-
PDF
- Split View
-
Views
-
Cite
Cite
Shira Natanson-Yaron, Eyal Y. Anteby, Caryn Greenfield, Debra Goldman-Wohl, Yaron Hamani, Drorit Hochner-Celnikier, Simcha Yagel, FGF 10 and Sprouty 2 modulate trophoblast invasion and branching morphogenesis, Molecular Human Reproduction, Volume 13, Issue 7, July 2007, Pages 511–519, https://doi.org/10.1093/molehr/gam034
Close - Share Icon Share
Abstract
Branching morphogenesis (BM) of the chorionic villous tree is a crucial component of early placental formation. Fibroblast growth factors (FGFs), their receptor tyrosine kinase (RTK) and negative regulators like Sprouty (Spry) proteins are pivotal factors in the development of diverse branching organ systems. The aim of this study was to examine the effect of FGF10 and Sprouty 2 on BM of the chorionic villi in vitro. Villous explants of first trimester placentas were cultured and their outgrowths were monitored. The effect of FGF10 was tested on matrigel migration/invasion assay, collagenolytic activity of single cell trophoblasts and on villous explants outgrowths. siRNA of Spry2 was used to reduce its expression and to investigate the role of Sprouty 2 in villous explants outgrowths. Quantitative RT–PCR and immunohistochemistry were performed to determine Sprouty 2 and HLA-G (a marker of invasion) expression. FGF 10 stimulated by 8-fold the migration/invasion of single cell trophoblast enhanced their collagenolytic activity. Reduction of Spry2 expression in villous explants showed a marked increase in villous outgrowths. This was accompanied by enhanced staining for HLA-G and by the reduction of Spry2 expression that was confirmed by immunohistochemistry and by quantitative RT–PCR. We conclude that trophoblast outgrowth and invasion (part of placental villi sprouting) at the fetal maternal interface is in part under delicate control of FGF 10 and Sprouty 2. FGF 10 promotes invasion and outgrowth of trophoblasts. In addition, it increases Spry2 expression, which attenuates trophoblast sprouting.
Introduction
The primary function of the placenta is to promote transport of nutrients and O2 essential for survival and growth of the fetus during gestation (Kingdom et al., 2000). The placenta, like many organs such as the lung, kidney and the vascular system, is composed of a network of tubes (Cross et al., 2003). The formation of these 3D structures is a common mechanism of development termed branching morphogenesis (BM).
Maldevelopment of the placental villous tree has been associated with pathogenesis of various gestational diseases. It has been shown that poor neonatal outcome associated with pre-eclampsia, intrauterine growth restriction (IUGR) or fetal asphyxia are closely related etiologically to abnormal placenta (Benirschke, 1995).
BM involves several cellular processes including migration, proliferation, cell-matrix interaction and repeated cycles of bud initiation, bud outgrowth and bud arrest (Davies, 2002). The branching process is initiated by selecting a subgroup of cells that are induced to become motile and invasive. This invasive behavior might be a general feature or even the driving force of BM, because branches enter surrounding tissues (Affolter et al., 2003). Signaling by fibroblast growth factors (FGFs) through their receptor tyrosine kinase (RTK) coordinates diverse biological processes including angiogenesis, proliferation, differentiation and BM. FGF10 is a key positive regulator of lung (Mailleux et al., 2005), limb (Min et al., 1998) and eye BM in mice (Tao et al., 2005) and in ovine placenta development (Chen et al., 2000).
Normal BM is tightly controlled by several regulatory proteins. Sprouty 2, a membrane-associated protein, has been characterized as a regulator of the FGF/EGF signaling pathway in modeling tissue growth and development (Sasaki et al., 2001). Historically, Sprouty 2 was identified as an antagonist of the RTK pathway in a number of developmental processes (Reich et al., 1999). Recently, it was demonstrated that an Src homology binding sequence on the C terminus of Sprouty 2 interacts with Grb2 resulting in the inhibition of phosphorylation of ERK (Lao et al., 2006).
However, other results indicate that in some physiological processes, Sprouty 2 positively modulates RTK signaling by binding to c-Cbl, an ubiqutin ligase that plays a role in down-regulation of RTK (Levkowitz et al., 1999).
In order to study human placenta BM, we previously examined the expression of FGFR 1–4 and FGF10 in placentas and in decidua. We found that FGFR 1–4 are expressed in placenta but not in the decidua, whereas FGF10 is expressed by decidual cells and by the placenta, especially the extravillous trophoblasts (EVT) of the columns (Anteby et al., 2005b). The expression of Sprouty 2 through the three trimesters was also examined. We showed that Spry2 is expressed throughout the three trimesters by placental villous macrophages [Hofbauer cells (HC)] (Anteby et al., 2005a) and that FGF10 induces Spry2 expression in HC culture.
These findings suggest that mesenchymal-epithelial interaction and cross-talk between the different cell types of the placenta may play a role in regulation of placental development.
In the present study, we focused on the two major processes of BM: migration and invasion. To this end, we investigated the placental development correlates of FGF10 and Sprouty 2 using villous organ culture. We demonstrated that exogenous FGF10 promotes invasion and outgrowth of villi in organ culture. FGF10 also up-regulates the activity of metalloproteases in trophoblast cell culture and increases the migration of single cell trophoblasts through matrigel. Silencing of Spry2 expression by siRNA also enhanced the outgrowth of trophoblasts.
Taken together, these findings suggest that Spry2 is a potential regulator of FGF10 activity. As FGF10 is a strong inducer of cell migration and collegenolytic activity (an imperative step of invasion), we hypothesize that trophoblast outgrowth and invasion at the fetal-maternal interface are in part under the delicate control of FGF10 as a positive regulator and Sprouty 2 as a negative regulator.
Material and Methods
Trophoblast single cell isolation
Trophoblast cells were isolated as described by Kliman et al. (1986) with some modifications. Villous tissues obtained from first trimester legal interruptions of pregnancy were obtained with approval of our Institutional Review Board. Placenta from weeks 7–10 was used. The tissue was rinsed with PBS and subjected to digestion with HBSS containing 0.125% trypsin (Sigma, St. Louis, USA) and 0.2 mg/ml DNase 1 (Sigma). The cells were then washed in DMEM:F12 medium containing 15% newborn calf serum (Biological industries, Beit Haemek, Israel). The collected cells were applied to 15–70% Percoll (Amersham Biosciences AB, Uppsala, Sweden) gradient and centrifuged at 1200g for 30 min at 30–34°C. The cells from layers 35–55% were collected, washed and resuspended in DMEM:F12, 15% FCS media. The cells were next incubated for 30 min on plastic plates at 37°C, 5% CO2 to reduce the monocytes. The medium containing the purified trophoblasts was removed for further use. The purity of the isolated trophoblasts was verified as we describe before (Hanna et al., 2006) by FACS analysis. These cells are 99% HLA-G positive, 98% Cytoceratin 7 positive and less then 0.7% Vimentin positive.
Ex vivo explant culture
Villous explant cultures from weeks 7–9 were established from first trimester human pooled placentas as described previously (Caniggia et al., 2000) with a number of modifications. Briefly, placental tissue was placed on ice-cold saline and processed within 2 h of collection. The tissue was aseptically dissected to remove decidual tissue and fetal membranes. Small fragments of placental villi were placed on four well glass slides (LAB-TEK®, Nalge Nunc, Naperville, IL, USA), precoated with growth factor reduced matrigel (BD biosciences, NJ, USA). Explants were cultured in serum-free DMEM:F12 (Biological industries) supplemented with 160 µg/ml gentamycin. The explants were incubated at 37°C in an atmosphere of 4–8% or 20% O2, 5% CO2. For further investigation, the explants were fixed for 2–4 h at 4°C in 4% (vol/vol) paraformaldehyde, embedded in paraffin and cut into 10-µm sections.
Migration-invasion assay
Transwell polycarbonate membrane filters measuring 6.5 mm in diameter with a 8-µm diameter pore (Costar, Corning Incorporated, NY, USA) were plated with 50 µl of 75% GFR-Matrigel (Growth Factor Reduced-Matrigel BD Biosciences, NJ, USA) diluted in ice-cold 1 × PBS and placed in a 37°C incubator. About 50 µl of serum- and antibiotics-free medium DMEM:F12 was added after 20 min.
Fifty microliter (2 × 105/well) of single cell trophoblasts suspended in serum/antibiotics-free medium DMEM:F12 was placed in the upper chamber.
The lower chamber was filled with 600 µl of serum/antibiotics-free medium DMEM:F12 as a control, or with 100 or 250 ng/ml FGF10 (human recombinant FGF10, R&D systems, Minneapolis, MN, USA). FGF10 concentration was chosen in accordance with the manufactures ED50. Heat denaturated FGF10 (boiled for 10 min) was used to specify the effect of FGF10. The chamber was placed in a 37°C incubator in an atmosphere of 5–8% O2.
The cells were allowed to migrate/invade for 4 days. The media with non-invaded cells and matrigel were then removed carefully from the upper chamber. The filter with the migrated cells was stained and fixed with crystal-violet solution for 1 h. After several washes with 1 × PBS, the filter was cut off of the transwell and placed on a glass slide, with the down side of the filter facing up. For quantification, the cells on the lower surface of the filter were counted under a microscope at 400 × magnification in three different fields. The assay was performed in triplicates and each experiment was repeated three times.
Zymography
Trophoblasts isolated from legal interruptions of first trimester pregnancy were suspended in DMEM:F12 medium with 15% FCS and grown for 96 h in 4–8% O2. The cells were then washed and resuspended in medium without serum for 8–24 h in the presence or absence of 250 ng/ml FGF10 (R&D Systems). Zymography was used to assess trophoblast plasminogen activator (uPA) and gelatinolytic metalloproteinase (MMP9 and MMP2) activity. βhCG levels in the medium were examined to validate that an equivalent number of cells were analyzed in each sample (Anteby et al., 2004). Media βhCG levels did not differ between the groups. Zymography of secreted proteins from various experiments was carried out by electrophoresis in 10% sodium dodecyl sulphate polyacrylamide (SDS–PAGE) gels impregnated with 3 mg/ml gelatin to demonstrate the presence of gelatinolytic metalloproteinase: MMP-9 and MMP-2 in conditioned media. Gels impregnated with casein (3 mg/ml) and plasminogen (5 mg/ml) were used to demonstrate the presence of uPA. Following incubation activation, gels were stained with 0.25% Coomasie Blue R-250 (Bio-Rad Labs, Ramsey, MN, USA) in 30% isopropanol and 10% glacial acetic acid. Destaining was preformed with 30% isopropanol and 10% acetic acid. The molecular weights were estimated by comparing their electrophoretic migration with that of protein standards. Enzyme activity was quantified by computerized image analysis with 2D scanning densitometry (Bio-Imaging System 202D).
Each experiment was standardized relative to control where control is considered 100. Results are presented as mean ± SE. We used the one sample t-test to compare the effect of FGF10 and control. The average results of each parameter are compared with a value of 100. For comparison of different doses, the Many–Whitney non-parametric test was applied. P < 0.05 was considered significant.
SiRNA design, transfection and silencing Spry2 expression
About 96 h villi explants cultured at 4–8% or 20% O2 were transfected with 200 nM of SMARTpool siRNA oligos (Dharmacon, Lafayette, CO, USA) in a serum-free media (DMEM:F12) using Oligofectamine reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions, based on published sequences of human Spry2 used as follows: 5́UCAGAGCCAUCCGAAACAC3́, 5́CUCGCAGGUCCAUUCUUCU3́, 5́AAUGUAAGGAGUGCACCUA3́, 5́UCACUGUUCUAAUGAGUAU3́.
Controls included non-specific control duplex (Dharmacon) and tranfection reagent lacking siRNA. About 72–96 h post-transfection, the explants were either fixed for 2–4 h at 4°C in 4% (vol/vol) paraformaldehyde and embedded in paraffin or used for total RNA purification.
cDNA preparation
Total RNA purification from villi explant culture was performed using RNAeasy (Qiagen, Hamburg, Germany) according to the manufacturer's instructions. Total RNA from each experiment (2 µg) was converted to single stranded cDNA using Superscript II (Invitrogen) with random primers (Promega, Madison, WI, USA) in a total volume of 20 µl.
Quantitative RT–PCR
About 100–250 ng of cDNA was used for quantitative RT–PCR (GeneAMP 5700, PE-Biosystems system, USA). SYBR Green fluorescent intercalating agent was used for Spry2 amplification, and TaqMan master mix for 18S and HLA-G amplification (Applied Biosystems, Foster City, CA, USA), according to the manufacturer's instructions. The 18S sequence was used as a reference. Quantification of relative gene expression was performed as described in the User's Bulletin. Results were normalized to the levels of 18S expression and are presented as mean ± SD fold-change. The results were analyzed using Student's t-test, with P < 0.05 determined significant.
Primers for spry2 were forward 5́CATGGGTGTCATGTCCCTCTT3́ and reverse 5́GCCTGTTAACCCGGTCATAACA3́. For 18S and HLA-G, we used ‘assay on demand’ Hs99999901s1 and Hs00365950q1, respectively (Applied Biosystems).
Immunohistochemistry
Immunohistochemistry was performed using the Histostain-Plus kit (Zymed laboratories Inc., South San Francisco, CA, USA). Briefly, frozen placental tissue/cells sections (5–6 µm) were fixed on ice-cold acetone for 10 min and quenched with 3% hydrogen peroxidase to eliminate endogenous peroxidase activity. The slides were washed, blocked and incubated at room temperature with primary antibodies using the dilutions stated below. Primary antibodies used rabbit polyclonal anti-human-Sprouty 2 (1:500) (Upstate Biotechnology, Lake Placid, NY, USA), mouse polyclonal anti-human HLA-G (1:25) (4H84, kind gift of M. McMaster), mouse anti-human CD68, ready to use, (Dako Corporation, USA) and (1:5) goat anti-human FGF-10 (R&D System). Secondary antibodies used Universal Immunoperoxidase polymer anti-goat (Histofine-Nichirei Corporation, Japan) and EnVision, Peroxidase, mouse/rabbit (DAKO Corporation, Glostrup, Denmark). The slides were then developed with a substrate-chromagen solution of aminoethyl carbazole—AEC (Sigma). The immunohistochemical analysis was repeated four times. For negative controls, we used each of the secondary antibodies.
For paraffin embedded samples, antigen was retrieved prior to the immunohistochemistry procedure. Antigen was retrieved by microwaving, the section in 10 mM sodium citrate for 5 min.
When initially characterizing the antibodies, isotype matched controls or preimmune serum were used to assure that there was no non-specific binding.
Results
FGF10 increases ex-vivo outgrowth of the villous explants
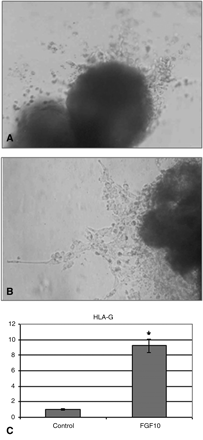
We tested the effect of addition of 250 ng/ml FGF10 on ex vivo BM of villous explants from 6–8 weeks gestation cultured under 4–8% O2 layered on growth factor reduced matrigel. Explants exposed to 250 ng/ml FGF10 displayed prominent outgrowth and increased number of cells migrating into the surrounding matrix 72 h after addition of FGF10 (Fig. 1B), whereas the non-treated culture showed less outgrowth and migration (Fig. 1A). A similar effect was demonstrated in culture under 20% O2 (data not shown). The expression of HLA-G in these explants was studied as well by quantitative RT–PCR. HLA-G expression was increased 9-fold (Fig. 1C) compared with their expression in the control culture.
The effect of FGF10 on EVT outgrowth and migration in villous explant culture. Representative pictures of villous explants from 6–9 weeks' gestation that were cultured under condition of 4–8% O2 for 72 h with media alone (A) or with the addition of 250 ng/ml FGF10 (B). Quantitative RT–PCR demonstrating the effect of FGF10 on HLA-G expression compared to the control (C). Results are normalized to the levels of 18S and are presented as mean ± SD fold-change. *P < 0.05 compared with control
FGF-10 increases the migration/invasion of single cell trophoblast
To investigate the effect of FGF-10 on trophoblast migration/invasion, cells were layered on Matrigel-coated filter in a transwell assay. The migration was tested with serum-free medium, heat inactivated FGF10, 100 or 250 ng/ml of FGF-10 in the lower chamber.
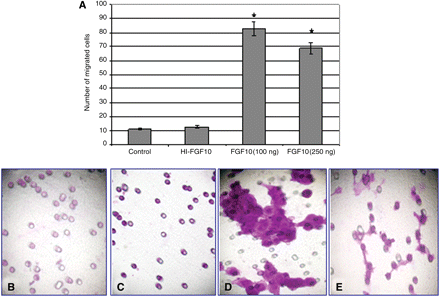
Compared to controls, 100 and 250 ng/ml of FGF-10 enhanced the ability of the cells to migrate through the Matrigel 7–8-fold and to make cell extensions (Fig. 2A). The formation of cell extensions seems to be slightly reduced using increased amounts of FGF10 (250 ng/ml). Addition of heat denaturated FGF10 led to results similar to control (Fig. 2B–E).
FGF 10 increases single cell trophoblasts (SCTs) migration and invasion through matrigel. SCTs were incubated on GFR-matrigel coated chamber with ‘heat inactivated’ FGF10 (HI FGF10), 100, 250 ng/ml FGF10 or with medium alone in the lower compartment. After 96 h, the cells on the lower surface of the filter were counted and the relative migration rate was determined. Graphs demonstrating the average migration rate from three migration/invasion assays (A). The Y-axis represents the average of cells that were counted in three different fields from three different tests. Representative migration pattern and extension formation in control, HI- FGF10, 100 and 250 ng/ml FGF10 (B–E), respectively
FGF10 stimulates uPA and Gelatinase activity
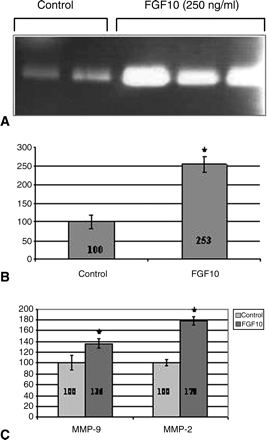
Since FGF10 increases the migration and invasion of trophoblasts and villous explants, we tested its effect on the uPA and gelatinase (MMPs) activity using zymography. Exposure to 250 ng/ml FGF10 increased the uPA activity by 153% (Fig. 3A and B) and the MMP-9 and MMP-2 activity by 36% and 78%, respectively, compared with the baseline level of activity (Fig. 3C).
FGF 10 (250 ng/ml) increases trophoblast proteases uPA and MMPs activity. Densitometry of three zymographic studies is presented as mean ± SE. Results are standardized relative to control where control is considered 100. Representative gel of uPA activity (A), the effect of FGF 10 on uPA activity (B) and the effect of FGF 10 on MMP-9, MMP-2 activity (C). *P < 0.05 compared with control
Reduction of Spry2 by siRNA silencing increases villous outgrowths
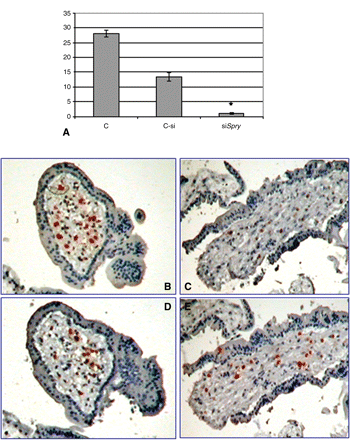
Quantitative RT–PCR on villous explants transfected with four siRNAs targeting human Spry2 revealed that 72 h following transfection, Spry2 expression was reduced 28-fold compared with the control, whereas the reduction was only ∼2-fold with non-specific siRNA (Fig. 4A).
Spry2 siRNA transfection reduced Spry2 expression in villous explants culture. Graphs summarizing three studies showing the reduction of Spry2 expression 72 h post-transfection (A). C, non-treated culture; Csi, non-specific siRNA; siSpry, Spry2 siRNA as determined by quantitative RT–PCR. Results are normalized to the levels of 18S and are presented as mean ± SD fold-change. *P < 0.05 compared with control. Representative immunohistochemistry using anti-Sprouty 2 (B and C) and anti-CD68 (D and E) antibodies on non-treated culture (B and D) and siSpry2 transfected culture (C and E).
Immunohistochemistry using anti-human Sprouty 2 demonstrated a similar picture: the staining intensity was markedly reduced in the villous explants 72 h post-Spry2 siRNA transfection when compared with the control (Fig. 4B and C).
Immunohistochemistry using anti-CD68 (marker for macrophages) showed no differences in staining between the control and treated cultures (Fig. 4D and E).
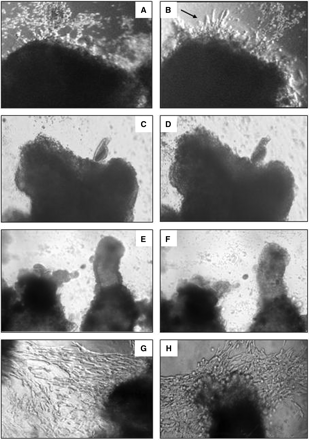
In order to determine the role of sprouty 2 in our model, we tested the effect of reduction of Spry2 expression on villous explants from 6–8 weeks' gestation cultured under condition of 4–8% or 20% O2. Under 20% O2 conditions, the reduction of Spry2 expression stimulated EVT outgrowth from distal ends of the villi, 96 h post-transfection (Fig. 5A and B), whereas in the control explants (Fig. 5C and D) and the non-specific siRNA (Fig. 5E and F), almost no budding or outgrowth was seen. The effect of the reduction of Spry2 expression on villous explants cultured under 4–8% O2 was hardly detected (Fig. 5G and H).
Reduction of Spry2 expression increases budding and outgrowth of EVT under condition of 20% O2 but is hardly detectable in culture under condition of 4–8% O2. Representative pictures of villous explants from 6–9 weeks' gestation cultured under 20% O2 (A–F) or 4–8% O2 (G and H) were transfected with Spry2 siRNA (A, B and G). Control experiments were run in parallel using explants from the same placentas: non-specific siRNA (E and F), without siRNA (C, D and H). Pictures taken 48 h (A, C, E and G) and 96 h (B, D, F and H) post-transfection
Reduction of Spry2 expression increased HLA-G expression in villous explant culture
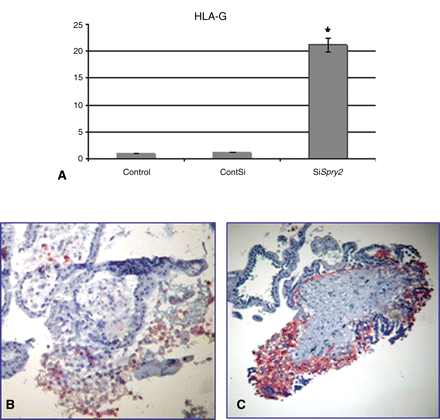
Since the reduction of expression of Spry2 increased villous outgrowth, we tested its effect on the expression of HLA-G. Quantitative RT–PCR revealed 21-fold enhancement of HLA-G expression compared with control and non-specific siRNA (Fig. 6A). In addition, immunohistochemistry using anti-HLA-G antibody demonstrated enhanced staining of HLA-G in the transfected villi compared with the control (Fig. 6B and C).
Reduction of Spry2 expression increases HLA-G expression in explants cultured under condition of 20% O2. Graphs summarizing three studies showing the effect of reduction in Spry2 expression by siRNA on HLA-G expression compared with control as determined by quantitative RT–PCR (A) control-non-treated culture, ContSI-none specific siRNA, SiSpry-Spry2 siRNA. Results are normalized to the levels of 18S and are presented as mean ± SD fold-change. *P < 0.05 compared to control. Immunohistochemistry using anti-HLA-G antibody on control culture (B) or Spry2 siRNA transfected culture (C)
Discussion
Successful pregnancy depends on proper placental growth and branching. These processes include differentiation, proliferation and bud initiation of the trophoblast cells. In addition, it involves matrix degradation to allow the migration and invasion of the trophoblasts into the decidua and maternal vessels (Red-Horse et al., 2005).
We have previously shown that FGF10, the FGF receptors 1–4 and Sprouty 2 are expressed in diverse cell types of the placenta. However, the correlation between these factors and their effect on the BM of the villi is not yet characterized. Herein, we suggest that FGF10 and Sprouty 2 are important regulators of placental BM, acting as positive and negative regulators of this process. Moreover, we demonstrate that placental BM is executed by mesenchymal-epithelial cell-to-cell talk as has been shown in BM of other systems (Hogan and Yingling, 1998).
We showed that stimulation of ex vivo explants with exogenous FGF10 not only increases the degree of EVT budding and outgrowth but also advances their formation in vitro by 1 or 2 days. The outgrowth of the villous explants imitates the migration and invasion of trophoblasts into uterine tissue.
This pivotal event of cell differentiation to an invasive phenotype is associated with major changes in the expression of markers of invasion (Caniggia et al., 1999).
Among these markers, HLA-G is an antigen of the major histocompatibility complex that is expressed by the invading EVT, and plays a major role in the protection of these cells from lysis by natural killer cells (Goldman-Wohl and Yagel, 2002).
We showed that the expression of HLA-G in the explant culture is 9-fold higher due to FGF10 stimulation. This demonstrates the differentiation of the villi more to the invasive phenotype.
The effect of FGF10 on trophoblast migration was tested on single cell trophoblast culture. We found FGF10 to be a powerful stimulator of trophoblast migration in vitro as it increases the migration and invasion of single cell trophoblasts 7–8-fold. The ability of trophoblasts to invade maternal tissue is known to be accompanied by degradation of the extracellular matrix. This process is mediated and controlled by proteases and their inhibitors. MMPs regulate BM in diverse organ systems (Pohl et al., 2000). In mammary epithelium, interplay between MMPs, growth factors and morphogenes is necessary for branching (Simian et al., 2001). Here, we demonstrate that stimulation of trophoblast cell culture with FGF10 increases uPA, MMP-9 and MMP-2 activity by 153%, 36% and 78%, respectively. These results exemplify the powerful positive effect of FGF10 on trophoblasts' invasive properties.
Sprouty 2 has both negative and positive roles in FGF/RTK signaling (Li et al., 2003). We decreased Spry2 expression and determined its role as a negative regulator of BM of the human placenta. Transfection of siRNA targeting hSpry2 to villous organ culture down-regulated Spry2 expression 28-fold, as revealed by real-time RT–PCR. Accordingly, immunohistochemistry using anti-hSprouty 2 showed reduced staining of Sprouty 2 whereas no differences in CD68 staining could be revealed.
The effect of the reduction in Spry2 expression in cultured villous explants was determined under the conditions of 20% O2 or 4–8% O2. Under 20% O2 conditions, inhibition of Spry2 expression increased the outgrowth and invasion of the EVT into the matrigel. Immunohistochemistry using anti-HLA-G demonstrated enhanced staining of HLA-G in the siRNA transfected culture. Determination of HLA-G expression by quantitative RT–PCR revealed that its expression was enhanced 21-fold. However, in this model, the effect of silencing Spry2 was hardly detected in the culture under reduced O2 tension (4–8%). One of the explanations maybe that low O2 tension is a strong positive inducer of trophoblast migration and invasion (Genbacev et al., 1997; Aplin, 2000). As can be seen in Fig. 5, the outgrowth at low O2 tension is much higher than that in 20% O2, before treatment (Fig. 5A and G). During early pregnancy when the trophoblasts start to invade the decidua of the uterus, the cells are exposed to variable O2 environments, partly depending on their position relative to arteries or veins (Benirschke, 1995). Low O2 tension is a trigger for the expression of many factors. Many of these factors have an overlapping function to ensure and support the beginning of placental development. In 20% O2, when there is only poor EVT outgrowth, partly owing to inhibitors such as Sprouty 2, silencing the inhibitor-Sprouty 2 promotes EVT outgrowth. Our results show that the outgrowth of EVT under low O2 tension is more evident than with the silencing of Spry2. The limited outgrowth of these siRNA treated explants, compared with explants that grow in low O2, may result from a redundancy among different factors that control this process.
Epithelial-mesenchymal cell interactions play a major role in the development of many organs.
As we previously showed, FGF10 is expressed by the proliferating/invading trophoblast cells, whereas the FGF antagonist Sprouty 2 is expressed by the mesenchymal-HCs. In addition, we showed that FGF 10 up-regulates Spry2 expression (Anteby et al., 2005a). These observations imply a mesenchymal/epithelial cell cross-talk that regulates the BM of the placenta. Similar cross-talk between different cell types has been shown during mouse lung development, where FGF10 secreted from mesenchymal cells increases Spry2 expression by epithelial cells (Mailleux et al., 2001). HC are a major cell type of the human placental mesenchyme (Castellucci and Zaccheo, 1989). They share a number of features with cells of the monocyte-macrophage lineage, such as their known phagocytic activity, yet have some distinctive properties (Zaccheo et al., 1989). The observation that HC comprise a major component of the non-trophoblast cells within the villi raised the hypothesis that in addition to their immunological activity these cells may have some roles in remodeling and regulating human placental development. The crucial role of macrophages in regulating BM has been evaluated in mammary gland development (Gouon-Evans et al., 2002). We hypothesize that FGF10 secreted from decidual and EVT cells promotes the growth and invasion of the developing placenta, while concomitantly it up-regulates the expression of its antagonist-sprouty 2 in HC. In Drosophila, tracheal branching loss of function mutation of Spry led to excessive FGF signaling and ectopic branching. We suggest that the role of Sprouty 2 in placental BM is to attenuate the invasion and migration of the cells to ensure the formation of the structural placental villous tree. This is in agreement with our previous observation that Sprouty 2 is localized in the HC of the stroma in areas of ‘hot-spot’ (Castellucci et al., 2000).
Sprouty 2 is an intercellular factor. The precise mechanism by which Sprouty 2 from the HC regulates the growth of trophoblast cells is yet to be tested. In Drosophila tracheal development, it was shown that Sprouty 2 from one cell functions in a non-cell-autonomous way to inhibit signaling in the neighboring cells (Kim and Bar-Sagi, 2004). We suggest that the location of HC at the ‘hot-spot’ of placental villous branching directs trophoblast growth and differentiation into an appropriately formed villous tree.
In conclusion, FGF10 and Sprouty 2 play a major role in BM of placental villi. FGF10 secreted by trophoblasts and other cells at the feto-maternal interface induces trophoblast invasion and migration and up-regulates the expression of Spry2 by HC, which in turn negatively control this process.
Acknowledgement
This work was supported by Hebrew University Hadassah Joint research fund and Hadassah Women's Center Health Fund.
References
†These authors contributed equally to the paper.