-
PDF
- Split View
-
Views
-
Cite
Cite
Sarah M. Mense, Amitabha Sengupta, Changgui Lan, Mei Zhou, Galina Bentsman, David J. Volsky, Robin M. Whyatt, Frederica P. Perera, Li Zhang, The Common Insecticides Cyfluthrin and Chlorpyrifos Alter the Expression of a Subset of Genes with Diverse Functions in Primary Human Astrocytes, Toxicological Sciences, Volume 93, Issue 1, September 2006, Pages 125–135, https://doi.org/10.1093/toxsci/kfl046
Close - Share Icon Share
Abstract
Given the widespread use of insecticides in the environment, it is important to perform studies evaluating their potential effects on humans. Organophosphate insecticides, such as chlorpyrifos, are being phased out; however, the use of pyrethroids in household pest control is increasing. While chlorpyrifos is relatively well studied, much less is known about the potential neurotoxicity of cyfluthrin and other pyrethroids. To gain insights into the neurotoxicity of cyfluthrin, we compared and evaluated the toxicity profiles of chlorpyrifos and cyfluthrin in primary human fetal astrocytes. We found that at the same concentrations, cyfluthrin exerts as great as, or greater toxic effects on the growth, survival, and proper functioning of human astrocytes. By using microarray gene expression profiling, we systematically identified and compared the potential molecular targets of chlorpyrifos and cyfluthrin, at a genome-wide scale. We found that chlorpyrifos and cyfluthrin affect a similar number of transcripts. These targets include molecular chaperones, signal transducers, transcriptional regulators, transporters, and those involved in behavior and development. Further computational and biochemical analyses show that cyfluthrin and chlorpyrifos upregulate certain targets of the interferon-γ and insulin-signaling pathways and that they increase the protein levels of activated extracellular signal–regulated kinase 1/2, a key component of insulin signaling; interleukin 6, a key inflammatory mediator; and glial fibrillary acidic protein, a marker of inflammatory astrocyte activation. These results suggest that inflammatory activation of astrocytes might be an important mechanism underlying neurotoxicity of both chlorpyrifos and cyfluthrin.
In the United States, approximately 800 million pounds of pesticides are used annually in agriculture, and 80–90% of households report using pesticides. Thus, it is not surprising that the American home environment is widely contaminated with pesticides, particularly insecticides (Eskenazi et al., 1999; Whyatt et al., 2002, 2003). Organophosphates, such as chlorpyrifos, and pyrethroids, such as cyfluthrin, are frequently detected in the house dust and indoor air of homes (Colt et al., 2004; Whyatt et al., 2002, 2003). In June 2000, the Environmental Protection Agency (EPA) entered into an agreement with pesticide manufacturers in order to begin phasing out residential uses of chlorpyrifos and to end all retail sales of chlorpyrifos for indoor use by December 2002 (USEPA, 2000). This action was taken by the EPA in part based on information gained from studies of chlorpyrifos in animal models and in cell lines suggesting that chlorpyrifos may cause developmental neurotoxicity (Barone et al., 2000; Eriksson and Talts, 2000; Pope, 1999; Slotkin, 1999). In replacement of the organophosphates chlorpyrifos and diazinon, the residential use of pyrethroids such as cyfluthrin in pest control has increased (Surgan et al., 2002).
Previous experiments have demonstrated the link between chlorpyrifos and adverse neurodevelopmental sequelae in rodents (Barone et al., 2000; Eriksson and Talts, 2000; Pope, 1999; Slotkin, 1999, 2004b). It was shown that subtoxic doses of chlorpyrifos inhibit DNA synthesis, mitosis, neurite outgrowth, neural cell replication, and neural cell differentiation and interfere with signaling cascades, including serotonergic, cholinergic, and catecholaminergic pathways (Eriksson and Talts, 2000; Pope, 1999; Slotkin, 1999, 2004b). Chlorpyrifos inhibits glial cell replication, gliogenesis, and glioma differentiation and disrupts the pattern of glial cell development in vivo (Garcia et al., 2001, 2002; Zurich et al., 2004). Because glial cells are targets of chlorpyrifos at subtoxic doses, the vulnerability of the developing brain to chlorpyrifos is increased (Garcia et al., 2001, 2002; Zurich et al., 2004).
Compared to the organophosphates, the pyrethroids are considered to be less toxic insect control agents because mammals have higher levels of the detoxifying enzymes than insects do (Aldridge, 1990; Soderlund et al., 2002). However, there is evidence suggesting that the detoxifying enzymes of pyrethroids are present at lower levels during fetal and early postnatal development than they are later in life (Cantalamessa, 1993; Sheets, 2000). Previous experiments showed that the toxicity of pyrethroids increases as the age of the animal decreases, so that neonates appear highly sensitive to pyrethroid exposure (Cantalamessa, 1993). A recent comprehensive review summarizing the existing 22 studies of the developmental neurotoxicity of pyrethroids suggests that pyrethroids may exert developmental neurotoxicity (Shafer et al., 2005). However, further studies are necessary to assess the neurotoxicity of pyrethroids.
In this report, we performed toxicogenomic and toxicological analyses to test the hypothesis that the representative type II pyrethroid cyfluthrin is less toxic than chlorpyrifos to human astrocytes at both the molecular and functional levels. We took advantage of the fact that much is known about the developmental neurotoxicity of chlorpyrifos and performed a comparative study of chlorpyrifos and cyfluthrin. Cyfluthrin is a representative type II pyrethroid, and the use of pyrethroids such as cyfluthrin is widespread (Surgan et al., 2002). We examined and compared the toxicogenomic profiles of chlorpyrifos and cyfluthrin in a major type of brain cells—primary human fetal astrocytes. Astrocytes play critical roles in the proper functioning of the brain (Magisretti and Ransom, 2002). They are important for maintaining ion and pH homeostasis, for the removal of neurotransmitters, and for providing glucose supply to the brain. Further, recent evidence indicates that astrocytes regulate synaptic activity, synaptogenesis, and neurogenesis (Ransom et al., 2003). Astrocytes play important roles in neuroprotection, and inflammatory activation of astrocytes or astrocyte dysfunction is believed to be associated with several chronic neurological diseases, including prion, Alzheimer disease, Parkinson disease, and HIV-1–associated dementia (Gebicke-Haerter, 2001; Wang et al., 2004). Clearly, astrocyte dysfunction causes neurological problems in adults. Very likely, astrocyte dysfunction would cause neurological problems in children because the developing nervous system is even more sensitive to environmental insults than that of the adult. Thus, examining and comparing the effects of chlorpyrifos and cyfluthrin on human fetal astrocytes might provide insights into the mechanism by which these insecticides cause neurotoxicity. Particularly, a comprehensive comparative toxicogenomic study may provide a global picture of their potential neurotoxicity.
MATERIALS AND METHODS
Human primary astrocytes and treatment.
Fetal astrocytes were isolated as previously described by Dr Volsky and colleagues (Canki et al., 2001; Wang et al., 2004). They were isolated from second trimester (gestational age, 16–19 weeks) human fetal brains obtained from elective abortions in full compliance with National Institutes of Health (NIH) guidelines and under an exemption from Institutional Review Board review. Homogenous preparations of astrocytes were obtained by using high-density culture conditions in the absence of growth factors in F12 Dulbecco's modified Eagles medium (GIBCO-Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum, penicillin, streptomycin, and gentamycin. Cells were maintained in this medium at 2 × 104 to 5 × 104 cells/cm2 and subcultured weekly up to six times. Cultures were regularly monitored by immunofluorescence staining for expression of the astrocytic marker glial fibrillary acidic protein (GFAP) and HAM-56 to identify cells of the monocyte lineage. Only cultures that contained ≥ 99% GFAP-positive astrocytes and no detectable HAM-56–positive cells were used in the experiments.
Cell growth, lactate dehydrogenase, and glutamate uptake assays.
Chlorpyrifos (99.5%) and cyfluthrin (98%) were purchased from ChemService (West Chester, PA) and dissolved in DMSO. They were initially dissolved in DMSO at a stock concentration of 500mM. Prior to addition to astrocytes, they were further diluted in DMSO to concentrations such that the eventual DMSO concentration in the medium was always 0.1%. All experiments were controlled such that 0.1% DMSO was present in all controls and all treatments. Primary human astrocytes (passage 4) were treated with DMSO (0.1% for control) or with the desired concentrations of chlorpyrifos or cyfluthrin (along with 0.1% DMSO) for 7 or 14 days. For 14-day treatments, media were refreshed after 7 days with the same media containing the appropriate concentration of chlorpyrifos, cyfluthrin, or 0.1% DMSO (for control). The effects of chlorpyrifos or cyfluthrin on astrocyte growth were examined by counting adherent, live cells after treatment with insecticides or untreated cells by using a hemacytometer. At least three independent sets of data were collected in each experiment. To further assess the viability of astrocytes, we measured the release of lactate dehydrogenase (LDH) into the medium. A total of 7.5 × 104 cells were plated on 24-well plates and treated with the desired concentrations of chlorpyrifos or cyfluthrin for 14 days. The medium was collected, and LDH activities were measured and calculated by using a kit purchased from Sigma (Saint Louis, MO). Glutamate uptake assays were performed by incubating treated or untreated cells with 0.5 μC of D-2,3-3H-aspartic acid for 5 min (Mutkus et al., 2005). Cells were collected. Radioactivity was measured by liquid scintillation spectroscopy, and proteins were measured by Bradford assays. The level of glutamate uptake was calculated as picomoles per milligram protein.
TUNEL assays.
The effect of chlorpyrifos or cyfluthrin on astrocyte survival was detected by using TUNEL (terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling) assays. Chlorpyrifos- or cyfluthrin-treated or untreated astrocytes were collected by scraping and centrifugation and fixed by 4% methanol-free formaldehyde solution. Cells were allowed to adhere to poly-L-lysine–coated slides. TUNEL assays were performed by using a kit from Promega, Inc. (Madison, WI). Fragmented DNA was labeled with fluorescein-12-dUTP. Images of cells were captured by fluorescence microscopy with a Nikon Eclipse E600 microscope with a ×20 objective and a digital camera. 4′,6-diamidino-2-phenylindole (DAPI) (for staining nucleus) images were captured by using excitation and emission wavelengths of 330–380 and 435–485 nm, respectively. TUNEL images were captured using excitation and emission wavelengths of 460–500 and 510–560 nm, respectively. These images were acquired and analyzed by using the SPOT Advanced 3.4.2 program (Diagnostic Instruments, Sterling Heights, MI).
RNA extraction and Affymetrix GeneChip expression analysis.
Primary human fetal astrocytes treated with 25μM chlorpyrifos or cyfluthrin or no insecticide for 7 days were collected. Total RNA was extracted by using TRIzol reagent (GIBCOBRL Life Technologies, Carlsbad, CA). The synthesis of cDNAs and biotin-labeled cRNAs was carried out exactly as described in the Affymetrix GeneChip Expression Analysis Technical Manual (2000). The human genome U133 plus 2.0 arrays were purchased from Affymetrix, Inc. (Santa Clara, CA) Probe hybridization and data collection were carried out by the Columbia University Affymetrix GeneChip processing center. Specifically, the Affymetrix GeneChip Hybridization Oven 640 and the next-generation GeneChip Fluidics Station 450 were used for hybridization and chip processing. Chip scanning was performed by using the GeneChip scanner 3000. Initial data analysis was performed by using the Affymetrix GCOS1.2 software. The original microarray data have been submitted to NIH Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/), accession number GSE5023.
Computational analysis of microarray data.
We analyzed microarray expression data by applying a series of quality control, statistical, filtering, gene ontology, and pathway analysis algorithms. First, by using GCOS1.2 with the advanced PLIER (probe logarithmic intensity error) algorithm, we calculated and examined the parameters reflecting the image quality of the arrays. Arrays with a high background level in any region were discarded and replaced. The average noise or background level was limited to less than 5%. The average intensity for genes judged to be present was at least 10-fold higher than that for genes judged to be absent. Also, arrays that deviated considerably in the percentage of present and absent genes from the majority of the arrays were replaced. Arrays with a β-actin 3′/5′ ratio greater than 2 were replaced. Next, microarray data were uploaded to GeneSpring 7.0 (Silicon Genetics, Santa Clara, CA) for further quality and statistical analysis. The data were normalized again by using the stringent per chip and per gene normalization algorithms. Genes with low control signal (less than average) or not present in 1/3 of the samples were dropped out before statistical analysis. The data were then analyzed by nonparametric two-way ANOVA (one parameter is cell and the other parameter is insecticide treatment). The Benjamini and Hochberg false discovery rate multiple testing correction was applied with a false discovery rate of less than 5%. Two-way ANOVA was chosen for statistical analysis because the variation between astrocytes from different subjects can be substantial and, in certain cases, may exert stronger effects than the insecticide treatment. By using two-way ANOVA, we were able to take the intersubject variability into account and identify statistically significant genes whose transcript level was consistently altered by chlorpyrifos or cyfluthrin. Moreover, to identify genes whose transcript level was significantly altered by chlorpyrifos or cyfluthrin, we applied four consecutive filtering processes, which identified genes whose overall expression level in three independent batches of human fetal astrocytes (HFAs) was altered at least 2-fold (insecticide treated vs. control) and whose expression level in each batch of HFAs was altered by more than 1.5-fold. The fold changes listed in the tables were calculated based on data from three batches of astrocytes by GeneSpring, which combined data from all three controls and three chlorpyrifos- or cyfluthrin-treated samples.
The identified chlorpyrifos- or cyfluthrin-altered genes were further analyzed and categorized by using various biological annotation programs including the Database for Annotation, Visualization and Integrated Discovery (DAVID)/the Expression Analysis Systematic Explorer program provided by The National Institute of Allergy and Infectious Diseases (http://apps1.niaid.nih.gov/david/upload.asp) and the Gene Ontology algorithm in GeneSpring. Data from Online Mendelian Inheritance in Man and the literature were also used to assist gene categorization. Our analyses led to the identification of six notable functional groups of genes whose expression is altered by chlorpyrifos or cyfluthrin (see the “Results” section). Finally, we used another computational program called PathwayAssist (Stratagene Software, La Jolla, CA) to analyze chlorpyrifos- or cyfluthrin-altered genes. This program uses information available in the current literature to identify common pathways, targets, or regulators that are associated with the altered genes.
Quantitative real-time reverse transcription–polymerase chain reaction.
Oligonucleotide primers for measuring transcript levels of genes were designed based on the sequences used to design microarray probes by using the primer 3 program (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). β-Actin was used as a control for the relative quantification of transcripts because our microarray data showed that the β-actin transcript level was unaffected by either chlorpyrifos or cyfluthrin. Reverse transcription–polymerase chain reactions (RT-PCR) were performed by using a Roche LightCycler and the SYBR green kit. Calculations were done by using the Roche LightCycler software. Primer sequences used for real-time PCR are available upon request.
Western blotting analysis.
Astrocytes were washed twice in PBS, and whole-cell extracts were prepared by adding 10 packed cell volumes of sample buffer (2% SDS, 100mM dithiothreitol, 60mM Tris, pH 6.8, and 10% glycerol) and boiled for 5 min. Protein concentrations were determined by using the bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Approximately 20 μg protein was analyzed on 9% SDS-PAGE and transferred onto the Immuno-Blot PVDF Membrane (Bio-Rad, Hercules, CA). The membranes were probed with polyclonal antibodies, followed by detection with a chemiluminescence Western blotting kit (Boehringer, Mannheim, Germany). Polyclonal antibodies against GFAP and interleukin 6 (IL-6) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) and those against phospho(Thr202/Tyr204)–extracellular signal-regulated kinase 1/2 (ERK1/2) and β-actin were purchased from Cell Signaling Technology (Danvers, MA).
RESULTS
Both Chlorpyrifos and Cyfluthrin Significantly Reduced Cell Growth and Survival and Induced Apoptosis
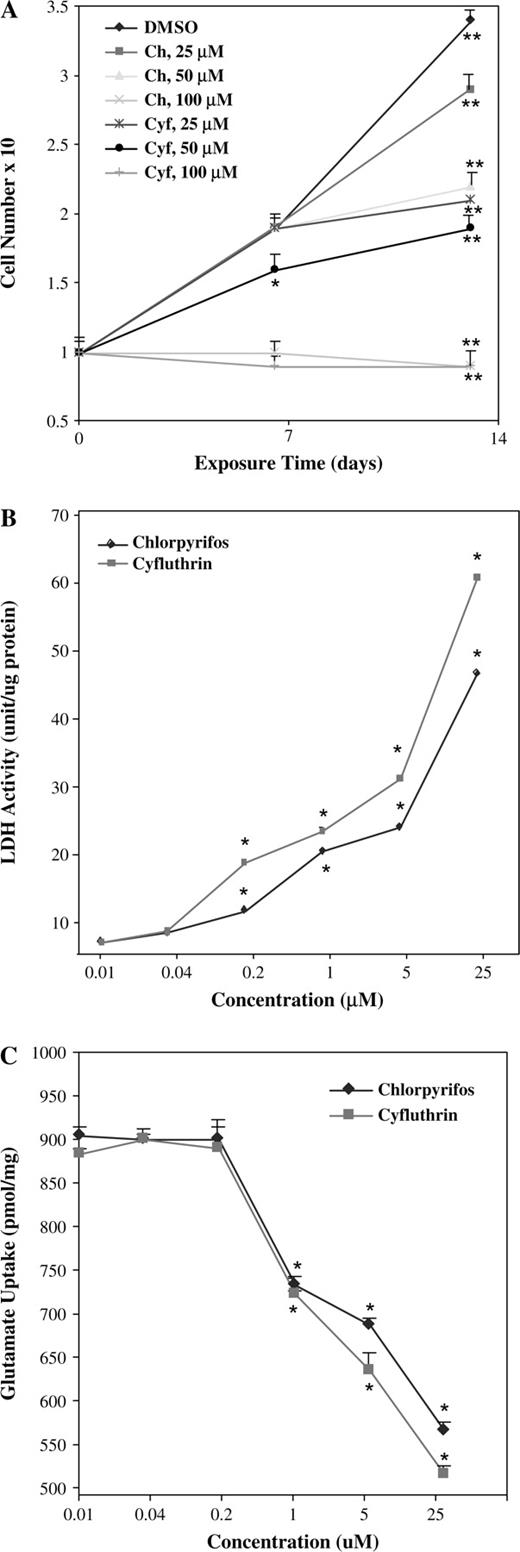
To gain insights into the potential neurotoxicity of cyfluthrin, we examined and compared the effects of chlorpyrifos and cyfluthrin on primary human astrocytes. We compared the effects of various concentrations of cyfluthrin and chlorpyrifos on the growth and survival of human astrocytes. We chose a concentration range that has previously been shown to affect rat neuronal and glial cell function. For example, in PC12 cells after 10 days of treatment with 50 μg/ml (140μM) chlorpyrifos, DNA synthesis is inhibited by 70%, RNA synthesis by 60%, and protein synthesis by 20% (Song et al., 1998). In rat cortical neurons, 50μM chlorpyrifos causes apoptosis in 70% of cells after 72 h (Caughlan et al., 2004). Our data showed that in primary human fetal astrocytes, 25μM or lower concentrations of chlorpyrifos or cyfluthrin did not considerably affect cell number after 1 week of treatment (Fig. 1A). After 2 weeks of exposure at 25μM, chlorpyrifos slightly affected cell growth, while cyfluthrin reduced cell number by 40%. The decrease in cell number brought about by 25μM cyfluthrin was somewhat greater than that caused by 50μM chlorpyrifos (Fig. 1A). One hundred micromolar chlorpyrifos or cyfluthrin resulted in a complete inhibition of astrocyte growth and survival (Fig. 1A).
(A) The effects of chlorpyrifos and cyfluthrin on astrocyte cell growth. Primary human fetal astrocytes were treated with 0, 25, 50, or 100μM of chlorpyrifos or cyfluthrin for 7 and 14 days and collected and counted by using a hemacytometer. The plotted numbers are averages from at least three independent experiments. Two-tailed t-tests were performed to compare insecticide-treated astrocytes (in 0.1% DMSO) with control astrocytes (in 0.1% DMSO) at the corresponding time points. The p values were calculated by using the SAS software. *p values of 0.0005; **p values of < 0.0001. (B) The effects of chlorpyrifos and cyfluthrin on LDH release. Primary human astrocytes were treated with the indicated concentrations of chlorpyrifos or cyfluthrin for 14 days. Then media were collected and subjected to LDH activity assays. The plotted numbers are averages from at least three independent experiments. Two-tailed t-tests were performed to compare insecticide-treated astrocytes (in 0.1% DMSO) with control astrocytes (in 0.1% DMSO). The p values were calculated by using the SAS software. *p values of < 0.0001. (C) The effects of chlorpyrifos and cyfluthrin on glutamate uptake by primary human astrocytes. Glutamate uptake was measured in primary human astrocytes treated or untreated with the indicated concentration of chlorpyrifos and cyfluthrin for 14 days. The plotted numbers are averages from at least three independent experiments. Two-tailed t-tests were performed to compare insecticide-treated astrocytes (in 0.1% DMSO) with control astrocytes (in 0.1% DMSO). The p values were calculated by using the SAS software. *p values of < 0.0001.
To further assess the effects of chlorpyrifos and cyfluthrin on cell viability, particularly at low concentrations, we measured the release of LDH activity to the medium. LDH release is a reliable and sensitive indicator of membrane failure. As shown in Figure 1B, both chlorpyrifos and cyfluthrin caused the release of LDH at a detectable level into the medium at a concentration as low as 0.2μM. At concentrations between 0.04 and 25μM, the effect of cyfluthrin on membrane was consistently more severe than that of chlorpyrifos, again indicating that cyfluthrin is more toxic to astrocytes than chlorpyrifos. We also examined the effects of chlorpyrifos and cyfluthrin on glutamate uptake by astrocytes because one important function of astrocytes is to maintain low levels of synaptic glutamate. We found that 1μM or higher concentrations of chlorpyrifos or cyfluthrin significantly reduced the capability of astrocytes to take up glutamate (Fig. 1C). Consistent with the data shown in Figures 1A and 1B, cyfluthrin exerted a somewhat stronger effect on glutamate uptake than chlorpyrifos (Fig. 1C).
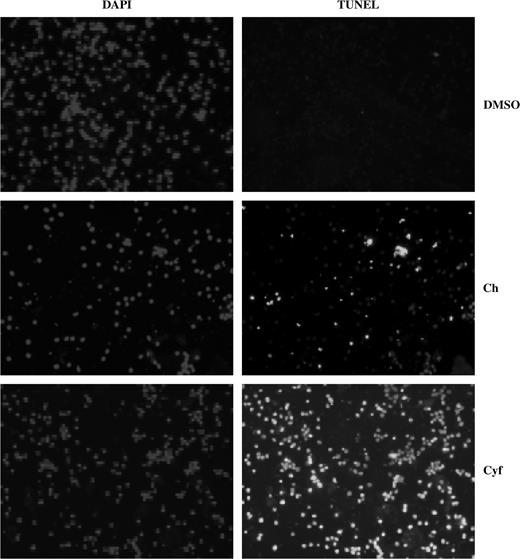
We next examined and compared the effects of chlorpyrifos and cyfluthrin on apoptosis. At 25μM concentrations, neither chlorpyrifos nor cyfluthrin generated clear apoptotic signals after 2 weeks of treatment (not shown). However, 100μM of either chlorpyrifos or cyfluthrin generated clear apoptotic signals in astrocytes treated for 2 weeks, as detected by TUNEL staining (Fig. 2). Note that cyfluthrin induced apoptosis in virtually all cells (98 ± 2%), whereas chlorpyrifos caused apoptosis in 35 ± 3% of the cells (Fig. 2).
Chlorpyrifos or cyfluthrin can cause apoptosis in primary human astrocytes. TUNEL assays were performed by using primary human astrocytes, treated with or without 100μM chlorpyrifos or cyfluthrin (in 0.1% DMSO) for 14 days. Fluorescent images for DAPI (nuclear staining, for controls) and TUNEL staining were captured and are shown here. Cell images were obtained by using Spot Advanced 3.4.2. The percentage of apoptotic cells was calculated by dividing the number of apoptotic cells by the number of total cells. Total cell number was counted from the DAPI images, while apoptotic cell number was counted from the TUNEL images.
About 1% of Transcripts Were Altered by Chlorpyrifos and Cyfluthrin in Human Fetal Astrocytes
To gain a broad idea about the molecular targets of chlorpyrifos and cyfluthrin, we examined and compared the effects of each insecticide on global gene expression in primary human astrocytes using a comparative toxicogenomic approach. We chose to treat astrocytes with 25μM concentrations of cyfluthrin or chlorpyrifos for 1 week based on astrocyte cell growth data, for example, those shown in Figure 1A. The data suggest that this treatment would likely affect most potential targets and allow us to identify them but would not cause the identification of many genes associated with cell death as targets.
We isolated RNA samples from three independent batches of primary human astrocytes treated with either chlorpyrifos or cyfluthrin for 7 days. Transcript levels were detected by using the human genome U133 plus 2.0 array. It includes most of the genes in the human genome and contains probe sets that allow the detection of over 47,000 transcripts. Microarray data were normalized and filtered followed by a series of statistical analyses, including nonparametric two-way ANOVA and the Benjamini and Hochberg false discovery rate multiple testing correction. Then, we identified target genes whose overall expression level in three independent batches of astrocytes was altered by at least 2-fold and whose expression level in each batch of astrocytes was altered by at least 1.5-fold. We used these stringent statistical criteria to minimize the identification of false-positive targets.
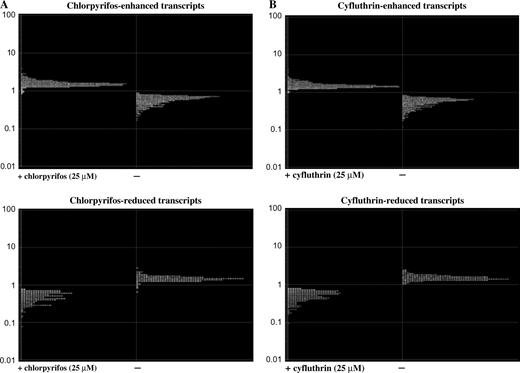
We identified 610 and 659 genes whose expression was selectively altered by chlorpyrifos and cyfluthrin, respectively. Of the genes whose expression was altered by chlorpyrifos, 433 were upregulated while 177 were downregulated (Table 1 and Fig. 3A). Among cyfluthrin-altered genes, 444 were upregulated and 219 were downregulated (Table 1 and Fig. 3B). A total of 290 chlorpyrifos-altered and 309 of cyfluthrin-altered genes are annotated (i.e., certain functional or structural information is known, Table 1). Note that about half the genes are not annotated, that is, no information regarding their structure or function is available (Table 1).
Graphic illustration of the normalized transcript levels of chlorpyrifos- (A) or cyfluthrin (B)-altered genes in primary human astrocytes. Microarray expression and statistical analyses of the data were performed, and the identification of chlorpyrifos- or cyfluthrin-altered genes is as described in the “Materials and Methods” section. The expression levels of upregulated genes (top) and downregulated genes (bottom) by chlorpyrifos (A) or cyfluthrin (B) were computed from three sets of independent astrocytes were plotted.
Numbers of Transcripts Altered by Chlorpyrifos or Cyfluthrin in Primary Human Astrocytesa
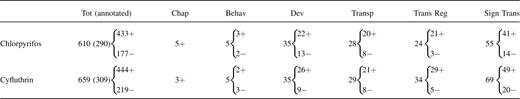 |
 |
The upregulated and downregulated genes in primary human astrocytes (Fig. 3) were categorized by using the NIH DAVID annotation program and the Gene Ontology algorithm in GeneSpring. Listed here are the numbers of altered genes encoding functions involved in behavior (Behav) and development (Dev) and those encoding chaperones (Chap), transporters (Transp), transcriptional regulators (Trans Reg), and signal transducers (Sign Trans). The total (Tot) numbers of altered transcripts by chlorpyrifos or cyfluthrin are also shown; the numbers of annotated (classified) genes are shown in parentheses. “+” indicates upregulated genes while “−” indicates downregulated genes.
Numbers of Transcripts Altered by Chlorpyrifos or Cyfluthrin in Primary Human Astrocytesa
 |
 |
The upregulated and downregulated genes in primary human astrocytes (Fig. 3) were categorized by using the NIH DAVID annotation program and the Gene Ontology algorithm in GeneSpring. Listed here are the numbers of altered genes encoding functions involved in behavior (Behav) and development (Dev) and those encoding chaperones (Chap), transporters (Transp), transcriptional regulators (Trans Reg), and signal transducers (Sign Trans). The total (Tot) numbers of altered transcripts by chlorpyrifos or cyfluthrin are also shown; the numbers of annotated (classified) genes are shown in parentheses. “+” indicates upregulated genes while “−” indicates downregulated genes.
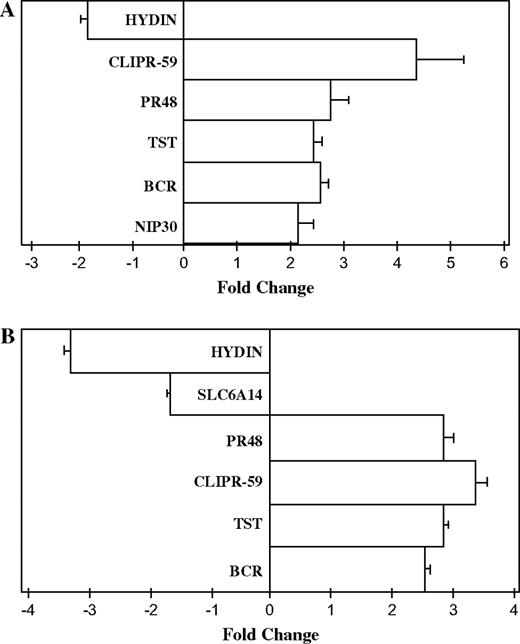
To confirm results from microarray gene expression profiling, we used real-time RT-PCR analysis to examine the effect of chlorpyrifos or cyfluthrin on the transcript levels of several genes. Figure 4A shows the effects of chlorpyrifos on the transcript levels of NIP30, BCR, PR48, CLIPR-59, and HYDIN. Figure 4B shows the effects of cyfluthrin on the transcript levels of BCR, TST, CLIPR-59, PR48, SLC6A14, and HYDIN. These changes detected in the transcript levels by real-time RT-PCR were largely consistent with the changes detected by microarray gene expression profiling.
Real-time RT-PCR analysis of the transcript levels of genes altered by chlorpyrifos (A) or cyfluthrin (B) in primary human astrocytes. Total RNA from three to six independent sets of human astrocytes was isolated, and real-time RT-PCR analysis was performed as described in the “Materials and Methods” section. β-Actin was used as an internal standard. The folds of induction of the indicated transcript levels were calculated by using Roche software. Abbreviations: NIP30, nucleobindin 2-interacting nuclear protein NIP30; BCR, breakpoint cluster region; TST, thiosulfate sulfurtransferase (rhodanese); PR48, protein phosphatase 2 regulatory subunit beta; CLIPR-59, CLIP-170–related protein; HYDIN, hydrocephalus inducing; and SLC6A14, solute carrier family 6 (amino acid transporter) member 14.
Chlorpyrifos and Cyfluthrin Altered the Expression of Diverse Genes Ranging from Chaperones to Signal Transducers
By using various gene ontology algorithms, we identified several functionally distinct classes of genes altered by chlorpyrifos or cyfluthrin. These include genes encoding chaperones, transporters, transcriptional regulators, and signal transducers, as well as genes encoding developmentally and behaviorally relevant functions (Table 1). Notably, our analysis identified 80 genes (57 upregulated and 23 downregulated) whose transcript levels were altered by chlorpyrifos and cyfluthrin in the same direction (Supplementary Data and S1B). The alteration of these genes might underlie the common neurotoxicity of both insecticides. Among them, several are known to play important roles in neural development and central nervous system (CNS) function (Supplementary Data and S1B). For example, the expression of CNR1, which plays important roles in signaling from the early stages of prenatal development (Mato et al., 2003), was upregulated by both chlorpyrifos and cyfluthrin. Four genes, including PRPH (Beaulieu et al., 1999), PRKCA (Birnbaum et al., 2004), STMN3 (Gavet et al., 1998), and CLIPR-59 (Perez et al., 2002), were upregulated by chlorpyrifos and cyfluthrin. The excessive activation or expression of these genes is known to cause serious dysfunction in the CNS (Beaulieu et al., 1999; Birnbaum et al., 2004; Gavet et al., 1998; Perez et al., 2002). In contrast, the transcript levels of HNT, HYDIN, and DNCH1 were downregulated by chlorpyrifos and cyfluthrin (Supplementary Data).
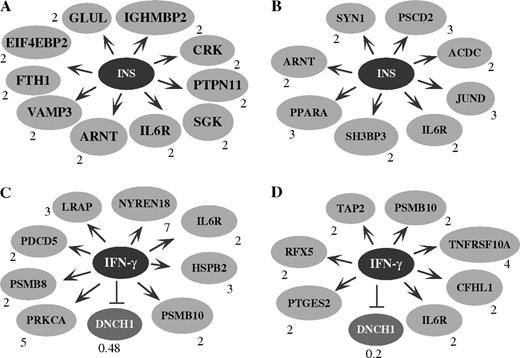
Four genes that act in insulin signaling—INSR, FRS2, PBEF, and SEC6L1 (Supplementary Data)—were selectively upregulated by chlorpyrifos or cyfluthrin. They all play positive roles in promoting insulin signaling in the CNS (Zick, 2004). Serendipitously, by using PathwayAssist, we also identified a series of chlorpyrifos or cyfluthrin upregulated genes, which are targets of the insulin-signaling pathway (Zick, 2004) (Figs. 5A and 5B). Chlorpyrifos caused upregulation of 10 genes that are targets of the insulin-signaling pathway, while cyfluthrin caused the upregulation of 8 insulin-targeted genes. The chlorpyrifos-upregulated genes (Fig. 5A) include GLUL, IGHMBP2, CRK, PTPN11, SGK, IL6R, ARNT, VAMP3, FTH1, and EIF4EBP2. The cyfluthrin-upregulated genes (Fig. 5B) include SYN1, PSCD2, ACDC, JUND, IL6R, SH3BP3, PPARA, and ARNT. The identification of these upregulated genes involved in insulin signaling suggests that both chlorpyrifos and cyfluthrin may affect insulin signaling.
The targets of the insulin- (INS, A and B) and IFN-γ (C and D)–signaling pathways are altered by both chlorpyrifos (A and C) and cyfluthrin (B and D). The chlorpyrifos- or cyfluthrin-altered genes in astrocytes were analyzed by using PathwayAssist software. Arrows indicate positive effects, while stopped lines indicate negative effects. The number next to each altered gene indicates the fold of change in the transcript level by chlorpyrifos or cyfluthrin. In A, it shows 10 chlorpyrifos-upregulated genes that are presumably positively affected by the insulin-signaling pathway. They are glutamine synthase (GLUL), immunoglobulin mu–binding protein 2 (IGHMBP2), v-CRK oncogene homolog (CRK), protein tyrosine phosphatase (PTPN11), serum- and glucocorticoid-regulated protein kinase (SGK), IL6R, aryl hydrocarbon receptor nuclear translocator (ARNT), vesicle-associated membrane protein (VAMP3), ferritin (FTH1), and translation initiation factor 4E–binding protein 2 (EIF4EBP2). In B, it shows eight cyfluthrin-upregulated genes that are presumably positively affected by the insulin-signaling pathway. They are synapsin I (SYN1); pleckstrin homology, Sec7 and coiled-coil domains 2 (PSCD2); adipocyte, C1Q and collagen domain–containing protein (ACDC); JUND, IL6R; SH3 domain–binding protein 3 (SH3BP3); peroxisome proliferative–activated receptor α (PPARA); and ARNT. In C, it shows eight chlorpyrifos-upregulated genes that are presumably positively affected by the IFN-γ–signaling pathway. They are leukocyte-derived arginine aminopeptidase (LRAP), NEDD8 ultimate buster 1 (NYREN18), HSPB2, proteosome subunit 10 (PSMB10), the α-subunit of protein kinase C (PRKCA), IL6R, proteosome subunit-8 (PSMB8), and programmed cell death 5 (PDCD5). The dynein cytoplasmic heavy polypeptide-1 (DNCH1) gene, which is presumably negatively affected by the IFN-γ–signaling pathway, was downregulated by chlorpyrifos. In D, it shows seven cyfluthrin-upregulated genes that are presumably positively affected by the IFN-γ–signaling pathway. They are the prostaglandin synthase (PTGES2), regulatory factor X (RFX5), transporter-2 ATP–binding cassette (TAP2), proteosome subunit 10 (PSMB10), IL6R, tumor necrosis factor receptor superfamily (TNFRSF10A), and complement factor H–related protein-1 (CFHL1). The DNCH1 gene was downregulated by cyfluthrin.
Beyond the 80 genes that were similarly altered by both insecticides, each specifically altered a series of genes encoding diverse functions (Table 1). For example, chlorpyrifos altered five genes encoding chaperones and five genes encoding functions relevant to behavior (Supplementary Data). Likewise, cyfluthrin altered three genes encoding chaperones and five genes encoding functions relevant to behavior (Supplementary Data). Chlorpyrifos upregulated heat shock protein (HSPB2), which accumulates in reactive astrocytes and degenerating neurons under various neuropathologies (Iwaki et al., 1997). Genes upregulated by cyfluthrin include TBCD and CRH. Overexpression of TBCD is associated with microtubule depolymerization and a progressive loss of microtubules (Martin et al., 2000). CRH acts as an important coordinator for neuroendocrine and behavioral responses to stress (McLean et al., 1995).
Another notable class of genes affected by chlorpyrifos and cyfluthrin are those involved in development (Table 1). Among chlorpyrifos-altered genes in this class, 22 were upregulated and 13 were downregulated (Table 1 and Supplementary Data). Two genes involved in cholinergic signaling, the cholinergic receptor CHRM3 and phospholipase C PLCE1, were altered upon exposure to chlorpyrifos (Supplementary Data). This is consistent with the previous finding that chlorpyrifos interferes with cholinergic signaling in animals (Garcia et al., 2001). Of the 35 genes altered by cyfluthrin, 26 were upregulated and 9 were downregulated (Table 1 and Supplementary Data). Some of them, such as NRXN3, EYA4, DRP2, and NR2E1, are known to play important roles in the CNS (Missler et al., 2003; Sherman et al., 2001; Shi et al., 2004; Wayne et al., 2001). Our analysis also revealed that chlorpyrifos altered the expression of 28 genes encoding transporters, while cyfluthrin altered the expression of 29 such genes (Table 1 and Supplementary Data and S4B). Chlorpyrifos upregulated SLC12A5, which functions as the main chloride extruder to promote fast-hyperpolarizing postsynaptic inhibition in the brain (Rivera et al., 1999) (Supplementary Data). Cyfluthrin upregulated the expression of AP2A1, HDLBP, and ABCD2 (Supplementary Data). Cyfluthrin downregulated TM4S11, which is a proteolipid protein with a role in the formation of ion channels (Fischer and Sapirstein, 1994).
The alteration of gene expression by chlorpyrifos and cyfluthrin is presumably mediated by their effects, directly or indirectly, on the levels or activities of transcriptional regulators and/or their upstream effectors, that is, signal transducers. Indeed, chlorpyrifos caused changes in the expression levels of 55 signal transducers (41 up- and 14 downregulated, Table 1 and Supplementary Data) and 24 transcriptional regulators (21 upregulated and 3 downregulated, Table 1 and Supplementary Data). Similarly, cyfluthrin caused the alteration of 69 genes encoding signal transducers (49 upregulated and 20 downregulated, Table 1 and Supplementary Data) and 34 transcriptional regulators (29 upregulated and 5 downregulated, Table 1 and Supplementary Data). Chlorpyrifos downregulated two important signal transducers, LRP2 and AKAP7. Notable signal transducers upregulated by cyfluthrin include HTR2A and HRH4. Further, cyfluthrin downregulated PMCHL1, TAC1, and TAS2R14, all of which play important roles in signal transduction in the CNS (Kinnamon, 2000; Pedeutour et al., 1994; Troger et al., 2001).
Both Chlorpyrifos and Cyfluthrin Upregulated the Expression of Proinflammatory Mediators
Intriguingly, by using the computational program PathwayAssist (Stratagene Software), we found a group of chlorpyrifos- or cyfluthrin-altered genes that are downstream targets of the proinflammatory interferon-γ (IFN-γ)–signaling pathway (Figs. 5C and 5D). Chlorpyrifos caused the upregulation of eight genes that are positively affected by the IFN-γ–signaling pathway and the downregulation of one negatively affected target (Schroder et al., 2004). These upregulated genes include LRAP, NYREN18, HSPB2, PSMB10, PSMB8, PRKCA, IL6R, and PDCD5 (Fig. 5C). The downregulated gene is DNCH1. Likewise, cyfluthrin caused the upregulation of seven downstream targets that are positively affected by the IFN-γ–signaling pathway (Schroder et al., 2004) and the downregulation of DNCH1. The upregulated genes are PTGES2, RFX5, TAP2, PSMB10, TNFRSF10A, IL6R, and CFHL1 (Fig. 5D).
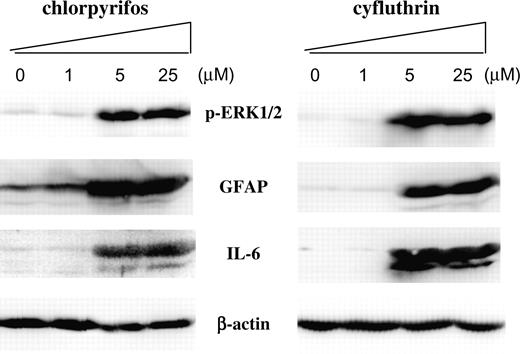
The upregulation of these proinflammatory targets suggests that chlorpyrifos and cyfluthrin may cause inflammatory activation of astrocytes. IL-6 is a major proinflammatory mediator, and its upregulation is often associated with brain inflammation. In addition, the upregulation of GFAP is also strongly associated with astrocyte activation (Wayne et al., 2001). Although the transcript levels of IL-6 and GFAP were not significantly affected by chlorpyrifos or cyfluthrin, these insecticides may alter their protein levels. We therefore examined the effects of chlorpyrifos and cyfluthrin on IL-6 and GFAP protein levels (Fig. 6). Indeed, we found that both chlorpyrifos and cyfluthrin significantly increased the protein levels of IL-6 and GFAP, at 5 and 25μM concentrations. As controls, we showed that these insecticides did not considerably affect β-actin protein levels (Fig. 6).
The effects of chlorpyrifos and cyfluthrin on the protein levels of IL-6, GFAP, phosphorylated extracellular signal–regulated kinase 1/2 (p-ERK1/2), and β-actin. Human fetal astrocytes were treated with the indicated concentrations of chlorpyrifos or cyfluthrin, as described in the “Materials and Methods” section. Then protein extracts were prepared and subjected to Western blotting analysis. The PVDF membranes were probed with polyclonal antibodies against IL-6, GFAP, phospho(Thr202/Tyr204)-ERK1/2 (p-ERK1/2), and β-actin, respectively. Equal amounts of cellular proteins were loaded in every lane, as confirmed by the levels of β-actin.
Notably, we also found that both chlorpyrifos and cyfluthrin significantly enhanced the levels of activated (phosphorylated) ERK1/2 (Fig. 6). Because ERK1/2 is a key component of insulin signaling, this increase is consistent with the finding that targets of the insulin-signaling pathway were upregulated by the insecticides, as shown above. The results from Western blotting analysis show that chlorpyrifos and cyfluthrin exerted similar effects on the expression of inflammatory markers IL-6 and GFAP, as well as on the signaling component ERK1/2.
DISCUSSION
In this report, to gain insights into the neurotoxicity of cyfluthrin, we compared the toxicological and toxicogenomic effects of chlorpyrifos and cyfluthrin on primary human fetal astrocytes. Although these studies do not address the issues of absorption, accumulation, and distribution in the brain, previous studies in animals have shown that cyfluthrin, like chlorpyrifos, can be delivered to the brain and affect brain function (Shafer et al., 2005). Thus, the present comparative studies of chlorpyrifos and cyfluthrin may provide important insights into the neurotoxic potential of these insecticides.
Our results provide several important clues about the potential neurotoxicity of the commonly used pyrethroid cyfluthrin and perhaps other pyrethroids. First, the evidence presented here suggests that cyfluthrin is at least as toxic as, if not more toxic than, chlorpyrifos to human astrocytes. Toxicological data (Figs. 1 and 2) suggest that cyfluthrin exerted somewhat stronger cytotoxic effects on primary human astrocytes. Likewise, toxicogenomic data showed that cyfluthrin altered the transcript levels of as many genes as did chlorpyrifos (Fig. 3). Note that a significant reduction in astrocyte cell number and a clear induction of apoptosis occurred only at high concentrations of chlorpyrifos and cyfluthrin (Figs. 1A and 2). Such concentrations are unlikely to be directly relevant to human exposures. Nevertheless, the relative toxicity of chlorpyrifos and cyfluthrin reflected by these effects at high concentrations is consistent with their degrees of toxicity revealed by more sensitive assays, including glutamate uptake and LDH release, at lower concentrations. Taken together, both toxicological and toxicogenomic data support the idea that cyfluthrin is somewhat more toxic than chlorpyrifos across a range of concentrations.
Second, both chlorpyrifos and cyfluthrin appeared to alter a limited number of, but diverse, genes with functions critical for neural development and CNS function (see Supplementary Data, S1B, S3A, and S3B). This is consistent with previous studies in animals showing that chlorpyrifos interferes with many processes of neural development by multiple mechanisms (Eriksson and Talts, 2000; Pope, 1999; Slotkin, 1999, 2004a,b). Of particular interest are those genes whose altered expression is known to cause problems in neural development or CNS function, for example, PRPH, PRKCA, STMN3, and CLIPR-59 (Beaulieu et al., 1999; Birnbaum et al., 2004; Gavet et al., 1998; Perez et al., 2002). Although it remains to be determined whether the alteration of these identified genes occurs in humans, the finding that the transcript levels of diverse genes were altered by chlorpyrifos and cyfluthrin (Table 1 and Supplementary Data–S7) in human fetal astrocytes may provide a plausible reason for a further examination and assessment of the neurotoxicity of cyfluthrin and other pyrethroids.
Third, results from both biochemical and microarray data analyses suggest that chlorpyrifos and cyfluthrin promote inflammatory activation of astrocytes. Several targets of the IFN-γ– and insulin-signaling pathways were upregulated. IFN-γ is the principal macrophage-activating cytokine and plays a critical part in innate immunity and adaptive cell-mediated immunity (Schroder et al., 2004). It also plays a major immunomodulatory role in astrocytes, and the activation of its signaling pathway leads to the expression of various inflammation-associated molecules (Schroder et al., 2004). Indeed, some of the genes upregulated by chlorpyrifos or cyfluthrin (Figs. 5C and 5D), such as HSPB2, IL6R, and RFX5, are known to be associated with activated astrocytes or are proinflammatory (Durand et al., 1997; Iwaki et al., 1997). More importantly, the proinflammatory mediator IL-6 and the marker of astrocyte activation GFAP were both strongly activated by chlorpyrifos and cyfluthrin (Fig. 6).
Inflammatory activation of astrocytes or astrocyte dysfunctions have been shown to be associated with many CNS diseases, such as bacterial or viral infection, multiple sclerosis, prion infection, Parkinson disease, Alzheimer disease, and ischemia (Gebicke-Haerter, 2001; Wang et al., 2004). Likewise, uncontrolled astrocyte activation in the developing brain of children is likely to cause neurological problems. The observed effects of chlorpyrifos and cyfluthrin on the proinflammatory functions in purified, homogeneous populations of primary human astrocytes raise the possibility that astrocyte activation may be a mechanism causing neuronal dysfunction and neuronal injuries in the human brain. Furthermore, our computational and biochemical analyses suggest that insulin signaling is enhanced by chlorpyrifos and cyfluthrin. During chronic inflammation, insulin action may exacerbate the inflammatory response and increase oxidative stress (Craft and Watson, 2004). The activation of the insulin-signaling pathway by the insecticides may synergize with the IFN-γ–signaling pathway to promote inflammatory action of astrocytes and ultimate neuronal injuries.
In summary, our comparative toxicogenomic, molecular, and toxicological studies of chlorpyrifos and cyfluthrin provide a global view of their potential neurotoxic actions in primary human fetal astrocytes. Such global insights would not have been gained without the use of genomic approaches. Clearly, further studies are necessary to assess the neurotoxicity of insecticides in humans and to understand the mechanisms of insecticide toxicity. Nonetheless, our studies may serve as a starting point for further detailed analysis and assessment of neurotoxicity of cyfluthrin and related insecticides.
This work was supported in part by public health grants HL65568 (L.Z.) and NS31492 (G.B. and D.J.V.) and pilot project funds from 5P30 ES009089 and 5P01 ES009600. We are grateful to Dr Michael Aschner for advice on toxicological assays.
References
Aldridge, W. N. (
Barone, S., Jr, Das, K. P., Lassiter, T. L., and White, L. D. (
Beaulieu, J. M., Nguyen, M. D., and Julien, J. P. (
Birnbaum, S. G., Yuan, P. X., Wang, M., Vijayraghavan, S., Bloom, A. K., Davis, D. J., Gobeske, K. T., Sweatt, J. D., Manji, H. K., and Arnsten, A. F. (
Canki, M., Thai, J. N., Chao, W., Ghorpade, A., Potash, M. J., and Volsky, D. J. (
Cantalamessa, F. (
Caughlan, A., Newhouse, K., Namgung, U., and Xia, Z. (
Colt, J. S., Lubin, J., Camann, D., Davis, S., Cerhan, J., Severson, R. K., Cozen, W., and Hartge, P. (
Craft, S., and Watson, G. S. (
Durand, B., Sperisen, P., Emery, P., Barras, E., Zufferey, M., Mach, B., and Reith, W. (
United States Environmental Protection Agency (USEPA) (
Eriksson, P., and Talts, U. (
Eskenazi, B., Bradman, A., and Castorina, R. (
Fischer, I., and Sapirstein, V. S. (
Garcia, S. J., Seidler, F. J., Crumpton, T. L., and Slotkin, T. A. (
Garcia, S. J., Seidler, F. J., Qiao, D., and Slotkin, T. A. (
Gavet, O., Ozon, S., Manceau, V., Lawler, S., Curmi, P., and Sobel, A. (
Gebicke-Haerter, P. J. (
Iwaki, A., Nagano, T., Nakagawa, M., Iwaki, T., and Fukumaki, Y. (
Magisretti, P. J., and Ransom, B. R. (
Martin, L., Fanarraga, M. L., Aloria, K., and Zabala, J. C. (
Mato, S., Del Olmo, E., and Pazos, A. (
McLean, M., Bisits, A., Davies, J., Woods, R., Lowry, P., and Smith, R. (
Missler, M., Zhang, W., Rohlmann, A., Kattenstroth, G., Hammer, R. E., Gottmann, K., and Sudhof, T. C. (
Mutkus, L., Aschner, J. L., Syversen, T., Shanker, G., Sonnewald, U., and Aschner, M. (
Pedeutour, F., Szpirer, C., and Nahon, J. L. (
Perez, F., Pernet-Gallay, K., Nizak, C., Goodson, H. V., Kreis, T. E., and Goud, B. (
Pope, C. N. (
Ransom, B., Behar, T., and Nedergaard, M. (
Rivera, C., Voipio, J., Payne, J. A., Ruusuvuori, E., Lahtinen, H., Lamsa, K., Pirvola, U., Saarma, M., and Kaila, K. (
Schroder, K., Hertzog, P. J., Ravasi, T., and Hume, D. A. (
Shafer, T. J., Meyer, D. A., and Crofton, K. M. (
Sheets, L. P. (
Sherman, D. L., Fabrizi, C., Gillespie, C. S., and Brophy, P. J. (
Shi, Y., Chichung Lie, D., Taupin, P., Nakashima, K., Ray, J., Yu, R. T., Gage, F. H., and Evans, R. M. (
Slotkin, T. A. (
Slotkin, T. A. (
Slotkin, T. A. (
Soderlund, D. M., Clark, J. M., Sheets, L. P., Mullin, L. S., Piccirillo, V. J., Sargent, D., Stevens, J. T., and Weiner, M. L. (
Song, X., Violin, J. D., Seidler, F. J., and Slotkin, T. A. (
Surgan, M. H., Congdon, T., Primi, C., Lamster, S., and Louis-Jacques, J. (
Troger, J., Neyer, S., Heufler, C., Huemer, H., Schmid, E., Griesser, U., Kralinger, M., Kremser, B., Baldissera, I., and Kieselbach, G. (
Wang, Z., Trillo-Pazos, G., Kim, S. Y., Canki, M., Morgello, S., Sharer, L. R., Gelbard, H. A., Su, Z. Z., Kang, D. C., Brooks, A. I., et al. (
Wayne, S., Robertson, N. G., DeClau, F., Chen, N., Verhoeven, K., Prasad, S., Tranebjarg, L., Morton, C. C., Ryan, A. F., Van Camp, G., et al. (
Whyatt, R. M., Barr, D. B., Camann, D. E., Kinney, P. L., Barr, J. R., Andrews, H. F., Hoepner, L. A., Garfinkel, R., Hazi, Y., Reyes, A., et al. (
Whyatt, R. M., Camann, D. E., Kinney, P. L., Reyes, A., Ramirez, J., Dietrich, J., Diaz, D., Holmes, D., and Perera, F. P. (
Zick, Y. (
Author notes
*Department of Environmental Health Sciences, Columbia University, Mailman School of Public Health, New York, New York 10032; and †Molecular Virology Division, St Luke's–Roosevelt Hospital Center and College of Physicians and Surgeons, Columbia University, New York, New York 10019




Comments