
| ECG abnormal | 1.73* | 1.02–2.93 | 1.11 | 0.64–1.91 |
| Cardiomegaly | 2.31** | 1.38–3.86 | 1.81* | 1.07–3.06 |
| GFR <80% | 3.56*** | 2.17–5.84 | 2.49*** | 1.49–4.16 |
Coefficients derived from separate Cox regression models for each antibody.
HR, hazard ratio, relative risk of death if organ involvement is present; PF, pulmonary fibrosis.
aCovariates corrected for age and sex.
*P < 0.05; **P < 0.01; ***P < 0.001.
Cox regression analyses of mortality with respect to organ involvement in 276 SSc patients
| HR | 95% CI | HRa | 95% CI | |
| Organic vasculopathy | 1.24 | 0.70–2.19 | 1.41 | 0.79–2.50 |
| Vasospasm | 1.10 | 0.54–2.25 | 1.03 | 0.50–2.11 |
| Pulmonary fibrosis | 2.10** | 1.29–3.45 | 1.22 | 0.73–2.06 |
| VC <80% | 2.48*** | 1.52–4.04 | 1.93** | 1.17–3.18 |
| DLCO <80% | 1.57 | 0.61–4.02 | 1.94 | 0.75–4.99 |
| Cst <60% | 2.71*** | 1.54–4.78 | 2.47** | 1.40–4.37 |
| Pulmonary hypertension | ||||
| Isolated PHT | 4.10** | 1.64–10.23 | 5.03*** | 1.98–12.81 |
| PHT associated with PF | 2.48 | 0.93–6.66 | 3.68* | 1.31–10.40 |
| ECG abnormal | 1.73* | 1.02–2.93 | 1.11 | 0.64–1.91 |
| Cardiomegaly | 2.31** | 1.38–3.86 | 1.81* | 1.07–3.06 |
| GFR <80% | 3.56*** | 2.17–5.84 | 2.49*** | 1.49–4.16 |
| HR | 95% CI | HRa | 95% CI | |
| Organic vasculopathy | 1.24 | 0.70–2.19 | 1.41 | 0.79–2.50 |
| Vasospasm | 1.10 | 0.54–2.25 | 1.03 | 0.50–2.11 |
| Pulmonary fibrosis | 2.10** | 1.29–3.45 | 1.22 | 0.73–2.06 |
| VC <80% | 2.48*** | 1.52–4.04 | 1.93** | 1.17–3.18 |
| DLCO <80% | 1.57 | 0.61–4.02 | 1.94 | 0.75–4.99 |
| Cst <60% | 2.71*** | 1.54–4.78 | 2.47** | 1.40–4.37 |
| Pulmonary hypertension | ||||
| Isolated PHT | 4.10** | 1.64–10.23 | 5.03*** | 1.98–12.81 |
| PHT associated with PF | 2.48 | 0.93–6.66 | 3.68* | 1.31–10.40 |
| ECG abnormal | 1.73* | 1.02–2.93 | 1.11 | 0.64–1.91 |
| Cardiomegaly | 2.31** | 1.38–3.86 | 1.81* | 1.07–3.06 |
| GFR <80% | 3.56*** | 2.17–5.84 | 2.49*** | 1.49–4.16 |
Coefficients derived from separate Cox regression models for each antibody.
HR, hazard ratio, relative risk of death if organ involvement is present; PF, pulmonary fibrosis.
aCovariates corrected for age and sex.
*P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
In the present study the serological profile of Swedish SSc patients did not differ significantly from findings in Caucasian populations from other parts of the world [9–13], but the results may not be applied to other races since great racial differences in antibodies from Black, Japanese and Caucasian populations are described [25, 26]. Better survival rates, lower frequency of pulmonary involvement and less progressive pulmonary fibrosis are also described among North American Caucasians compared with Japanese or North American Black populations [27]. The population in the present study is university selected, which further precludes a generalization to other populations. This study also confirms the earlier observation that ACA and anti‐topo I seldom coexist [9, 28]. The negative association between the presence of the three antibodies, ACA, anti‐topo I and anti‐U1 RNP (Table 4), and the differences in organ involvement (Tables 2 and 3) support the concept that each antibody present in SSc serum is associated with a certain combination of clinical features. It has been assumed that anti‐RNAP [13], ACA and anti‐topo I antibodies relate to different subgroups of SSc. The lack of a negative correlation between anti‐RNAP and the other ANA in this study, however, indicates overlap between clinical subgroups. We could not confirm the earlier observation [13] of a decreased survival of patients positive for anti‐RNAP. In our study, the analysis of anti‐RNAP I, II and III antibodies was undertaken jointly. Three main groups of anti‐RNAP sera have been characterized: anti‐RNAP I/III sera, anti‐RNAP I/II/III sera and a group precipitating both RNAP II and topo I [10]. Our survival and organ involvement data may be influenced by these three major subsets of anti‐RNAP positivity, which could explain the absence of correlation.
ACA‐positive SSc patients were predominantly female, had less skin involvement and pulmonary fibrosis, but more vasculopathy and renal involvement, as measured by a decreased GFR. Trostle et al. [29] reported increased intimal thickening in small renal arteries in patients with lSSc. Clements et al. [30] reported reduced renal plasma flow in SSc, reflecting chronic renovascular disease, and all patients with a low 24‐h endogenous creatinine clearance also had a reduced renal plasma flow. However, these changes did not precede SRC, which supports the hypothesis that SRC occurs acutely and de novo without detectable long‐standing precursor factors. Findings concerning ACA‐positive patients may, therefore, be related to the vasculopathy commonly seen in these patients [29], and observed in this study. These manifestations are usually not complicated by severe, irreversible damage to the kidneys [30], and, in keeping with this, none of the ACA‐positive patients in this study developed SRC. An association was found between SRC and ANA attached to nucleoli. The nucleolar pattern correlated with the presence of anti‐topo I, but in this study none of the patients who developed SRC was anti‐topo I positive. It should be noted that nucleolar staining is a typical feature of anti‐topo I antibodies, mostly seen in conjunction with a fine grainy nucleoplasmic staining [21].
PHT occurred in 60 (41%) of the 147 patients examined, similar to the findings by Battle et al. [31] who found 12 of 34 patients to have PHT. MacGregor et al. [32] found 119 of 152 patients to have PHT in a study where only a minority (152/930) was ever examined with echocardiography, indicating a frequent occurrence of PHT in asymptomatic patients. There were considerable differences in pulmonary involvement between patients with different ANA. ACA‐positive patients had significantly less pulmonary fibrosis and less PHT associated with pulmonary fibrosis, but more often isolated PHT. Reverse findings were noted in patients positive for anti‐topo I (Tables 2 and 3), who had signs of interstitial lung disease, evidenced by decreased VC values and, sometimes, fibrosing changes seen on radiography. The pulmonary function test cut‐off values have been set to 80% of predicted value, a limit which affects the results but was chosen before the antibody analyses were done.
In earlier studies by Sato et al. [16] and Hasegawa et al. [17] the presence of AHA was associated with severe pulmonary fibrosis. In the present study, AHA‐positive patients had a reduced survival, and AHA were the only antibodies associated with pulmonary, cardiac and renal involvement. The importance of AHA in SSc patients should be emphasized, even though their occurrence is not specific to SSc. Hasegawa et al. [17] reported AHA to be heterogeneous, and analysis of antibodies to individual histone components and histone complexes may provide more information.
A nucleolar IIF pattern was also associated with a poorer survival (Table 5). Patients with this pattern did not have more pulmonary, cardiac or renal involvement than patients with ANA with a homogeneous pattern (Table 2). However, they seemed to carry a slightly increased risk of developing SRC. It is unclear what antibody subtype causes the poorer survival. A nucleolar pattern was associated with the presence of anti‐topo I, but these patients were few, which was why the CI for them was broader and did not reach a significant level.
The earlier observation of a decreased survival of patients positive for anti‐topo I [13] could not be confirmed in the study (Table 5). Patients with anti‐topo I were distinguished by pulmonary involvement (Table 3) and so received more immunosuppressive treatment. The differences in treatment may also influence the frequency of internal organ involvement. Most striking is the low frequency of SRC, possibly explained by the wide use of angiotensin‐converting enzyme (ACE) inhibitors. As more effective treatment is introduced the differences in survival between patients with poor or fair prognosis will diminish. The unexpectedly good prognosis among the patients with anti‐topo I might reflect the effectiveness of the treatment. Anti‐topo I positivity found once may be unreliable, since these antibodies are known to vary over time in some patients [33]. SSc patients who are positive for anti‐topo I, but later become negative, have a more favourable outcome [34].
When performing numerous statistical tests, positive ones are expected by chance. Findings consistent with previous studies reinforce their importance, whereas unexpected findings like lack of reduced survival in patients positive for topo‐I positive or RNAP, reduced survival and critical organ involvement in AHA‐positive patients, and renal involvement in ACA‐positive patients need to be confirmed in future studies.
It is possible that antibodies are a consequence of organ involvement and not a predictor. This is supported by case reports of SSc patients whose anti‐topo I titre increased markedly at the time of diagnosis of lung cancer [35].
In conclusion, this study shows that AHA and a nucleolar HEp‐2 cell pattern are associated with critical organ involvement and may predict a reduced survival in SSc patients. In our clinic we now analyse AHA in SSc patients, which we have not done previously.
Correspondence to: R. Hesselstrand, Department of Rheumatology, Lund University Hospital, S‐221 85 Lund, Sweden. E‐mail: r.hesselstrand@telia.com
This study was supported by grants from the Österlund Foundation, the Kock Foundation, Anna Greta Crafoord Foundation, The Swedish Rheumatism Association, Gustaf V's 80 years Fund, the Heart–Lung Foundation, the Medical Faculty of the University of Lund and the Swedish Medical Research Council (S0898 and 11550).
References
LeRoy EC, Black C, Fleischmajer R et al. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis.
Lee P, Langevitz P, Alderdice CA et al. Mortality in systemic sclerosis (scleroderma).
Bryan C, Howard Y, Brennan P, Black C, Silman A. Survival following the onset of scleroderma: Results from a retrospective inception cohort study of the UK patient population.
Hesselstrand R, Scheja A, Åkesson A. Mortality and causes of death in a Swedish series of systemic sclerosis patients.
Bennett R, Bluestone R, Holt PJL, Bywaters EGL. Survival in scleroderma.
Barnett AJ, Miller MH, Littlejohn GO. A survival study of patients with scleroderma diagnosed over 30 years (1953–1983): The value of a simple cutaneous classification in the early stages of the disease.
Medsger TA Jr, Masi AT, Rodnan GP, Benedek TG, Robinson H. Survival with systemic sclerosis (scleroderma). A life‐table analysis of clinical and demographic factors in 309 patients.
Giordano M, Valentini G, Migliaresi S, Picillo U, Vatti M. Different antibody patterns and different prognoses in patients with scleroderma with various extent of skin sclerosis.
Steen VD, Powell DL, Medsger TA Jr. Clinical correlations and prognosis based on serum autoantibodies in patients with systemic sclerosis.
Harvey GR, Butts S, Rands AL, Patel Y, McHugh NJ. Clinical and serological associations with anti‐RNA polymerase antibodies in systemic sclerosis.
Jacobsen S, Halberg P, Ullman S et al. Clinical features and serum antinuclear antibodies in 230 Danish patients with systemic sclerosis.
Bunn CC, Denton CP, Shi‐Wen X, Knight C, Black CM. Anti‐RNA polymerases and other autoantibody specificities in systemic sclerosis.
Kuwana M, Kaburaki J, Okano Y, Tojo T, Homma M. Clinical and prognostic associations based on serum antinuclear antibodies in Japanese patients with systemic sclerosis.
Nishimaki T, Aotsuka S, Kondo H et al. Immunological analysis of pulmonary hypertension in connective tissue diseases.
Fritzler MJ, Tan EM. Antibodies to histones in drug‐induced and idiopathic lupus erythematosus.
Sato S, Ihn H, Kikuchi K, Takehara K. Antihistone antibodies in systemic sclerosis. Association with pulmonary fibrosis.
Hasegawa M, Sato S, Kikuchi K, Takehara K. Antigen specificity of antihistone antibodies in systemic sclerosis.
Masi AT, Rodnan GP, Medsger TA Jr et al. Preliminary criteria for the classification of systemic sclerosis (scleroderma).
Åkesson A, Wollheim FA. Organ manifestations in 100 patients with progressive systemic sclerosis: A comparison between the CREST syndrome and diffuse scleroderma.
Scheja A, Eskilsson J, Åkesson A, Wollheim FA. Inverse relation between plasma concentration of von Willebrand factor and CrEDTA clearance in systemic sclerosis.
Humbel RL. Detection of antinuclear antibodies by immunofluorescence. In: van Venroij WJ, Maini RN, eds. Manual of biological markers of disease. Dordrecht, the Netherlands: Kluwer Academic Publishers,
Hoier‐Madsen M, Andersen MG. Microhemagglutination test for detection of antibodies against extractable nuclear antigen. Comparative study of different patient groups for ANA, DNA and ENA antibodies.
Jacobsen S, Ullman S, Shen GQ, van Venrooij WJ, Wiik A, Hallberg P. Influence of clinical features, serum antinuclear antibodies and lung function on survival of patients with systemic sclerosis.
Mouritsen S, Hoier‐Madsen M, Demant EJ, Permin H. Enzyme‐linked immunosorbent assay for determination of anti‐mitochondrial antibodies.
Tager RE, Tikly M. Clinical and laboratory manifestations of systemic sclerosis (scleroderma) in Black South Africans.
Kuwana M, Okano Y, Kaburaki J, Tojo T, Medsger TA Jr. Racial differences in the distribution of systemic sclerosis‐related serum antinuclear antibodies.
Kuwana M, Kaburaki J, Arnett FC, Howard RF, Medsger TA Jr, Wright TM. Influence of ethnic background on clinical and serologic features in patients with systemic sclerosis and anti‐DNA‐topoisomerase I antibody.
Tan EM, Rodnan GP, Garcia I, Moroi Y, Fritzler MJ, Peebles C. Diversity of antinuclear antibodies in progressive systemic sclerosis: Anti‐centromere antibody and its relationship to CREST syndrome.
Trostle DC, Bedetti CD, Steen VD, Al‐Sabbagh MR, Zee B, Medsger TA Jr. Renal vascular histology and morphometry in systemic sclerosis. A case–control autopsy study.
Clements PJ, Lachenbruch PA, Furst DE, Maxwell M, Danovitch G, Paulus HE. Abnormalities of renal physiology in systemic sclerosis. A prospective study with 10‐year follow‐up.
Battle RW, Davitt MA, Cooper SM et al. Prevalence of pulmonary hypertension in limited and diffuse scleroderma.
MacGregor AJ, Canavan R, Knight C et al. Pulmonary hypertension in systemic sclerosis: risk factors for progression and consequences for survival.
Henry PA, Atamas SP, Yurovsky VV, Luzina I, Wigley FM, White B. Diversity and plasticity of the anti‐DNA topoisomerase I antibody response in scleroderma.
Kuwana M, Kaburaki J, Mimori T, Kawakami Y, Tojo T. Longitudinal analysis of autoantibody response to topoisomerase I in systemic sclerosis.




Comments