-
PDF
- Split View
-
Views
-
Cite
Cite
Maria José de los Santos, Deborah J. Anderson, Catherine Racowsky, Joseph A. Hill, Presence of Fas-Fas Ligand System and Bcl-2 Gene Products in Cells and Fluids from Gonadotropin-Stimulated Human Ovaries1, Biology of Reproduction, Volume 63, Issue 6, December 2000, Pages 1811–1816, https://doi.org/10.1095/biolreprod63.6.1811
Close - Share Icon Share
Abstract
Apoptosis, or programmed cell death, is an important mechanism for the regulation of embryonic development and tissue homeostasis. It is coordinated by a number of molecules including the Fas-Fas ligand (FasL) system and bcl-2. The purpose of this study was to characterize the expression of these molecules in human oocytes and cumulus cells from gonadotropin-stimulated human ovaries and to determine whether the presence of soluble Fas (sFas), soluble FasL, or interferon-γ in follicular fluid (FF) correlated with apoptosis in cumulus cells, oocyte maturation, and embryo quality. Levels of sFas were significantly higher in FF containing immature oocytes compared with those containing atretic oocytes (P < 0.05; FF containing mature oocytes had highly variable levels of sFas. Levels of sFas in FF did not correlate with either fertilization, embryo quality resulting from fertilized oocytes, or apoptosis rate in cumulus cells. Fas was expressed in both unfertilized oocytes and cumulus cells, whereas FasL expression was not usually detected in these cell types. Messenger RNA for bcl-2 was detectable in both freshly isolated oocytes and cumulus cells but was not demonstrable following 24 h of culture that coincided with a significant increase of apoptosis in cumulus cells. Our results indicate that soluble forms of the Fas-FasL system are present in FF from gonadotropin-stimulated human ovaries and suggest that this system may play a role in preventing oocyte atresia during folliculogenesis but is probably not important for apoptotic events in cumulus cells and oocytes after fertilizaton failure. Apoptosis in this case may be facilitated by the downregulation of bcl-2. Further studies on the expression of these molecules in follicles containing atretic oocytes and immature oocytes are needed to confirm this new hypothesis.
Introduction
Apoptosis, or programmed cell death, is an important mechanism for the regulation of embryonic development and adult tissue homeostasis. It is especially prominent in organs and tissues with high cellular proliferation [1]. Apoptosis is also involved in ovarian physiology because follicular atresia occurs at all stages of development during reproductive life [2]. The process of apoptosis is coordinated by a number of molecules including Fas, Fas ligand (FasL), soluble (s)Fas, and bcl-2.
Human Fas, also known as CD95 or APO-1, is a 45-kDa transmembrane glycoprotein of 325 amino acids with a signal sequence at the NH2-terminus and belongs to the tumor necrosis factor (TNF) family [3]. FasL, with no signal sequence at the NH2-terminus, has a domain of hydrophobic amino acids in the middle of the molecule, indicating it is a type II membrane protein with its COOH-terminal region outside of the cell [4]. When FasL binds to Fas on the cell surface, apoptosis is initiated [5]. Fas has five isoforms of soluble variants generated by alternative splicing of the Fas gene that can block cell death in vitro through binding with either FasL or membrane-associated Fas [6–9]. FasL also has a soluble form; however, unlike the Fas antigen, its soluble variant is produced as a result of the action of matrix metalloproteinase-like enzymes [10]. Fas-mediated apoptosis requires the balancing of receptor-ligand interactions that can be modulated by the sFas that acts as a functional antagonist of FasL-mediated apoptosis [9]. FasL is elevated in some pathological conditions, and elevated levels of sFasL often accompany systemic tissue damage. Soluble Fas has been found in human sera and reproductive fluids including seminal plasma, oviductal fluid, and follicular fluid; however, sFasL is not normally present in sera of healthy individuals [11].
In the human ovary, the Fas-FasL system may be regulated by gonadotropin-dependent mechanisms and may play a role during attrition, follicular regression, and atresia, because Fas antigen has been detected in oocytes from fetal and adult ovaries and in granulosa cells during the luteal phase [12, 13]. Fas also mediates apoptosis in human granulosa luteal cell cultures when these cells are pretreated with the Th1 cytokine, interferon-gamma (IFN-γ) [14].
Bcl-2 is an oncoprotein localized on the cytoplasmic side of the mitochondrial outer membrane, endoplasmic reticulum, and nuclear envelope [15]. Bcl-2 promotes cell survival by suppressing apoptosis [16]. Cyclic expression of bcl-2 has been described in human endometrium [17, 18] and has been detected in human granulosa cells from antral follicles [19] and in human embryos [20]. Fas-transduced cell death is only partially inhibited by overexpression of bcl-2 [21]. Ablation of bcl-2 expression in transgenic mice is associated with decreased oocytes and primordial follicles [22], whereas overexpression of bcl-2 results in decreased follicle apoptosis, enhanced folliculogenesis, and increased germ cell tumorigenesis [23].
There has been no systematic investigation of these important regulators of cell death in cells and fluids collected from exogenous gonadotropin-stimulated human ovaries. Therefore, the purpose of this study was to characterize the Fas-FasL system and bcl-2 in human oocytes and cumulus cells (CC) following exogenous gonadotropin stimulation and to determine whether the presence of sFas, sFasL, or IFN-γ in follicular fluids (FF) correlated with apoptosis in CC, oocyte maturation, fertilization, and embryo quality. We hypothesized that high levels of sFasL and/or low levels of sFas in FF would be correlated with a higher incidence of apoptosis and poor oocyte and embryo quality.
Materials and Methods
Follicular Fluids, Oocytes, CC, and Embryos
After obtaining informed consent, 53 individual FF were aspirated from 31 women participating in the assisted reproductive technology program within the Center for Reproductive Medicine at Brigham and Women’s Hospital. Oocytes (n = 53) were scored for maturation (GV, MI, MII, and atretic), and fertilization (0PN, 1PN, 2PN, 3PN, and degenerated). Resulting embryos (n = 20) were assessed for quality (number of blastomeres and the degree of fragmentation). Embryos were scored based on fragmentation as follows: 0 if no fragments were observed, 1 if 10% of the volume consisted of fragments; 2 if between 11% and 25%, 3 if between 26% and 50%, and 4 if more than 50% of the volume of the embryo was fragmented.
After follicle aspiration, all FF were centrifuged at 200 × g for 10 min at room temperature, and the supernatants were stored at −80°C until used. Cumulus cells were mechanically removed from the oocytes 15 to 30 min after oocyte retrieval. They were then divided and placed in either Tissue-Teck O.C.T. compound (Sakura Finetek USA Inc., Torrance, CA) and stored at −80°C, so that cryosections (5 mm) for immunostaining (Fas, FasL, and bcl-2) and apoptosis experiments or Trizol so that reverse transcription-polymerase chain reaction (RT-PCR) for RNA analysis could be later performed on the same CC complexes for direct comparison.
An additional 70 oocytes and 80 CC complexes were assessed for Fas-FasL system and bcl-2 by immunohistochemistry (n = 28 and n = 32, respectively) and RT-PCR (n = 35 and n = 55, respectively).
Patient Gonadotropin Stimulation Protocol
Briefly, induction of ovulation was performed using human postmenopausal gonadotropin (Pergonal; Serono Laboratories, Inc., Randolph, MA) or FSH (Metrodin; Serono Laboratories) separately or combined in conjunction with the GnRH agonist (GnRH-a) leuprolide acetate (Lupron; TAP Pharmaceuticals, Deerfield, IL) initiated in the preceding midluteal phase. When the estradiol level was ≥650 pg/ml and at least two follicles had diameters of >18 mm × 12 mm, hCG was administred. Transvaginal ultrasound-guided oocyte retrieval was performed 34–36 h after this injection.
Enzyme-Linked Immunosorbent Assay
The concentration of free sFas, free sFasL, and IFN-γ in FF were determined using commercially available ELISA kits according to the manufacturer’s directions. The ELISA kits for sFas and sFasL were obtained from MBL (Nagoya, Japan) and IFN-γ from Genzyme Corporation (Cambridge, MA). The inter- and intra-assay coefficients of variation for these kits were <12%. The sensitivity of the assays was 312 pg/ml for sFas, 100 pg/ml for sFasL, and 3 pg/ml for IFN-γ. Samples containing less than these amounts were given a value of 0 pg/ml. Data are presented as median and range.
RNA Isolation from Human Oocytes and CC
Twenty-four hours following retrieval, unfertilized oocytes were washed in Ham’s F-10 Hepes-buffered medium and placed in Tyrode’s salt solution, pH 2.1 (both from Gibco BRL Life Technologies, Grand Island, NY) for no more than 20 sec to remove the zona pellucida. Freshly isolated immature oocytes were processed in a similar fashion. The oocytes were stored at −80°C in 19 μl of an RT mixture, as described below, containing 0.5% Nonidet P-40 until processing. Extraction of RNA from CC was performed using Trizol reagent according to the manufacturer’s instructions (Gibco BRL Life Technologies).
Reverse Transcription-PCR
Total RNA from single oocytes and 0.5 μg of RNA from CC and for control, peripheral blood mononuclear cells (PBMC), were reverse transcribed in 20 μl of buffer consisting of 5.2 mM MgCl2, 10.5 mM Tris-HCl, 52.6 mM KCl, 20 U of RNase inhibitor, 0.05 nmol of random hexameres, 50 U of Moloney murine leukemia virus (MMLV) reverse transcriptase (Perkin Elmer, Branchburg, NJ), and 5.3 nm each of deoxyribonucleoside triphosphate mixture (dNTP). The reaction condition was 10 min at 22°C and 42 min at 42°C, followed by a final incubation step of 5 min at 95°C to inactivate the MMLV.
Polymerase chain reaction was performed with cDNA from single oocytes, CC, and PBMC using 0.5 mM of transmembrane domain-specific upstream primer 5′-GCACACTCACCAGCAACACC-3′ and downstream primer 5′-GGTTTTCCTTTCTGTGCTTT-3′ for Fas, upstream primer 5′-CACCAGATCCTGGGGATACTTAGG-3′, downstream primer 5′-CACCAGATCCTGGGGATACTTAGAG-3′ for FasL, and upstream primer 5′-GTGGCCTTCTTTGAGTTCGGTG-3′, downstream primer 5′-TTGGCAGTAAATAGCTGATTCGACG-3′ for bcl-2 (both designed with the help of the Biomolecular Research Tools program), by means of Amplitaq Gold Polymerase (Perkin-Elmer) in a reaction mixture of 30 ml containing 1.67 mM MgCl2, 10 mM Tris-HCl, 50 mM KCl, and 200 mM of digoxigenin (DIG)-labeled nucleotide mixture. The cycle profile consisted of denaturation at 95°C for 30 sec, an annealing temperature that varied from 56 to 60°C, depending on the transcript analyzed (56°C for Fas, 57°C for bcl-2, and 60°C FasL) for 30 sec, and 72°C for 30 sec, with a final extension at 72°C for 7 min.
Heminested PCR was performed with 1/50th of the first amplification mixture, using the same upstream primers and the following inner-downstream primers: 5′-TCATGTAGACCTTGTGGCTCAGG-3′, 5′-CTGCATGTTTCTGTACTTC-3′ for Fas and FasL, respectively, and the inner upper primer 5′-CACCTGGATCCAGGATTACGGA-3′ for bcl-2. Similar amplification conditions were used, except that DIG-labeled nucleotides were not required and the annealing temperature for Fas was 54°C. For the semiquantitative PCR, amplification was performed in the exponential phase of the reaction. Primers were chosen to span introns to avoid genomic DNA template contamination. Histidyl tRNA synthetase was used for semiquantitative PCR analysis. The PCR products were visualized on 1.5–3% agarose gel by staining with ethidium bromide. In addition, CD45 was assessed by immunohistology as a control for the presence of white blood cells in CC.
Polymerase Chain Reaction-ELISA Detection
The PCR-ELISA assays were performed in duplicate as described in the manufacturer’s manual (Boeringher Mannheim, Indianapolis, IN), with slight modifications. Briefly, 5 μl of the PCR product was denatured with 20 μl of denaturation buffer. After 5 to 10 min, single DNA strains were hybridized with the following specific biotin-labeled probes for the transmembrane domain (exon 6) of Fas: 5′-AAGACAAAGCCACCCAAGTTAGATCTGGA-3′, FasL: 5′-CCTCAGCTCCTTTTTTTCAGGGGGTGGACT-3′, bcl-2 5′-TGGGGCAGGCATGTTGACTTCCACTTGTG-3′. Denatured PCR products were incubated for 3 h at 55°C in avidin-coated wells. Each well was washed three times and then incubated for 30 min in the presence of 200 ml (10 mU/ml) of anti-DIG peroxidase-conjugated antibody. Following another three washes, the wells were incubated with 200 μl of ABTS (2,2′-azinobis[3-ethylbenzothiazoline-6-sulfomic acid]) substrate, for 20–30 min. Absorbance was measured in an ELISA reader plate at 405 nm (reference filter 492 nm).
Immunohistochemistry
Immunohistochemical staining was performed by the alkaline phosphatase procedure. Briefly, oocytes and CC were preincubated with 2% human serum in Tris buffer for 30 min to block nonspecific antibody binding. Nine oocytes were tested for Fas expression, 11 oocytes for FasL and 8 for bcl-2, using rabbit antihuman polyclonal IgG for Fas (C-20) at 1/20 dilution and FasL (Q-20) at 1/50 dilution, and mouse monoclonal IgG1 for bcl-2 at 1/50 for 30 min at room temperature in a humidified atmosphere (Santa Cruz Biotechnology Inc., CA). Samples were washed three times and then incubated with goat serum for 10 min at room temperature, followed by 30 min incubation with the secondary antibody, ready-to-use biotin-conjugated goat antirabbit and antimouse antibodies (BioGenex, San Ramon, CA). After washing, alkaline phosphatase streptavidin link (BioGenex) was added for 30 min at room temperature. Fast red substrate (Dako Corp., Carpinteria, CA) was added and incubated until color appeared. Rabbit and mouse immunoglobulins (BioGenex) were used, instead of the primary antibodies, for negative control in an additional 15 unfertilized human oocytes (11 for Fas and FasL and 4 for bcl-2).
An average of 134 ± 26 cells per CC complex was scored for the expression of Fas, and the data were expressed as the percentage of the total number of cells counted.
In Situ Cell Death Detection in CC
Thirty-two individual CC complexes were analyzed for apoptosis using terminal deoxynucleotidyl transferase incorporating fluorescein-labeled nucleotides to DNA strand breaks in situ (Boeringher Mannheim). Briefly, 5-μm cryosections of CC complexes were washed with PBS for 30 min at room temperature and incubated with 0.1% of Triton X-100 permeabilization solution for 2 min at 4°C. After rinsing the slides twice with PBS, CC were incubated in a humidified chamber for 60 min at 37°C. The slides were rinsed three times with PBS, mounted with Vectashield mounting medium with propidium iodide (PI) (Vector Laboratories, Inc., Burlingame, CA), and analyzed under a fluorescence microscope. An average of 129 ± 33 cells were counted per CC complex.
Statistical Analysis
Nonparametric Mann-Whitney U and Kruskal-Wallis tests of significance were used to compare the concentration of cytokines in FF samples associated with oocyte quality. Linear regression analysis was used to analyze the relationship between follicular levels of sFas and both the percentage of the TUNEL-positive cells in CC complexes and with embryo quality.
Results
Soluble Fas was detected in the majority (94%) of individual FF samples (median = 1110 pg/ml, range = 495–2744 pg/ml). In contrast, sFasL was detected in only 3 of 31 (10%) FF samples analyzed (one MII oocyte [sFasL = 322.0 pg/ml], one oocyte MII that failed to fertilize [sFasL = 746.2], and one atretic oocyte [sFasL = 582.9 pg/ml]).
Interferon-γ was detected in 25 (47.5%) of all FF analyzed (median = 41.9 pg/ml, range = 5.0–227.1 pg/ml). Levels of sFas in FF with detectable concentrations of IFN-γ were not significantly different from sFas levels in FF samples with undetectable levels of IFN-γ.
Follicles containing atretic oocytes (n = 5) contained significantly lower concentrations of sFas than follicles containing immature oocytes (n = 5; median = 1052.1 pg/ml, range = 748.2–1860.3 pg/ml vs. median = 1252.5 pg/ml, range = 1012.6–1796.1, P < 0.05). However, follicles with mature oocytes (n = 43) contained a wide range of sFas concentrations (152.3–4956.3 pg/ml) that were not statistically different when compared with follicles containing either atretic or immature oocytes. Levels of sFas did not correlate with either fertilization or embryo quality resulting from mature fertilized oocytes.
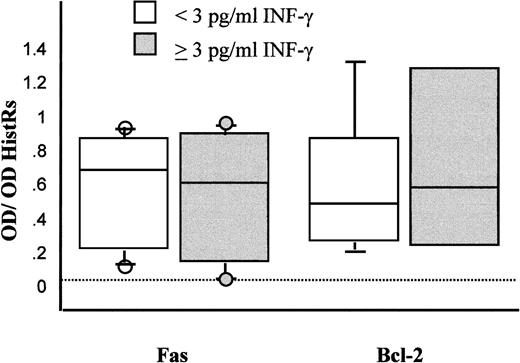
The presence of IFN-γ in FF did not correlate with either the maturation stage of oocytes or the outcome of fertilization. Similarly, neither the number of blastomeres nor the fragmentation rate was altered by the presence of IFN-γ in FF. Semiquantitative PCR combined with ELISA detection revealed that the expression of the Fas gene was independent of the presence of INF-γ in FF (Fig. 1).
Results of semiquantitative RT-PCR analysis of levels of Fas and bcl-2 transcripts in fresh CC
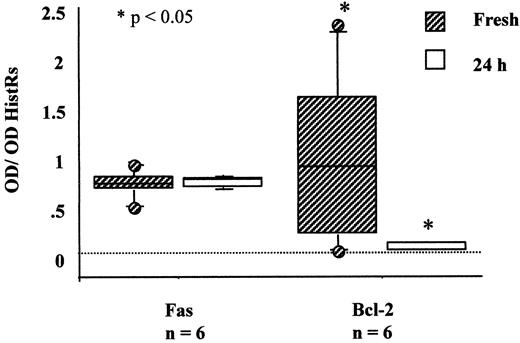
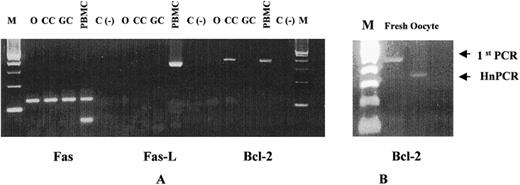
Expression of Fas mRNA was detected in CC, even after 24 h of in vitro culture in the absence of spermatozoa (Fig. 2). Low transcriptional activity for FasL was detected in 2% of the freshly isolated CC complexes, and no de novo transcription was found when cultured for 24 h. Using transmembrane domain-specific primers, neither CC, granulosa cells, nor oocytes expressed the soluble variant of Fas that occurs when the transmembrane domain of 62 base pairs (bp) is deleted (FasTMDel). The PBMC used for positive control showed successful transcription for Fas (including the soluble splice variant of 68 bp of the Fas antigen), FasL (342 bp), and bcl-2 (373 bp) size gene products (Fig. 3).
Result of semiquantitative RT-PCR analysis of transcription activity for Fas and bcl-2 in freshly isolated CC (shaded bars) and after 24 h of culture in vitro (empty bars) in Ham’s F-10 supplemented with 10% synthetic serum
A) Ethidum-bromide-stained agarose gel showing the heminested PCR products in unfertilized oocytes (O), CC, and purifed freshly isolated granulosa cells (GC). B) Bcl-2 mRNA expression in fresh isolated oocytes
The bcl-2 mRNA was present in fresh CC. However, transcriptional levels of bcl-2 did not change in those CC derived from follicles with detectable immunoreactive levels of IFN-γ (Fig. 3). The bcl-2 gene products were absent after 24 h of culture (Fig. 2A). Transcripts for Fas antigen were detected in unfertilized oocytes, whereas mRNA for bcl-2 was detected only in freshly isolated oocytes (Fig. 2B). Messenger RNA for FasL was not present in oocytes. After detection of Fas PCR products by ELISA in unfertilized oocytes, heminested PCR was conducted to confirm the PCR products.
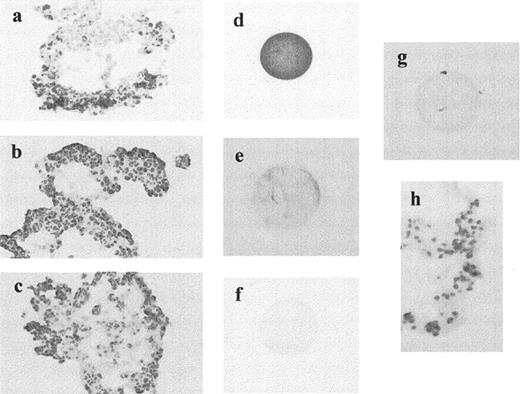
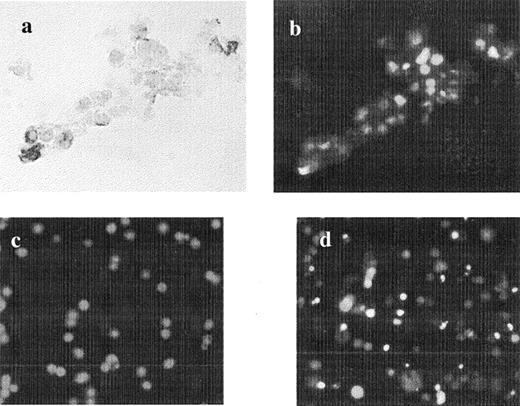
Immunostaining revealed the presence of Fas in both CC and unfertilized oocytes (Fig. 4). The percentage of Fas-positive cells per CC complex analyzed was 18.2 ± 9.6 (mean ± SD) and did not correlate with the concentration of IFN-γ or sFas, fertilization outcome, or poor embryo quality. The goat polyclonal antibody raised against human FasL (C-20) that recognizes the extracellular domain gave a strong staining pattern in both oocytes and CC. However, the use of the antibody Q-20 was consistent with our RT-PCR data. Like FasL, bcl-2 protein was absent in all the CC complexes and on unfertilized oocytes (Fig. 4).
Immunostaining of the Fas antigen, FasL, and bcl-2 on freshly isolated CC and unfertilized oocytes. Fas protein expression was observed on CC (a) and unfertilized oocytes (d). However, neither FasL (b, e), nor bcl-2 (c, f) were detected in both types of cells. Replacement of the primary antibodies with rabbit and/or mouse immunoglobulins showed lack of staining in oocytes and CC (g, h)
The DNA fragmentation analysis revealed 12% (range 1.4 to 39%) apoptotic cells per CC and was independent of the level of sFas or IFN-γ found in FF. There was no correlation between the percentage of TUNEL-positive cells and the percentage of Fas-positive cells per CC complex. Seven colocalization experiments showed the existence of Fas-negative cells undergoing apoptosis by TUNEL assay (Fig. 5, a and b). The rate of apoptosis was influenced by time in in vitro culture. The percentage of TUNEL-positive cells increased from a median of 7% (range 2–32%) in freshly isolated CC complexes to 32% (range 7–46%) observed in the same CC complex (n = 10) 24 h later (P < 0.05). Soluble FasL was undetectable in the culture media.
Colocalization for Fas receptor and TUNEL assay in freshly isolated CC. a) Light microscopy (×20) showing four CC, three of them positive for Fas receptor. b) Same picture showing DNA fragmentation (fluorescein isothiocyanate) by fluorescent microscopy in Fas-negative cells. Example of apoptosis in c) freshly isolated and d) 24-h cultured CC (×p20). The nuclei of the cells (red dots) are stained with PI
Discussion
The Fas-FasL system appears to be involved in the apoptotic events of luteal regression and follicular atresia in humans and other species. Recently, soluble components of the Fas-FasL system that play a functional role in regulating apoptosis have been described in human FF [11]. Fas is expressed in human immature oocytes from primordial and primary follicles [12]. Therefore, we hypothesized that high concentrations of sFas and low concentrations or absence of sFasL in FF may be associated with good quality oocytes. To our knowledge, this is the first report correlating levels of soluble components of the Fas-FasL system in FF with oocyte quality, fertilization outcome, and subsequent embryo quality. Our results indicated that levels of sFasL were beneath the assay detection limit in FF containing mature oocytes, and sFasL was detected in only one (20%) of five FF-containing atretic oocytes. We observed that the level of sFas in FF with immature oocytes was significantly higher than that in FF with atretic oocytes. However, no difference was found when the level of sFas in FF containing mature oocytes was compared with that of sFas in FF containing immature oocytes. The lack of a significant difference was probably attributed to the small number of oocytes used in this study and to the wide range of follicles containing immature and atretic oocytes. Therefore, levels of sFas may not be critical in controlling the quality of oocytes once the oocytes overcome meiotic arrest and achieve the metaphase II stage of development. Lower sFas concentrations in FF did not impact negatively on the outcome of fertilization of mature oocytes or on embryo quality. Similarly, we found no association between the concentration of sFas in FF and the rate of apoptosis, Fas mRNA, percentage of cells expressing Fas in CC, or concentration of IFN-γ in the FF.
Although apoptosis was observed in CC complexes shortly after oocyte harvesting, no trace of FasL was detected in these cell complexes. The significant increase of apoptosis in CC after 24 h of culture in the absence of de novo transcription of FasL compared with freshly isolated ones and the presence of Fas-negative cells undergoing apoptosis suggests that apoptosis in CC is not induced through the Fas system. Because CC are distinct from mural granulosa cells, the mechanisms regulating apoptosis in these cells may also be different, such as increased expression of Fas [14] or new expression of FasL in luteinized granulosa cells [24].
The presence of bcl-2 is thought to be important for cell survival. The majority of the freshly isolated CC analyzed in our study (86%) expressed bcl-2 mRNA. However, bcl-2 mRNA and protein were not detectable in these cells after 24 h of culture. We hypothesize that the absence of bcl-2, rather than the percentage of Fas-positive cells, is related to apoptosis in this type of cells. A similar hypothesis has been postulated in human endometrium, where Fas is present throughout the menstrual cycle, but bcl-2 is reduced beginning by the third postovulatory day and disappears in glandular endometrial cells by the late secretory phase, consistent with the appearance of apoptotic cells [17, 18]. In mice, mechanisms of apoptotic signal transduction in the male reproductive tract is postulated to be through downregulation of bcl-2 that also precedes Fas expression [25].
Oocytes from primordial to antral follicles express Fas antigen, whereas oocytes from preovulatory follicles in nonstimulated ovaries do not [12]. We detected Fas at both the mRNA and protein level in mature and immature unfertilized oocytes. The presence of Fas antigen in unfertilized oocytes may be the result of translation during culture or translation induced by supraphysiological levels of gonadotropin during ovarian stimulation. Expression of bcl-2 may be important in the maintenance of the number of oocytes and primordial follicles [22]. Similarly, bcl-2 overexpression increases folliculogenesis and germ cell tumorigenesis [23]. Bcl-2 may be important for in vitro survival of human embryos, because it is detected as early as the 2-cell stage of development and is expressed more abundantly in good quality embryos [20]. Bcl-2 was not detected in unfertilized oocytes in our study, although it was detected in freshly isolated oocytes. We therefore propose that maternal bcl-2 gene products persist in oocytes unless fertilization is not achieved, and in the absence of fertilization, bcl-2 gene products are rapidly degraded facilitating cell death as we observed in CC after 24 h of culture.
In summary, our results indicate that soluble forms of the Fas-FasL system are present in FF from gonadotropin-stimulated ovaries. The presence of significantly higher concentrations of sFas in FF containing immature oocytes compared to FF containing atretic oocytes, and the detection of sFasL in FF containing atretic oocytes suggests that this system plays a role in preventing oocyte atresia during folliculogenesis but may be not important for apoptosis after fertilization failure. Following ovulation, apoptosis in cumulus cells and in unfertilized oocytes may be facilitated by downregulation of bcl-2, rather than directly through the Fas-FasL system. Further studies analyzing follicles containing atretic oocytes and immature oocytes will be needed to confirm this new hypothesis.
References
Author notes
This work was supported in part by grant AI3851502 from the National Institute of Health, Bethesda, MD, by the Fearing Research Laboratory Endowment, and by the Instituto Valenciano de Infertilidad.