-
PDF
- Split View
-
Views
-
Cite
Cite
Mikihiko Higa, Hiromi Kanda, Takashi Kitahashi, Michihiko Ito, Tadayoshi Shiba, Hironori Ando, Quantitative Analysis of fushi tarazu Factor 1 Homolog Messenger Ribonucleic Acids in the Pituitary of Salmon at Different Prespawning Stages, Biology of Reproduction, Volume 63, Issue 6, December 2000, Pages 1756–1763, https://doi.org/10.1095/biolreprod63.6.1756
Close - Share Icon Share
Abstract
Steroidogenic factor 1 (SF-1) or Ad4BP is a member of the fushi tarazu factor 1 (FTZ-F1) family and an orphan nuclear receptor that plays an important role in the hypothalamus-pituitary-gonadal axis and the adrenal cortex. Although its critical role in the differentiation of adrenals, gonads, and pituitary gonadotropes has been well demonstrated, regulatory function of SF-1 during sexual maturation is yet to be examined. To investigate the potential role of SF-1 in sexual maturation, expression of two salmon FTZ-F1 homolog genes, sFF1-I and sFF1-II, was examined in the pituitaries of chum and sockeye salmons, using specific and sensitive RNase protection assays. Only sFF1-I mRNA was found in the pituitary and other organs, such as the ovary, spleen, liver, brain, and skeletal muscle. In chum salmon during upstream migration from the bay to the hatchery, the level of sFF1-I mRNA in the male fish was increased on the midway in the river, where the levels of gonadotropin α- and IIβ-subunit mRNAs were increased. In maturing sockeye salmon, the expression of the sFF1-I gene was elevated in the mature male fish, but the administration of GnRH analog did not further enhance the expression. These results indicate that sFF1-I gene expression in the pituitary is upregulated in maturing salmon, and this upregulation may not depend on GnRH.
Introduction
Mammalian steroidogenic factor 1 (SF-1), also known as Ad4BP [1], is a homolog of Drosophila fushi tarazu factor 1α (dFTZ-F1α) that is crucial for activation of the homeobox segmentation gene fushi tarazu in early embryogenesis [2, 3]. Steroidogenic factor-1 is an orphan nuclear receptor that plays a pivotal role in transcriptional regulation of genes coding for several steroidogenic enzymes, glycoprotein hormones, and hormone receptors in the hypothalamus-pituitary-gonadal axis [4]. The role of SF-1 has been highlighted in the development and differentiation of adrenals, gonads, and pituitary gonadotropes, because an SF-1 null mouse lacked these endocrine organs [5, 6]. In murine gonadotrope-derived αT3–1 cells, SF-1 increased the promoter activity of genes encoding GnRH receptor [7], glycoprotein α-subunit [8], and LH β-subunit [9] through binding to the gonadotrope-specific elements in their promoters. These reports suggested that SF-1 mediates tissue-specific expression of the three genes in the gonadotropes directly related with reproductive function.
Transcriptional regulation of gonadotropin (GTH) subunit genes by SF-1 involves several additional transcription factors. For example, SF-1 and early growth response protein 1 (Egr-1) acted synergistically to stimulate the LHβ promoter [10–13]. The activity of SF-1 on the LHβ promoter was unmasked by the interaction between pituitary homeobox 1 (Ptx1) and the ligand binding domain of SF-1 [14]. The SF-1 and estrogen receptor (ER) also acted in synergism to regulate the expression of the salmon (s)GTHIIβ subunit gene [15]. Consequently, SF-1 is considered to coordinate gene expression in gonadotropes by cooperation with other transcription factors. Although these results from in vitro studies increased information on the molecular mechanisms of SF-1 action, the physiological significance of SF-1 in the control of expression of gonadotrope-specific genes in maturing animals is yet to be determined.
Several studies showed that SF-1 may be a critical component in the stimulation of GTH subunit gene transcription by GnRH in the pituitaries of rat [16], sheep [17], and transgenic mice containing bovine LHβ promoter [18]. However, recent in vitro studies showed that SF-1 might not respond to GnRH stimulation [10–13]. Thus, the regulatory function of SF-1 in gonadotropes in maturing animals is still obscure.
In the pituitary of chum salmon (Oncorhynchus keta) during upstream migration, sGTHα and sGTHIIβ but not sGTHIβ mRNAs were increased at the spawning ground [19]. This elevation corresponds well with the increased GnRH production in the preoptic area [20]. Furthermore, in maturing sockeye salmon (Oncorhynchus nerka), implantation of a GnRH analogue (GnRHa) increased expression of both sGTHα and sGTHIIβ genes but not of the sGTHIβ gene [21]. It is therefore interesting to know whether SF-1 is involved in the elevation of sGTHα and sGTHIIβ subunit gene expression in response to GnRH during salmonid sexual maturation.
To investigate the potential role of SF-1 in sexual maturation, two types of genes for salmon FTZ-F1 homologs (sFF1-I and sFF1-II) were partially isolated from both chum and sockeye salmons. The levels of sFF1-I mRNA were determined in the pituitaries of chum salmon at the different stages during upstream migration and of sockeye salmon implanted with GnRHa. The measurement was carried out using highly specific and sensitive RNase protection assays that distinguish the sFF1-I and -II mRNAs. The amounts of sGTH subunit mRNAs were determined by quantitative dot-blot analyses.
Materials and Methods
Animals for the Measurement of sFF1 and sGTH Subunit mRNAs
Prespawning chum salmon of the Ishikari River (Hokkaido, Japan) were caught along their homing pathway at three key locations in 1995. Seawater (SW) fish were captured at Atsuta in Ishikari Bay, 20 km to the mouth of the river. Freshwater (FW) fish were captured at Ebetsu in the midway of the river and were also obtained at the Chitose Salmon Hatchery. Hereafter, the fish caught at Atsuta, Ebetsu, and Chitose are referred to as maturing SW fish, maturing FW fish, and matured fish, respectively. The maturing SW fish showed apparent silver color, and the mean gonadosomatic indices (GSI) were 5.4% and 15.5% in males and females, respectively [22]. The matured males and females that were spermiated and ovulated had the nuptial color with 4.5% and 19.0% of GSI, respectively. The maturing FW fish showed intermediate color but similar GSI to the maturing SW fish.
Anadromous maturing sockeye salmon were caught in the Bibi River, Hokkaido, Japan in 1996 and were kept in a holding pond in the Chitose Salmon Hatchery under natural environmental conditions. At the beginning of September, fish received in their dorsal muscle a 2-mm implant capsule containing 75 or 150 μg of GnRHa, (Des-Gly10, d-Ala6, Pro9)-GnRH ethyl amide in an ethylene vinyl acetate copolymer matrix [23]. The fish sampled on the day of implantation were assigned as initial controls, and sham-operated fish served as controls. The control and GnRHa-implanted fish were sampled 3 wk after the implantation. No milt and ovulated eggs were found in the initial control fish, whereas spermiation and ovulation were observed in both control and GnRHa-implanted fish [24].
RNA Preparation
The pituitaries of chum and sockeye salmons were removed upon decapitation, frozen in liquid nitrogen, and stored at −80°C. Total RNA was extracted from individual pituitaries by the guanidium thiocyanate/hot phenol method [25] for chum salmon and by the acid guanidium thiocyanate-phenol-chloroform (AGPC) method [26] for sockeye salmon. For Northern blot analysis, poly(A)+ RNA was prepared from the maturing SW chum salmon using a QuickPrep Micro mRNA purification kit (Amersham Pharmacia Biotech, Buckinghamshire, UK). To examine the tissue distribution of sFF1-I mRNA, total RNAs of various tissues were additionally extracted by the AGPC method from matured chum salmon that homed to the Otsuchi River, Iwate, Japan.
Genomic DNA Preparation
Genomic DNAs for Southern blot analysis and partial isolation of sFF1 genes were prepared according to the method by Sambrook et al. [27] from chum salmon of the Ishikari River and sockeye salmon that were kept in the Toya Lake Station for Environmental Biology, Hokkaido, Japan.
Northern and Southern Blot Analyses
For Northern blot analysis, poly(A)+ RNA (2 μg) from chum salmon pituitary was electrophoresed on a 0.8% agarose/formaldehyde gel and capillary transferred to a nylon membrane (Hybond N+; Amersham Pharmacia Biotech). A partial cDNA encoding FTZ-F1 homolog was cloned from a testicular cDNA library of rainbow trout (Oncorhynchus mykiss) (in preparation, partial sequence was deposited in the GenBank database, accession no. AB038151). The cDNA encodes 472 amino acids from the putative N-terminus of the rainbow trout FTZ-F1 homolog (rtFTZ-F1) (Fig. 1A). The RNA probe that corresponded to 282 base pairs (bp) of the 3′ end of rtFTZ-F1 was transcribed with [α-32P]UTP (800 Ci/mmol, Amersham Pharmacia Biotech) using a MAXIscript in vitro transcription kit (Ambion, Austin, TX). After prehybridization at 42°C for 3 h in 5× SSPE (1× SSPE: 0.18 M NaCl, 10 mM NaH2PO4, pH 7.4, and 1 mM EDTA), 0.5% SDS, 5× Denhardt solution (1× Denhardt solution: 0.02% ficoll, 0.02% BSA, and 0.02% polyvinylpyrrolidone), and yeast tRNA (0.02 g/L), hybridization was performed at 42°C for 20 h in the same buffer with the probe denatured at 95°C for 5 min. The membrane was washed in 2× standard saline citrate (SSC) (1× SSC: 0.15 M NaCl and 15 mM sodium citrate, pH 7.0)/0.1% SDS at room temperature for 15 min, and washed twice in 0.1× SSC/0.1% SDS at 65°C for 30 min. The membrane was exposed to a Fuji imaging plate (Fuji Photo Film, Tokyo, Japan) for 24 h and was analyzed by a Bioimaging analyzer system, Fuji BAS 2000 (Fuji Photo Film).
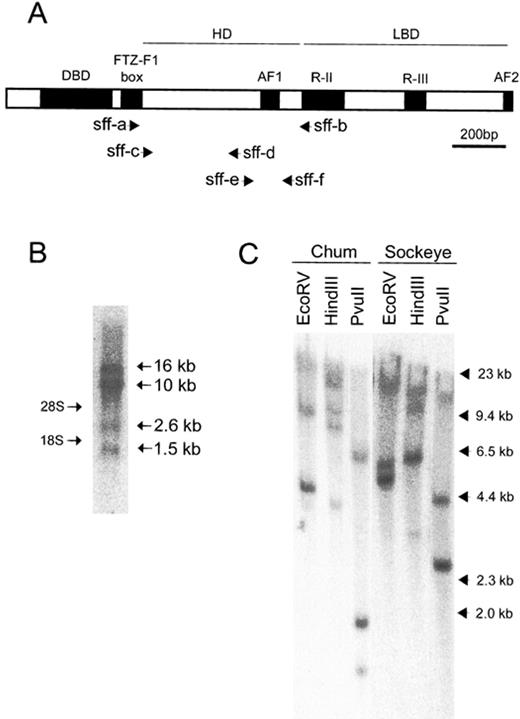
The FTZ-F1 homolog genes in salmonids. A) A schematic representation of the structure of rtFTZ-F1. Primer positions for partial isolation of genes encoding sFF1 in chum and sockeye salmons (see text) are indicated. AF1 and 2, activation function 1 and 2; DBD, DNA binding domain; HD, hinge domain; LBD, ligand binding domain; R-II and -III, region-II and -III. The scale bar represents 200 bp. B) Northern blot analysis of sFF1 mRNA in chum salmon pituitary. Poly(A)+ RNA (2 μg) were hybridized with a radiolabeled RNA probe of the C-terminus 282-bp fragment of rtFTZ-F1. The molecular sizes of multiple transcripts on the right are based on the positions of 28S and 18S rRNAs on the left. C) Southern blot analysis of sFF1 genes in chum and sockeye salmon genomic DNAs. Genomic DNA (15 μg) as hybridized with a radiolabeled DNA probe that corresponded to the middle 606-bp fragment (from sff-a to sff-b) of rtFTZ-F1. Molecular size markers from HindIII-digested lambda DNA are indicated on the right
For Southern blot analysis, genomic DNAs of chum and sockeye salmon were digested with EcoRV, HindIII, or PvuII, then electrophoresed on 0.8% agarose gels and capillary transferred to Hybond N+ membranes (Amersham Pharmacia Biotech). The 606-bp DNA fragment of the rtFTZ-F1 (from FTZ-F1 box to R-II domain in Fig. 1A) was labeled with [α-32P]dCTP (3000 Ci/mmol; Amersham Pharmacia Biotech) using a Megaprime DNA labeling kit (Amersham Pharmacia Biotech). Hybridization and the following analysis were carried out as described in the Northern blot analysis, except for the hybridization temperature at 65°C.
Partial Isolation of sFF1-I and -II Genes by Polymerase Chain Reaction
Two primers, sff-a (5′-AA[C/T]AA[A/G]TT[C/T]GG[C/G]CCATGTACAAG-3′) and sff-b (5′-AC[A/G]ATGGAGA[A/T][C/G]A[A/G]GTCTGGTC-3′) were designed based on the structure of rtFTZ-F1 (Fig. 1A). A reaction mixture (100 μl) contained 10 ng of genomic DNA from chum or sockeye salmon, 20 pmol of each primer, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.25 mM of dNTP, and 1 U of Taq DNA polymerase of an Expand high fidelity polymerase chain reaction (PCR) system (Roche Molecular Biochemicals, Mannheim, Germany). The amplification profile was 95°C for 1 min, 35 cycles of 95°C for 30 sec, 50°C for 30 sec, and 72°C for 1 min, and finally 72°C for 10 min. The PCR products were ligated to a pCRscript Amp SK(+) cloning vector (Stratagene, La Jolla, CA). The nucleotide sequence was determined by the dideoxy chain-termination method [28] using an SQ-5500 DNA sequencer (Hitachi, Tokyo, Japan). Two types of FTZ-F1 homolog genes of 606 bp were amplified in both chum and sockeye salmons and designated as csFF1-I and csFF1-II in chum salmon and ssFF1-I and ssFF1-II in sockeye salmon.
Ribonuclease Protection Assay
To prepare antisense probes for sFF1 mRNAs, partial cDNA fragments were further amplified by PCR using primers sff-c (5′-GAATTCCATGACCCAGGTGATGCAGGCG-3′) and sff-d (5′-GTCGACGGGTCCGGGTACTCGGACTTG-3′) for csFF1-I and ssFF1-I, and sff-e (5′-GTCGACCAGCTCGCCCGAGTCCATCATG-3′), and sff-f (5′-AAGCTTCGGCTGGCCTGCTCCTGCTGCAG-3′) for csFF1-II and ssFF1-II (Fig. 1A). The reaction was performed using 10 ng of each sFF1 cDNA as a template DNA in the same condition as described above except for the extension time at 72°C for 30 sec. The products (276 bp for csFF1-I and ssFF1-I and 168 bp for csFF1-II and ssFF1-II) were ligated to a pGEM-T easy vector (Promega, Madison, WI). The linearized template DNAs were transcribed with [α-32P]UTP (800 Ci/mmol, Amersham Pharmacia Biotech) as described above. The probes were then purified by 5% polyacrylamide/6 M urea denaturing gel electrophoresis. Reference sense RNAs were transcribed using each 606-bp sFF1 DNA fragment as a template DNA, and in vitro transcription reactions were stopped by digesting the templates with an RNase-free DNase I (Ambion). The purified RNAs were quantified by a spectrophotometer and were serially diluted to prepare standard curves.
For solution hybridization, total RNA was incubated with the probe (2 × 104 cpm) in a final volume of 10 μl of hybridization solution (Ambion) for 16–18 h at 55°C. After hybridization, the samples were digested at 30°C for 45 min using 140 μl of digestion mix (5 mM EDTA, pH 8.0; 10 mM Tris-HCl, pH 8.0; 200 mM sodium acetate, pH 8.0; and 70 U of RNase I [Ambion]). The products were recovered by ethanol precipitation and were analyzed by 5% polyacrylamide/6 M urea denaturing gel electrophoresis. After the electrophoresis, the gels were mounted on 3MM paper filters (Whatman, Clifton, NJ), exposed to Fuji imaging plates (Fuji Photo Film) for 72 h, and were analyzed by the Fuji BAS 2000 system (Fuji Photo Film). The lowest detection limit in this assay was 0.036 amol (Fig. 4). The intra-assay variations ranged from 7.5% to 23.0% and interassay variation was 7.1%.
Quantitative Dot-Blot Analysis
The amounts of sGTH subunit mRNAs were measured in the individual pituitaries of chum salmon as previously described [19]. Briefly, total RNA (1 μg) was blotted onto Hybond-N+ nylon membranes (Amersham Pharmacia Biotech) using MilliBlot-D (Millipore, Bedford, MA). The probes and reference single-stranded sense DNAs were prepared by primer extension using specific primers. After hybridization, the signals were analyzed by the BAS2000 system (Fuji Photo Film). Specificity of the probes and practically low levels of variation were verified as previously described [19].
Statistical Analysis
The data are presented as means ± SEM. An ANOVA was used to test for statistically significant differences among groups, and when appropriate the Games/Howell’s post hoc test for multiple comparison was applied.
Results
Northern and Southern Blot Analyses of sFF1 Genes in Chum and Sockeye Salmons
Northern blot analysis of sFF1 mRNA in the chum salmon pituitary with the rtFTZ-F1 as a probe showed multiple sizes of transcripts, even after stringent washing condition (Fig. 1B). The analysis showed expression of four transcripts in approximate sizes of 1.5, 2.6, 10, and 16 kb, indicating that sFF1 mRNA consists of multiple transcripts, possibly generated by alternative splicing and/or duplicate genes in salmon. Therefore, Southern blot analysis was carried out to examine the multiplicity of sFF1 genes in the salmon genome. The genomic DNAs of chum and sockeye salmons showed two major and one weak bands (Fig. 1C), suggesting that there are at least two copies of sFF1 genes in each of chum and sockeye salmons. The additional band of weak signal suggested the presence of another related gene in chum and sockeye salmons.
Partial Isolation of sFF1-I and -II Genes
The results of Northern blot analysis led us to use an RNase protection assay that can determine the level of sFF1 mRNAs more specifically. To prepare the specific probes for sFF1 mRNAs of chum and sockeye salmons, sFF1 genes were partially isolated by PCR using primers based on the sequence of rtFTZ-F1 (Fig. 1A). The sequence analyses of the PCR products of predicted size (606 bp) showed two types of sFF1 genes (sFF1-I and sFF1-II) in both chum and sockeye salmons, consistent with the result of Southern blot analysis. The two genes were designated as csFF1-I and csFF1-II in chum salmon and ssFF1-I and ssFF1-II in sockeye salmon. The sequence similarities between the two PCR products are 93.4% and 93.2% in chum and sockeye salmons, respectively. The similarities of sFF1 genes between chum and sockeye salmons are 99.1% and 98.0% for sFF1-I and sFF1-II, respectively. The previously isolated rtFTZ-F1 belongs to the group of sFF1-I.
Tissue Distribution of csFF1-I and -II mRNAs
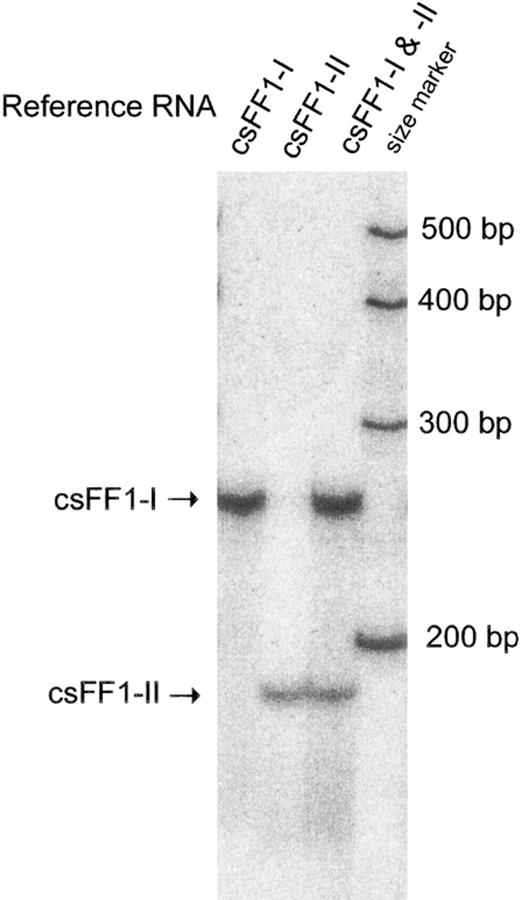
Sensitive RNase protection assays that allowed specific detection of the sFF1-I and -II mRNAs were developed for both chum and sockeye salmons. The assay used RNase I that digested efficiently the heterologous hybrids with a number of single mismatches between the probe and target mRNA. As shown in Figure 2, only homologous hybrids were protected from the RNase digestion after the csFF1-I and -II probes were hybridized with either csFF1-I or -II reference RNA.
Ribonuclease protection assay for specific detection of csFF1-I and csFF1-II mRNAs. Quantities of 1.2 amol of csFF1-I, csFF1-II, or a mixture of two reference RNAs were hybridized with the mixture of the two antisense probes, followed by RNase digestion. Size markers were transcribed from Century Marker template (Ambion)
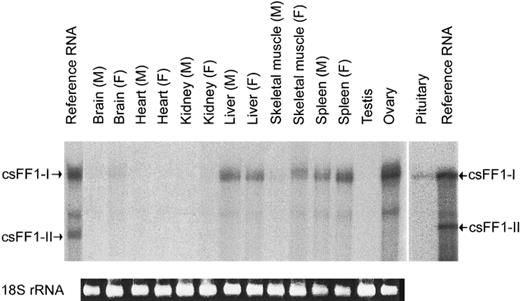
To examine whether the csFF1-I and -II genes were expressed in the matured chum salmon, total RNAs from various tissues were analyzed by RNase protection assay. The csFF1-I mRNA was found in the brain, liver, skeletal muscle, spleen, ovary, and pituitary, but not in the heart, kidney, and testis (Fig. 3). The brain and the pituitary had low levels of csFF1-I mRNA, while the liver, the spleen, and the ovary showed high levels of csFF1-I mRNA. Interestingly, the female skeletal muscle showed abundant csFF1-I mRNA, while the level in the male was low. In contrast to csFF1-I, csFF1-II mRNA was not detected in any tissues examined.
Tissue distribution of csFF1-I mRNA in chum salmon. Total RNAs (20 μg) from various tissues were hybridized with the csFF1-I antisense probe, followed by RNase digestion. M and F in parentheses indicate male and female, respectively. The 18S rRNA bands of total RNAs (0.5 μg) are indicated at the bottom
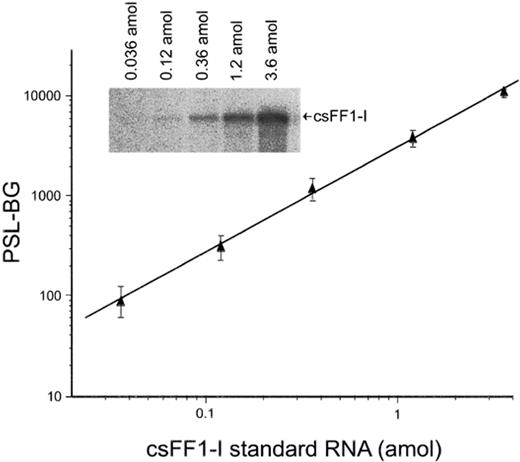
Representative standard curve in RNase protection assay for csFF1-I mRNA. Serially diluted reference RNA was hybridized with antisense probe, followed by RNase digestion. The linear standard curve was obtained by plotting the background (BG)-subtracted value of photostimulated luminescence (PSL) of the protected band. The bands are shown in the inset against the respective amounts of the csFF1-I reference RNA
Changes in the Level of csFF1-I mRNA During Upstream Migration
Because of the undetectable level of csFF1-II mRNA, we considered that csFF1-I mRNA is the major sFF1 mRNA expressed in chum salmon and measured only the amounts of csFF1-I mRNA by the RNase protection assay. A typical standard curve for csFF1-I mRNA is shown in Figure 4. The significance level (R2) of the linear regressions calculated for the standard curves was greater than 0.99.
For the measurement of sFF1-I mRNA levels by the RNase protection assay, internal standards of some housekeeping genes, such as β-actin, glyceraldehyde phosphate dehydrogenase, and rRNA, were not included for the following two reasons. First, recent studies showed that expression of these genes are not always expressed at constant levels and can be affected by experimental treatment and living conditions [29–31]. Indeed, GnRHa increased the content of β-actin mRNA in the pituitary of striped bass [32]. Second, we measured sFF1-I mRNA levels in a whole pituitary that contains various cell types. The sFF1-I is considered to be expressed in only a fraction of pituitary cells, i.e., gonadotropes. Because sFF1-I nonexpressing other cells also contribute to the levels of housekeeping transcripts, normalization using a housekeeping gene as an internal standard is not adequate. Thus, the absolute mRNA content in a pituitary is considered to be more appropriate for the evaluation of the changes in the sFF1-I mRNA levels.
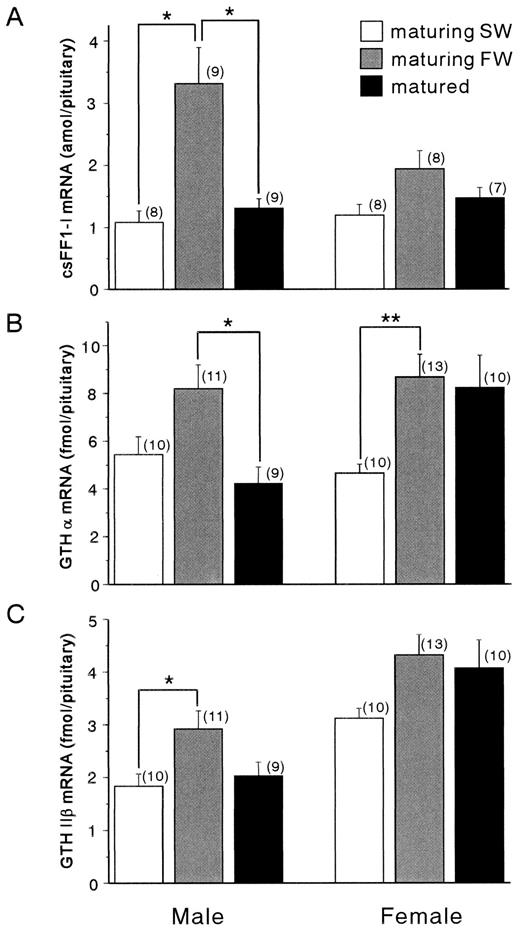
To investigate the potential role of csFF1-I in sexual maturation of chum salmon, the levels of csFF1-I and sGTH subunit mRNAs were measured at three different reproductive stages during upstream migration. The csFF1-I mRNA level was threefold higher in the maturing FW males compared to the maturing SW and matured fish (Fig. 5A). The females also showed the same tendency as observed in the males, but the changes were not statistically significant. It is noteworthy that in the maturing FW males both of the sGTHα and sGTHIIβ mRNA levels were also significantly elevated (Fig. 5, B and C), while the level of sGTHIβ mRNA did not change significantly (data not shown). The changes were moderate in the females, except for the sGTHα mRNA, that showed significant accumulation in the maturing FW fish. These results indicated that the expression of csFF1-I, sGTHα, and sGTHIIβ genes were mostly elevated in the maturing FW fish in which the final maturation progressed on the way to the spawning ground.
Changes in the levels of csFF1-I (A), sGTHα (B), and sGTHIIβ (C) mRNAs in the pituitaries of maturing SW (white column), maturing FW (gray column), and matured (black column) chum salmon. Total RNAs (30 μg) from pituitaries were hybridized with antisense probes, followed by RNase digestion. Values are mean ± SEM, and the number of samples is indicated in parentheses. Asterisks indicate statistically significant differences between means: *P < 0.05; **P < 0.01
Effect of GnRHa Implantation on ssFF1-I Gene Expression
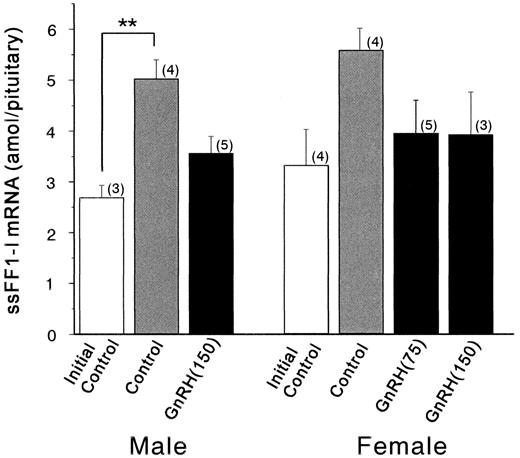
Because it is difficult to obtain chum salmon at the early stage of sexual maturation in large numbers, sockeye salmon that migrate to a river at the early stage and stay there for a few months until they mature were used for the experiment of GnRH analog implantation. The level of ssFF1-I mRNA was measured in the pituitaries of maturing sockeye salmon that had been treated with GnRHa implants. We previously demonstrated the increase of sGTHIIβ gene expression in the pituitaries of sockeye salmon by the administration of GnRHa [21]. We used the same pituitaries for the measurement of ssFF1-I mRNA levels in this study. As shown in Figure 6, the level of ssFF1-I mRNA was significantly elevated in the control males compared to the initial control. However, the ssFF1-I mRNA level was not further enhanced by the implantantion of GnRHa. The females also showed the same tendency as observed in the males, but the changes were not statistically significant. These results indicated that, in maturing sockeye salmon, the gene expression of ssFF1-I was augmented toward spawning, but GnRH may not contribute to the activation of the ssFF1-I gene.
Pituitary contents of ssFF1-I mRNA in initial control (white column), control (gray column), and GnRHa-implanted fish (black column). GnRH (75), 75 μg of GnRHa implantation; GnRH (150), 150 μg of GnRHa implantation. Total RNAs (20 μg) from pituitaries were hybridized with antisense probes, followed by RNase digestion. Values are mean ± SEM, and the number of samples is indicated in parentheses. Asterisks indicate statistically significant differences between means: **P < 0.01
Discussion
In the present study, we found for the first time the elevated expression of sFF1-I genes in the pituitaries of salmon during sexual maturation. We established the highly specific, sensitive, and accurate RNase protection assays for sFF1-I and -II genes. In the pituitaries of male prespawning chum salmon, the sFF1-I mRNA levels increased significantly during the final stage of sexual maturation. Furthermore, in maturing sockeye salmon, a significant increase in the ssFF1-I mRNA level was observed in the control male fish, in which the final maturation progressed toward spawning. The females of both chum and sockeye salmon showed moderate changes with the same tendency as observed in the males. These changes occurred along with the elevation of sGTHα and sGTHIIβ mRNA levels in chum salmon (Fig. 5, B and C) [19] and also in sockeye salmon that had been kept in a holding pond [21]. Therefore, we consider that progress of sexual maturation rather than migratory behavior triggered the augmentation of sFF1-I gene expression.
These findings support the idea that the sFF1-I may have a crucial role in the activation of sGTH subunit gene expression. LeDrean et al. [15] indicated that mammalian SF-1 activated the transcription of sGTHIIβ gene in synergism with ER by cotransfection study using heterologous cells, and that this activation was dependent on the quantities of both SF-1 and ER expression vectors. Therefore, the amount of sFF1 protein seems critical in the activation of sGTH gene expression. Changes in the levels of SF-1 mRNA have also been reported in other animals in relation to sexual maturation. In the pituitary of a ewe, a correlation between SF-1 and LHβ mRNA levels was observed in the luteal phase but not in the follicular phase [33]. Gonadotropin releasing hormone stimulation of SF-1 gene expression was reported in the pituitaries of rat [16] and ewe [17]. This correlation between SF-1 and GTH subunit mRNA levels suggests a common molecular mechanism underlying the regulation of SF-1 and GTH subunit genes in the pituitary.
The transcriptional regulation of SF-1 gene has been investigated with respect to cell-type-specific expression. Several reports showed that an E-box motif in the proximal promoter of SF-1 gene was necessary for steroidogenic cell-specific expression [34, 35]. Harris and Mellon [36] showed that a transcription factor USF (upstream stimulatory factor), a member of the helix-loop-helix (HLH) family of transcription factors, bound the E-box and further suggested that a subset of HLH proteins in gonadotrope were important for gonadotrope-specific expression of SF-1 gene. Nomura et al. [37] showed that SF-1 protein itself bound to an element in the first intron of rat FTZ-F1 gene, suggesting that an autoregulatory loop determines the tissue-specific expression of SF-1 gene. Although the function of these cis-acting elements and binding factors needs to be defined further, these molecular mechanisms that direct tissue-specific expression of the SF-1 gene appear to be under the control of extracellular stimulation, i.e., from hormones, to achieve the crucial role of SF-1 in the control of reproductive functions.
In addressing the hormonal regulation of sFF1 gene expression, we examined the possible roles of GnRH, which is a major regulator of the sGTH subunit gene expression [21, 38]. In contrast to the results observed in the rat [16] and the ewe [17], exogenous GnRHa did not enhance ssFF1-I gene expression in the pituitary of maturing sockeye salmon but tended to decrease its level. This discrepancy might be due to the fact that in the pituitary of control fish, the sFF1-I gene was already stimulated by the elevation of endogenous GnRH during sexual maturation, and exogenous GnRH did not further enhance ssFF1-I gene expression in the GnRHa-implanted fish. Nevertheless, the present results suggest that sFF1-I gene expression is not dependent on GnRH.
A number of in vitro studies have shown that GnRH stimulation does not alter the expression of the SF-1 gene but increases Egr-1 gene expression [10–13]. The SF-1 gene has been reported to act through a synergistic interaction with Egr-1 to activate the LHβ promoter in response to GnRH. Furthermore, in steroidogenic cells, the levels of SF-1 mRNA were mostly unaffected by the administration of peptide hormones, such as ACTH [39]. Hammer et al. [40] suggested that the phosphorylation of SF-1 is important in the regulation of SF-1-dependent genes in response to extracellular signals. Therefore, transcriptional regulation of SF-1 gene in response to peptide hormones is currently in doubt.
The present study showed clearly the elevation of sFF1-I gene expression in the pituitary of maturing salmon. We can only speculate on the regulation of the sFF1 gene, but one of the strong candidates of regulatory factors is gonadal steroids that are well-known modulators of GTH subunit genes [41, 42]. Indeed, estradiol was found to suppress SF-1 gene expression in the sheep pituitary [33] and in the rat testis [43]. In salmonids, the plasma levels of sex steroid hormones changed dramatically during sexual maturation [24, 44]. Therefore, it is of considerable interest to determine if gonadal steroids are involved in the control of the sFF1 gene in the pituitary of salmon during sexual maturation.
In conclusion, the expression of sFF1-I genes was upregulated in the pituitary of salmon during sexual maturation, and this upregulation may not be dependent on GnRH. Because the amount of sFF1 protein is considered to be critical in the activation of sGTH subunit gene expression, the elevated expression of the sFF1-I gene in the pituitary of maturing salmon certainly contributes to the GnRH stimulation of sGTH subunit gene expression, even though the expression of the sFF1 gene is not stimulated by GnRH. Thus, the present results support the idea of the role of sFF1 as being crucial in the control of sGTH subunit gene expression and further suggest that the regulatory mechanisms of sFF1 gene expression are complex in the pituitaries of salmon during sexual maturation.
Acknowledgments
We are very grateful to Dr. A. Urano (Hokkaido University) for his encouragement and helpful discussion. The authors thank Dr. H. Ueda (Hokkaido University), Dr. M. Kaeriyama (Hokkaido Tokai University), M. Ban (National Salmon Resources Center, Hokkaido), H. Aihara (Atsuta Fisherman’s Cooperative Association, Japan) for their help in sampling, and Dr. Y. Zohar (University of Maryland) for providing the GnRHa implants. We also thank Dr. S. Itoh (Kyowa Hakko Kogyo Co., Ltd.) for the chum salmon GTH subunit cDNAs.
References
Author notes
This study was supported by Grants-in-Aid from the Fisheries Agency and Ministry of Education, Science, Sports and Culture, Japan. The nucleotide sequences of csFF1-I, csFF1-II, ssFF1-I, and ssFF1-II reported in this paper have been deposited in the GenBank database with the respective accession numbers AF242223, AF242224, AF242225, and AF242226.