-
PDF
- Split View
-
Views
-
Cite
Cite
Ann M. Clark, Kristin K. Garland, Lonnie D. Russell, Desert hedgehog (Dhh) Gene Is Required in the Mouse Testis for Formation of Adult-Type Leydig Cells and Normal Development of Peritubular Cells and Seminiferous Tubules, Biology of Reproduction, Volume 63, Issue 6, December 2000, Pages 1825–1838, https://doi.org/10.1095/biolreprod63.6.1825
Close - Share Icon Share
Abstract
Testes from adult and prepubertal mice lacking the Desert hedgehog (Dhh) gene were examined in order to describe further the role of Dhh in spermatogenesis because, in a previous report, Dhh-null male mice were shown to be sterile. Dhh is a signaling molecule expressed by Sertoli cells. Its receptor, patched (Ptc), has been previously localized to Leydig cells and is herein described as being localized also to peritubular cells. Two phenotypes of the mice were observed: masculinized (7.5% of Dhh-null males) and feminized (92.5%), both of which displayed abnormal peritubular tissue and severely restricted spermatogenesis. Testes from adult feminized animals lacked adult-type Leydig cells and displayed numerous undifferentiated fibroblastic cells in the interstitium that produced abundant collagen. The basal lamina, normally present between the myoid cells and Sertoli cells, was focally absent. We speculate that the abnormal basal lamina contributed to other characteristics, such as extracordal gonocytes, apolar Sertoli cells, and anastomotic seminiferous tubules. The two Dhh-null phenotypes described have common peritubular cell defects that may be indicative of the essential role of peritubular cells in development of tubular morphology, the differentiation of Leydig cells, and the ultimate support of spermatogenesis.
Introduction
The Hedgehog (Hh)-signaling pathway is involved in a number of processes during embryonic development, including limb morphogenesis [1], neural patterning [2], and development of hair follicles [3] and lungs [4]. The various Hh proteins, Desert, Sonic, and Indian, act through the patched receptor (Ptc) that, when not bound by Hh, represses the action of Smoothened (Smo) [5]. Smoothened is a transmembrane protein that mediates the Hh signal that includes upregulation of the transcription factor Gli [6].
The function of the Hh proteins has been examined in a myriad of developing tissues, but information about the importance of Hh signaling in reproductive organs has emerged only relatively recently. Bitgood and coworkers showed that Desert hedgehog (Dhh) is expressed by Sertoli cells [7] and follows closely the expression of the testis-determining factor Sry in the embryonic testes. Bitgood and coworkers [8] subsequently generated knockout mice that were genetically null for the Dhh gene; although the homozygous null females showed normal reproduction, the males were sterile. The severity of the phenotype varied depending upon the genetic background of the mice. Those on an inbred background of the 129/Sv strain showed development of germ cells up to primary spermatocytes, whereas germ cells in some of the 129/Sv-C57BL/6J F1 hybrids developed through meiosis to become step 15 spermatids.
In our colony of Dhh-null mice bred on a mixed genetic background, the phenotypic outcome of the Dhh-null condition is more severe than previously described. In this paper, we present the characterization of the abnormal morphology of the testis where Dhh is absent. In addition, we correlate this morphology with the cell types that express the Ptc receptor, presumably the targets of Dhh action, by localizing Ptc in the testis of the ptc-LacZ mouse. Lastly, we determine which abnormalities in the testis are due to a lack of Dhh versus a lack of testosterone by comparing the testicular morphology of the Dhh-null mouse with that of the Tfm mouse. These studies are important because the Dhh-null mouse provides a useful model for increasing our understanding of the fundamental interactions between testicular epithelium and mesenchyme and emphasizing the importance of peritubular cell physiology in the development of tubular morphology, the differentiation of adult-type Leydig cells, and the support of spermatogenesis.
Materials and Methods
Animals
Generation of Dhh-null mice was described previously [8], and original breeding mice for our colony were kindly provided by Dr. Andrew McMahon (Harvard University). Mice were bred on a mixed background of 129/Sv, C57BL/6, and Swiss Webster. The Dhh genotype was determined by polymerase chain reaction (PCR) of tail DNA. Because most homozygous-null males were phenotypic females, PCR for Zfy [9] or Sry (sense: GAGAGCATGGAGGGCCAT; antisense: CCACTCCTCTGTGACACT [10]) of tail DNA was used to determine gender.
The ptc-LacZ mice were generated as described by Goodrich and coworkers [11] and were kindly provided by Dr. Matthew Scott (Stanford University). Heterozygous males were bred to B6D2F1/J females (The Jackson Laboratory, Bar Harbor, ME), and the newborn progeny were genotyped by incubation of tail tips in X-gal staining buffer (see below) overnight at 37°C and visualizing subsequent β-gal blue staining.
Testicular feminized (Tfm) mice (63 days of age) were kindly given by Dr. Michael Griswold (Washington State University, Pullman), who originally obtained them from The Jackson Laboratory.
All experimental protocols involving animals were approved by the Institutional Animal Care and Use Committee and were in accordance with National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Preparation of Tissue
For fixation of testes for electron microscopy, adult (>2 mo old) and weanling (∼20 days old) mice were anesthetized with pentobarbital and perfused through the heart [12] with 0.05 M Na-cacodylate buffer containing 5% glutaraldehyde. Testes from neonatal mice were immersion-fixed in a buffer of 0.1 M Na-cacodylate and 1 mM CaCl2 containing 4% glutaraldehyde. After fixation, testes were excised, trimmed of epididymis, weighed, cut transversely with a razor for cross-sectioning of seminiferous tubules, and then cut into 1-mm3 tissue blocks. The tissue blocks were postfixed in osmium:ferrocyanide [13], dehydrated, infiltrated, and embedded in Araldite 502 epoxy resin (Electron Microscopy Sciences, Ft. Washington, PA).
For light microscopy of these tissues, sections 1 μm in thickness, were mounted onto glass slides and stained with 1% toluidine blue. For electron microscopy, thin sections showing silver-gold interference colors were prepared using an ultramicrotome with a diamond knife (Delaware Diamond Knives, Wilmington, DE) and examined with an H500 transmission electron microscope (75 kV, Hitachi, Japan).
The ptc-LacZ mice were killed by CO2 asphyxiation. Testes were removed, decapsulated, immersion fixed for 1 h at ambient temperature in a buffer of 0.1 M phosphate (pH 8.0), 0.5 mM EDTA (pH 8.0), 2 mM MgCl2 containing 0.2% glutaraldehyde, washed three times for 15 min in wash buffer containing 2 mM MgCl2, 0.01% deoxycholate, 0.04% NP40, 0.1 M phosphate (pH 8.0), stained overnight at 37°C in X-Gal stain (wash buffer containing 1 mg/ml X-Gal, 2.12 mg/ml K-ferrocyanide and 1.64 mg/ml K-ferricyanide), and postfixed overnight at 4°C in PBS containing 4% paraformaldehyde. Tissues from adult animals were dehydrated, infiltrated, and embedded in paraffin. Sections (5 μm) were mounted onto glass slides, deparaffinized, and rehydrated, counterstained with nuclear fast red, and covered with Universal mounting media (Research Genetics, Huntsville, AL). Tissues from 2-wk-old animals were embedded in JB4+ (Polysciences, Inc., Warrington, PA), sectioned at 10 μm, mounted onto glass slides, counterstained with nuclear fast red, and cover-slipped with Permount.
Radioimmunoassays
Testosterone in serum of adult mice was measured by an ELISA using a commercial kit for testosterone (Oxford Biomedical, Oxford, MI). Each 50-μl aliquot of serum was combined with 50 μl of PBS and extracted with 1 ml ethyl ether in 12 × 75 glass tubes as instructed by the manufacturer. The organic phase was transferred to a new tube, evaporated overnight, and the residue was reconstituted in 100 μl of buffer that was provided in the kit. The extract was then diluted 100-fold in the same buffer and assayed in 50 μl, along with a standard curve of testosterone doses ranging from 0.002 to 0.2 ng/ml, according to manufacturer’s instructions. The intra-assay coefficient of variation was 4.7%.
Luteinizing hormone and FSH in serum of adult mice were measured using Amersham Pharmacia Biotech (Piscataway, NJ) enzyme immunoassay Biotrak kits for rat hormones. Serum was diluted 5-fold for the LH assay and 10-fold for the FSH assay in buffer provided in the kit. Duplicate 50-μl aliquots of each diluted serum sample were assayed according to manufacturer’s protocols, along with a standard curve of 6.25 to 400 ng/ml and 0.4 to 100 ng/ml for FSH and LH, respectively. The intra-assay coefficient of variation was 9.2% for FSH and 15.7% for LH.
The optical densities for standards and samples in each assay plate were read in a Thermomax Microplate Reader (Molecular Devices, Sunnyvale, CA) at wavelengths suggested by the kit manufacturers. Hormone concentrations were calculated using a Log-Logit transformation of the data from the standard curves.
Statistical Analyses
Possible differences in testicular weight and hormonal concentrations between Dhh-null and wild-type/heterozygous mice were determined by Student’s t-test (α = 0.05).
Results
External Phenotype, Genitalia, and Hormones
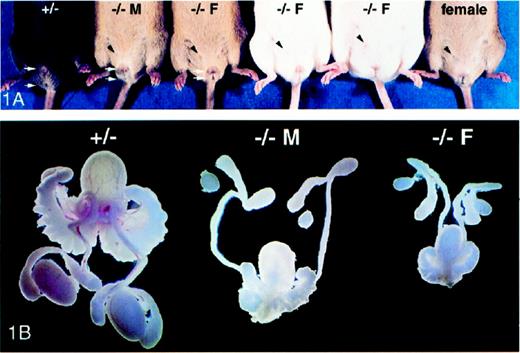
Of the 120 Dhh homozygous-null male mice that have been born in our colony, 111 (92.5%) were pseudohermaphrodites with an external female phenotype, evidence of mammary teats, and a blind vaginal opening (Fig. 1A). Identification of feminized mice as males was accomplished by genotyping for the presence of the Zfy or Sry genes that are located on the Y-chromosome [14]. Internally, the testes of these mice were undescended, extremely small, and were embedded in the fat pads located next to the kidneys (Fig. 1B). The mean paired testis weight (±SEM) in heterozygous animals (n = 5) was 213 ± 12 mg and was significantly greater (P < 0.05) than that of feminized Dhh-null (n = 9) animals, which was 11 ± 3 mg. The external phenotype of the Dhh-null masculinized males was characterized by a relatively longer urogenital-anal distance compared to that of feminized Dhh-null males and little evidence of teats (Fig. 1A). Internally, the masculinized Dhh-null mice were similar to the feminized mice except that the overall size of the reproductive tract was relatively larger, although still smaller, than that of wild-type or heterozygous animals (Fig. 1B). The external phenotype and testicular size of Dhh heterozygous males (Fig. 1) were similar to that of wild-type males (not shown).
A) Ventral view of five male and one female Dhh mice: heterozygous (+/−), masculinized Dhh-null (−/− M), and feminized Dhh-null (−/− F). One of the mammary teats evident in each of the Dhh-null males and the normal female is marked with a black arrowhead. The urogenital-anal distance is marked with white arrows. B) Isolated testes and associated reproductive organs from heterozygous (+/−), masculinized Dhh-null (−/− M), and feminized Dhh-null (−/− F) mice
Assays for LH, FSH, and testosterone were conducted for six control animals (adult wild type and heterozygotes) and three adult Dhh-null feminized males. Mean concentrations (±SEM) of FSH were not significantly different (P > 0.05) between control males (212.3 ± 38.9 ng/ml) and feminized Dhh-null males (281.3 ± 80.1 ng/ml). Concentrations of LH were significantly lower (P < 0.05) in control males (4.9 ± 1.0 ng/ml) than Dhh-null feminized males (14.1 ± 2.5 ng/ml). The mean concentrations of testosterone in control males (3.6 ± 0.64 ng/ml) was significantly higher (P < 0.05) than in feminized Dhh-null males (1.39 ± 0.05 ng/ml).
Light Microscopic Testicular Morphology
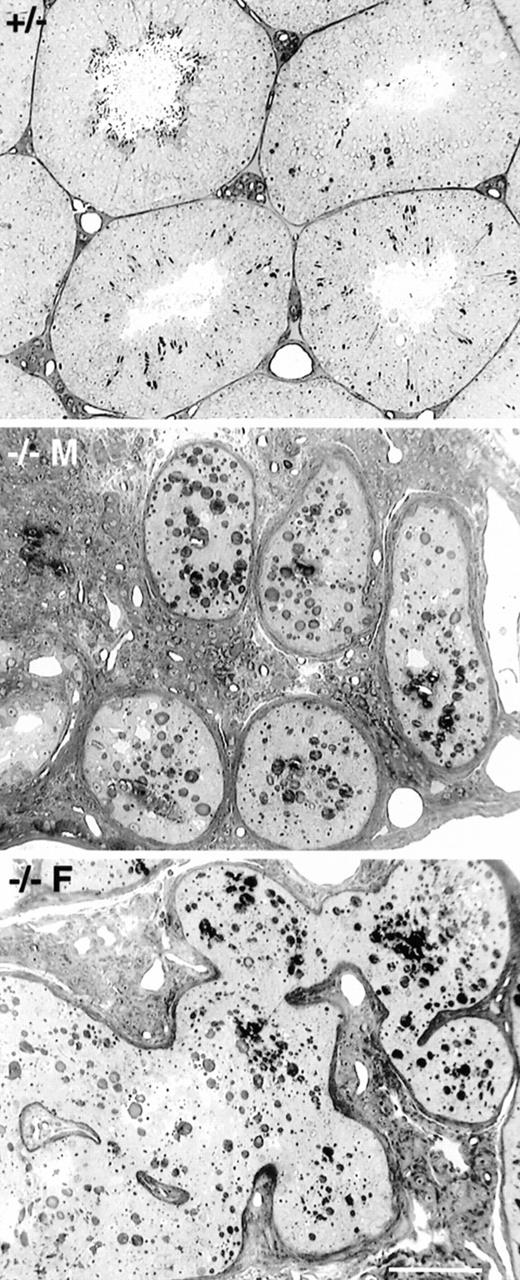
Masculinized Dhh-null adult males (7.5% of all Dhh-null males) showed severely depressed spermatogenesis. Spermatogonia were evident, but only occasional spermatocytes were seen. Other than a lack of spermatogenesis, the seminiferous tubules appeared to be relatively normal, although it was evident that Sertoli cells were small and lacked their normal architecture in the relative absence of germ cells. The interstitial lymphatic space was partially occupied by abundant adult-type Leydig cells (Fig. 2). Feminized Dhh-null adult males also showed depressed spermatogenesis, with rare spermatogonia and spermatocytes. Tubular profiles were not rounded but appeared irregular and anastomotic. The interstitial region of the testes showed few Leydig cells and was largely occupied by fibroblast-like cells (Fig. 2).
Light microscopy of adult spermatogenesis in heterozygous (+/−), masculinized Dhh-null (−/− M), and feminized Dhh-null (−/− F) mice. Tubular profiles in masculinized mice were rounded and contained primarily Sertoli cells and spermatogonia, although occasional spermatocytes could be found. Abundant Leydig cells were noted in the intertubular region. In feminized males seminiferous tubules were anastomotic. The intertubular tissue contained few Leydig cells but contained extensive fibroblastic tissue. Bar = 100 μm
Although we have described two phenotypes above, we have found instances where some Dhh-null male animals were only partially feminized, for example, with colored teat hairs but lacking a blind vaginal opening. As regards testicular structure, we have occasionally found features that were a mixture of the two phenotypes, or one testis of an animal showed the phenotype described for masculinized males and the other testis of the same animal showed the phenotype described for feminized males.
Electron Microscopic Observations of Testicular Morphology
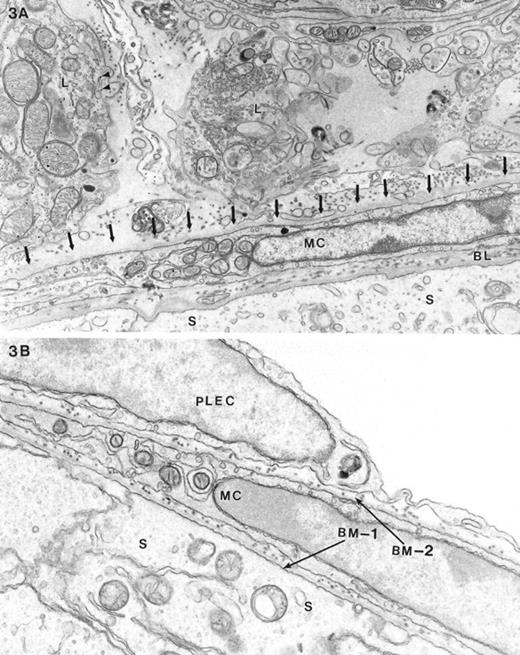
Masculinized Dhh-null adult male mice displayed adult-type Leydig cells characterized by numerous features that differed from fetal-type Leydig cells. Features of adult-type Leydig cells included a dense cytoplasmic matrix, an abundance of smooth endoplasmic reticulum, the presence of whorled endoplasmic reticulum, and a relative paucity of glycogen [15, 16] (Fig. 3A). The myoid cell layer appeared to be relatively normal; however, external to the myoid cell layer there was an incomplete layer of parietal endothelial cells of the lymphatic space. In some regions this layer was present or poorly represented, whereas in other regions it was absent (Fig. 3A). The continuous layer of flattened parietal lymphatic endothelial cells normally found in the wild-type testis (Fig. 3B) was not seen in the masculinized Dhh-null testis. Most of the area normally occupied by the parietal lymphatic endothelial layer was absent. In some regions where the parietal cell of the lymphatics was expected, processes from what appeared to be fibroblast-like elements were noted (Fig. 3A).
Electron microscopy of the testis of A) a masculinized Dhh-null mutant animal and B) a control heterozygous mouse. The Sertoli cell (S), myoid cell (MC), and intervening basal lamina (BL) are indicated. The major difference between the masculinized Dhh-null and heterozygous animals was the absence, in the former, of the parietal layer of the lymphatic endothelium, its normal position indicated by arrows in A. Typical Leydig cells (L) characteristic of adult-type are shown outside the seminiferous tubule of the masculinized animals. The main feature characterizing these cells was the relatively increased cytoplasmic density of the cells compared with fetal-type Leydig cells, abundance of SER and mitochondria, and a relative scarcity of lipid and glycogen (arrowheads). In B, two seminiferous tubules are juxtaposed. Indicated are the Sertoli cells (S), intervening basement membrane (BM-1) between Sertoli cells and myoid cells (MC), the basement membrane external to the myoid cell (BM-2), and the parietal lymphatic endothelial cells (PLEC). Magnification of A = ×15 000; B = ×18 000
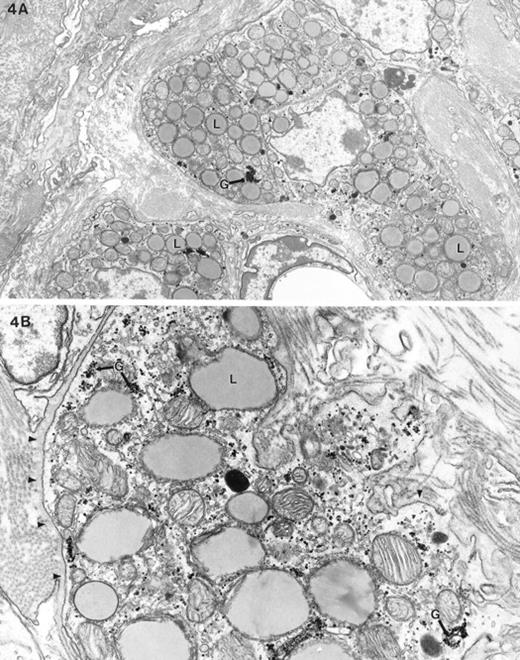
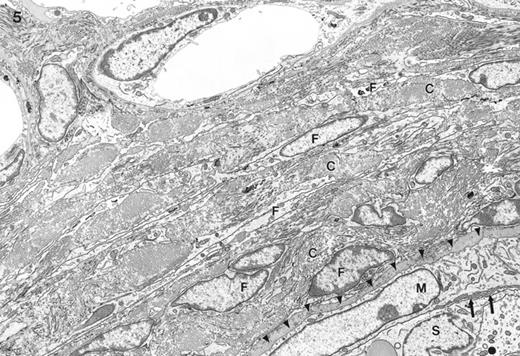
The most pronounced ultrastructural feature of feminized Dhh-null mutant testis was the absence of adult-type Leydig cells. A few aggregations of fetal-type Leydig cells were present in the interstitium (Fig. 4). The fetal-type Leydig cells showed relatively less smooth endoplasmic reticulum (SER) and a less dense cytoplasmic matrix compared to adult-type Leydig cells. They contained no whorled SER [17] and abundant lipid and glycogen. There was generally a basal lamina surrounding clumps of fetal-type Leydig cells. Other than a few clusters of the fetal-type Leydig cells, the interstitium was filled with fibroblast-like cells and abundant intervening collagen and some basal lamina material (Fig. 5). The fibrosis produced by the accumulation of collagen fibers in the interstitium of the feminized mice increased with age. There was no apparent lymphatic endothelial space and no evidence of the peritubular lymphatic endothelial cells.
Electron micrograph of fetal-type Leydig cells in Dhh-null feminized mice. A) This low-power view of Leydig cells shows a cluster of fetal-type Leydig cells, abundant lipid (L), no swirled endoplasmic reticulum, and dense clumped glycogen (G). B) The higher magnification micrograph shows these aforementioned features as well as the abundant glycogen (G). Note the cytoplasmic density of fetal-type Leydig cells is low compared with the adult-type Leydig cells shown in Figure 3A. A basal lamina (arrowheads) surrounds the clump of Leydig cells. Magnification of A = ×6500; B = ×18 900
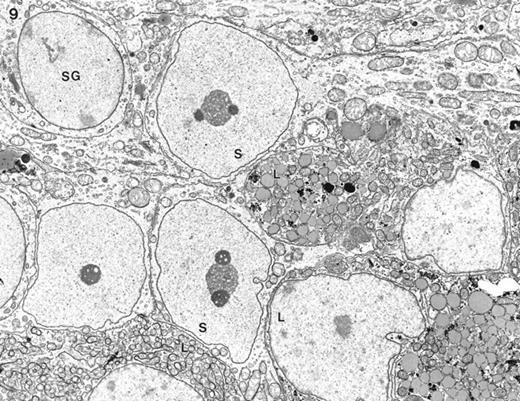
Electron micrograph of the interstitium and border of the seminiferous tubule (lower right) typically seen in feminized animals. External to the seminiferous tubule are numerous layers of fibroblast-like cells (F) with abundant intervening collagen (C). Identified is the myoid cell (M), Sertoli cell (S), and an interstitial capillary (top center). No lymphatic space was found outside the seminiferous tubule. The myoid cell appeared to be large and immature. No parietal cell of the lymphatic space was found external to the myoid cell (arrowheads show its expected position). There was no basal lamina between the myoid cell and the Sertoli cell, instead some microvillous processes were noted in one region (arrows). Magnification = ×4600.
Myoid cells of feminized testes generally appeared to be immature (Fig. 5). They were abnormally thickened instead of being flattened and were without organized actin filaments and the pinocytotic vesicles that are seen in these cells in normal testes. Sometimes the myoid cell layer around the seminiferous tubules was discontinuous, with only collagen in the place of absent cells (not shown).
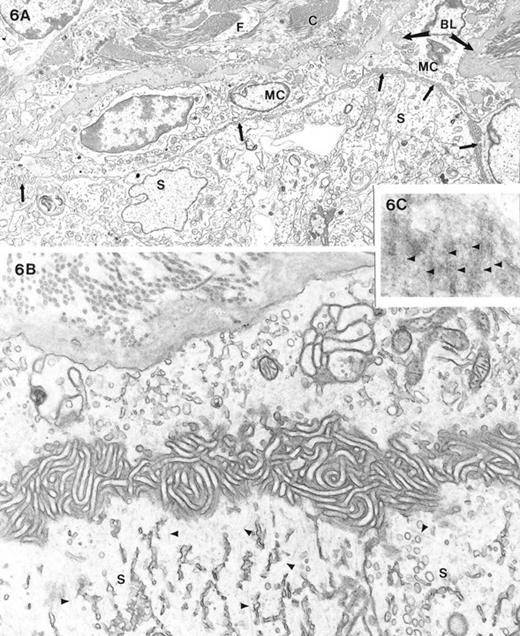
There was a basement membrane between myoid cells and Sertoli cells in most regions of seminiferous tubules, although the lamina densa of the basal lamina was frequently duplicated. In some regions of feminized testes, there was no basement membrane between the myoid cell and cells of the seminiferous tubules (Fig. 6A). Instead, the myoid cells faced and closely apposed the Sertoli or spermatogonial cells with no apparent intercellular, intervening material. In other regions, the myoid cells gave rise to numerous microvilli that impinged upon the Sertoli cells. The apposing Sertoli and myoid cells sometimes sent out microvillous processes that were interdigitated (Fig. 6B). The body of the Sertoli cells in these particular regions of the tubules displayed numerous microtubules in their basal region (Fig. 6B). Microtubules of Sertoli cells in the heterozygous and wild-type mice were situated normally in the apical region as has been previously described [18]. Thus, it appeared that the absence of a basement membrane resulted in a loss of polarity by the Sertoli cells.
A) Low-power electron micrograph showing a seminiferous tubule and interstitial region of a feminized animal. There was no basal lamina between the myoid cell and the Sertoli cells comprising the seminiferous tubule contents. Microvillous processes (arrows) of the large, immature-appearing myoid cells (MC) commonly projected toward the Sertoli cells (S). The interstitium showed fibroblastic cells (F) and collagen accumulation (C) as well as excessive basal lamina (BL) material. B) High magnification micrograph of the relationship of the myoid cell (upper part of image) and the Sertoli cell (S) showing the absence of a basal lamina and the presence of microvillous (mv) processes primarily emanating from the myoid cell. Note that microtubules (arrowheads) were commonly seen in the basal cytoplasm of the Sertoli cell. C) Region of Sertoli-Sertoli cell junctions sectioned en face shows electron-opaque linearities (arrowheads) that represent occluding junction strands. Magnification of A = ×4600; B = ×21 000; C = ×47 000
Examination of en face sections of Sertoli-Sertoli interfaces at the bases of the seminiferous tubules revealed the presence of tight junctional strands. Tight junctional strands were seen as electron-translucent lines in the region where Sertoli-Sertoli interfaces were lined by ectoplasmic specialization (Fig. 6C).
Testes from Neonatal Mice
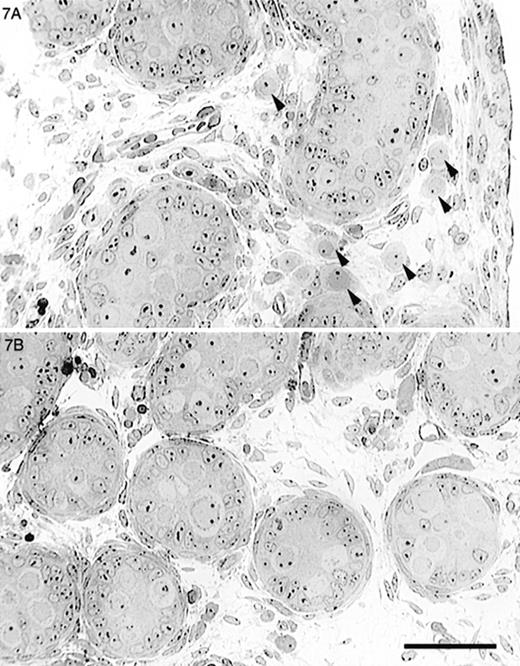
Because many of the testicular abnormalities seen in the Dhh-null mice worsened with age, we examined testes from neonatal mice in order to pinpoint the timing of the abnormal development and determine whether it occurred before or after birth. Testes from newborn (1-day-old) Dhh-null mice were examined by light and electron microscopy. Light microscopy revealed that seminiferous tubules were irregular and sometimes anastomotic but not as extensively anastomotic as seen in adult animals. The basal lamina of the seminiferous cords was not always continuous as noted in adult animals (not shown). In some instances, myoid cells could not be recognized. Relatively undifferentiated cells as well as islands of fetal-type Leydig cells occupied the interstitium. Occasional interstitial mast cells were also noted (not shown). Germ cells were largely present within the seminiferous cords, but many were also seen outside the cords (Fig. 7A). Electron microscopy revealed that germ cells outside the cords were similar in appearance to gonocytes. No synaptonemal complexes were noted in extracordal germ cells, indicating that meiotic development had not occurred. In some instances, somatic cells of an unknown type were partially surrounding these extracordal germ cells. By 8 days of age, nearly all extracordal germ cells had been lost (not shown).
Light micrographs of testes from newborn mice. A) Newborn Dhh-null mouse showed slightly irregular seminiferous tubules and gonocytes (arrowheads) outside of the seminiferous tubules. B) Control mouse testis showed rounded tubular profiles and no extratubular gonocytes. Bar = 50 μm
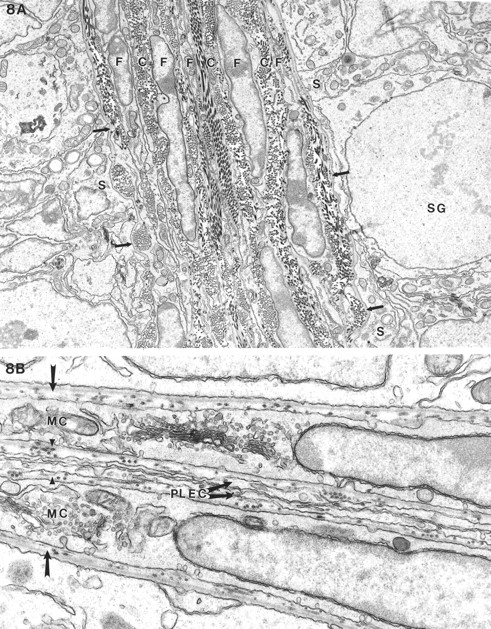
The peritubular and intertubular tissue in a Dhh-null feminized mouse and heterozygous mouse, both at 20 days of age, are compared in Figure 8. Only fibroblastic elements and islands of fetal Leydig cells were seen among tubules of the Dhh-null animals. It appeared that a thin basal lamina was present adjacent to the Sertoli cell. No myoid cells or parietal lymphatic cell could be identified in the Dhh-null testes (Fig. 8A). In contrast, prominent lymphatic space was seen in the heterozygous male outside the typical myoid and parietal cell of the lymphatics (Fig. 8B).
Electron microscopy of seminiferous tubules and interstitial cells of feminized Dhh-null (A) and heterozygous (B) males at 20 days of age. A) Adjacent seminiferous tubules showed a thin basal lamina (arrows) abutting the Sertoli cells (S) and a spermatogonium (SG). External to the basal lamina were numerous layers of fibroblastic (F) cells with intervening collagen (C) with no evidence of a lymphatic space. B) In a heterozygous animal where two tubules adjoined, the typical basal lamina (arrow) was seen between the myoid cells (MC) and the Sertoli cells. External to the basal lamina was yet another basal lamina (arrowheads) separating the myoid cell from the parietal lymphatic endothelial cell (PLEC). Because the two tubules were closely apposed, there was no lymphatic space. Magnification of A = ×8500; B = ×4600
In some cases where the basement membrane was absent, there was a direct intermingling of Leydig cells with Sertoli cells without any intervening peritubular tissue or extracellular basal laminae (Fig. 9). This feature was noted in animals at 20 days of age, yet was also found in adult animals (not shown).
Electron micrograph of feminized Dhh-null testis of 20 days of age. At the periphery of some seminiferous tubules no peritubular tissue was seen, allowing direct contact between fetal-type Leydig cells (L) and Sertoli cells (S). SG, Spermatogonium. Magnification = ×3500
Testes from Tfm Mice
We compared the testicular morphology of the Dhh-null animals with that of Tfm mice that lack a functional androgen receptor and are androgen insensitive [19]. Testes from adult Tfm mice (not shown) contained abundant adult-type Leydig cells. Similar to a previous report [20], we found that these cells were relatively undifferentiated, lacking smooth endoplasmic reticulum. An extra layer of parietal lymphatic cells was present, but the cells appeared to be normal. Myoid cells were also normal. Peritubular fibrosis was evident by light microscopy. By electron microscopy, peritubular fibrosis was characterized by a few extra layers of fibroblastic-like cells around the seminiferous tubules, although this fibrosis was not as severe as that seen in the Dhh-null testis. Collagen and basal lamina material between Sertoli and myoid cells extended around the entire seminiferous tubule unlike that of the patchy basal lamina seen in Dhh-null mutants. Germ cell development progressed to spermatocytes.
Cellular Location of the Ptc Receptor in the Testis
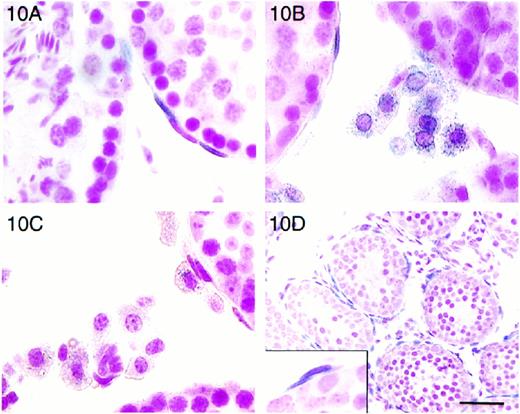
In the ptc-LacZ “knock-in” mice, β-galactosidase is coexpressed with the Ptc receptor. Intracellular location of the LacZ β-galactosidase enzyme is directed to the nucleus of expressing cells by a nuclear-targeting sequence [11]. Staining of tissues from these mice with X-gal helped to identify cells that expressed Ptc, labeling them as targets of Hh signaling. In the testis of neonatal (not shown), 20-day-old, and adult ptc-LacZ mice, ptc was expressed in peritubular cells, possibly Leydig cells (Fig. 10), and in tunica propria cells of epididymal tubules (not shown). In a close examination of Leydig cells, ptc-LacZ mice showed a greater β-galactosidase staining (Fig. 10B) than nontransgenic mice (Fig. 10C); however, most of this staining appeared to be cytoplasmic. Thus, we remained uncertain about the presence of the Ptc receptor in Leydig cells.
Localization of β-galactosidase in ptc-LacZ “knock-in” mice (A, B, D) and nontransgenic littermate (C), adult (A, B, C), and 13-day-old (D) mice. A) β-Galactosidase-positive nuclei were seen in peritubular cells. When two peritubular cells overlay each other, the innermost cell was presumed to be the myoid cell and was always the cell that was LacZ positive. B) LacZ-positive Leydig cells showed β-galactosidase staining primarily in the cytoplasm. C) Endogenous β-galactosidase activity was demonstrated by staining in Leydig cells of control animals but was not as intense as that seen in ptc-LacZ animals. No staining was seen in myoid cells or overlapping peritubular cells in LacZ-negative control animals. D) β-Galactosidase staining in testes from neonatal animals localized ptc expression to all myoid and peritubular lymphatic cells (inset). Bar = 50 μm in D and 17 μm in A, B, C, and D-inset
Because there are two flattened cell types around seminiferous tubules, it was important to distinguish them to determine the precise site of ptc expression. We identified myoid cells as the innermost flattened cells in tubular profiles where parietal cells of the lymphatic endothelium lay adjacent to or on top of myoid cells. When images of this orientation were noted, only the myoid cells of the adult animals were LacZ-positive (Fig. 10A). In 2-wk-old animals, most peritubular cells were LacZ-positive, including those lying peripheral to the innermost layer (Fig. 10D). Neither myoid nor peritubular cells showed blue staining in testes from nontransgenic animals (Fig. 10C).
Discussion
In the present study we have described several features of a testicular phenotype that have not been reported previously. We are not aware of genetic models in which the adult population of Leydig cells is missing, although transgenic mice that have strong overexpression of anti-Müllerian hormone do have a reduction in the number of adult-type Leydig cells that differentiate from mesenchymal cells [21]. To our knowledge, anastomotic and highly irregular seminiferous tubules have never been described nor have the types of abnormalities described here in the peritubular tissue. Finally, we are not aware of genetic models in which large numbers of gonocytes reside outside the seminiferous tubules in postnatal life. Each of these apparently unique features results directly or indirectly from the lack of Dhh. Although there is no reported human condition that is similar to the Dhh-null mouse, we believe that the testicular characteristics displayed by this mouse make it a useful model with which to study testicular growth and differentiation.
For this model, we have reported here two phenotypes of which one commonality was the abnormal peritubular tissue. Because Dhh-null mice have deficiencies in both Dhh and testosterone, we examined testes of the Tfm mouse to determine whether the abnormalities in the peritubular tissue of the Dhh-null testes are due to insufficient androgen that is independent of Dhh. Although some interstitial fibrosis was evident in the Tfm testis, the basal lamina, and peritubular cell layers were normal. Therefore, a main function of Dhh in the testis appears to involve the proper development of peritubular tissue.
The peritubular tissue of the testis is the least studied of all of the testicular components. There are two cell layers in this tissue that normally form the tunica propria. In the present study, we have shown that both of these cellular layers in the neonate express ptc, the receptor for Hh, and the inner layer expresses ptc in the adult.
In the wild-type testis, the outermost of the two peritubular layers is comprised of highly flattened and poorly differentiated cells termed the parietal endothelium of the lymphatic space [22]. The role of the parietal lymphatic endothelial cells is unknown, but these cells are believed to delimit the seminiferous tubule from the lymphatic space. In the mouse and rat, there is no well-defined lymphatic space because the cells comprising the visceral layer of the lymphatic space, although described [22], are not generally found in the normal animal (L.D.R., personal observations). The innermost cells of the peritubular tissue are more differentiated and are known as the myoid cells. Traditionally, the myoid cells have been described as contractile [23] and important in compressing the tubule to move fluid [24].
A better-characterized function of the myoid cell is its contribution to the formation of the basal lamina. The basal lamina is known to be secreted cooperatively by both Sertoli cells and myoid cells [25] and primitive tubules can be formed by coculture of peritubular cells and Sertoli cells [26]. The consequences of the loss of the basal lamina in feminized animals are revealing. In regions where the basal lamina was absent, the myoid cells showed microvillous processes in the position where a basal lamina should have been found. Such a finding suggests that the myoid cells had partially taken on an epithelioid characteristic, forming an apical surface directed toward the Sertoli cells. The presence of basally positioned microtubules in Sertoli cells situated at areas where the basal lamina was lacking suggests that these Sertoli cells no longer had a distinct basal-apical polarity. The polarity of the Sertoli cell, like that of many cell types in the body, is largely governed by the presence of a basal lamina [27]. In addition to unpolarized Sertoli cells, a patchy basal lamina in the feminized Dhh-null animals may have allowed both the direct apposition of Sertoli cells to Leydig cells and the anastomosis of the seminiferous tubules.
How does tubular anastomosis occur developmentally in these mice? We suggest that tubular anastomosis is related to the focal loss of the basement membrane. The basement membrane represents a physical boundary that delimits the contents of the seminiferous epithelium from the peritubular tissue. Because there is not a basal lamina in some regions of the tubules, there may be no barrier to the fusion of juxtaposed tubules or cords. Because the basal lamina is formed as cords develop prenatally and Dhh is one of the earliest genes to be expressed in male development [7], our objective will now be to examine earlier periods of testicular development in the Dhh-null mouse to determine the nature and timing of the primary defect.
The loss of a continuous basal lamina may have also contributed to the migration of large numbers of germ cells from the primitive cords to the interstitium in the Dhh-null testis. McLaren and Buehr [28] have proposed from in vitro data that when Sertoli cells do not form cords, primordial germ cells enter meiosis shortly thereafter. In addition, ectopic positioning of embryonic germ cells from either XX or XY animals prompts these cells to enter meiosis [29–31]. A factor hypothesized to be secreted by the genital ridge at about the 12th postcoital day prohibits entry of primordial germ cells into meiosis [31]. In testes of the Dhh-null animals, cords were formed but extracordal germ cells had not entered meiosis, suggesting that the presence of seminiferous cords is important to prevent primordial germ cells from entering into meiosis. The extracordal germ cells died shortly after birth, coinciding with the initial divisions of murine spermatogenesis.
The question as to what leads to defective spermatogenesis in the two phenotypes of Dhh-null animals remains open. In the phenotype where mature Leydig cells are absent, it is tempting to speculate that testosterone is insufficient, resulting in the failure of germ cells to progress beyond spermatocytes, as is normally the case in the absence of gonadotropin or androgen [32]. In addition to inadequate amounts of testosterone, the testes of both phenotypes are abdominal. Thus, spermatogenesis may be suppressed by the intra-abdominal milieu. Although suppression of spermatogenesis would occur in this condition, the degree of suppression was greater in Dhh-null animals than in Tfm animals—animals that lack androgen action and have abdominal testes. Therefore, we suggest that a disruption of the physiology of the peritubular cells due to the loss of action of Dhh is the cause of defective spermatogenesis in the Dhh-null mice. This disruption of peritubular physiology in the Dhh-null testis may have negative consequences on communication from the peritubular cells back to the Sertoli cells, perhaps in the form of P-mod-S that is secreted by the peritubular cells and stimulates Sertoli cell function [33].
Although it is apparent from the present data that Dhh acts upon the peritubular tissue, effects on development of adult-type Leydig cells were also seen in the Dhh-null mice. Expression of ptc in Leydig cells has been reported [8, 34]. In the present study, we could not be certain of the expression of ptc in Leydig cells when localizing blue staining in testes from ptc-LacZ mice because the staining, although more intense than that from endogenous β-galactosidase activity in wild-type cells, was not localized to the nucleus. If Leydig cells do not express Ptc, then Dhh is probably not required for the proper function of adult-type Leydig cells once they have developed. Development of functional adult-type Leydig cells in the wild-type mouse occurs postnatally, beginning at approximately 15–20 days of age (L.D.R., personal observations). This process is dependent upon LH and androgen [35]. In the Tfm mouse testis, adult-type Leydig cells develop in the absence of androgen action, although the cells are functionally immature (present study) [20]. Thus, the lack of development of adult-type Leydig cells in the Dhh-null testis is not a consequence of insufficient androgen. Nor is it due to an insufficiency in LH, as we detected elevated concentrations of LH in serum of Dhh-null mice that was expected based on the relatively low concentrations of circulating testosterone (present study). Therefore, the absence of adult-type Leydig cells in the Dhh-null mouse testis must be due to the lack of Dhh.
The cellular origin of adult-type Leydig cells is possibly from the peritubular cells or fibroblastic cells in the interstitium and/or from fibroblastic cells around the vessels [36]. Most investigators suggest a peritubular site for the origin of most of the adult population of Leydig cells, and a recent paper supports this for the rat [37]. Leydig cells are frequently formed in the area of the peritubular tissue external to the myoid cell or between the myoid cell and the parietal lymphatic cell. Thus, the parietal lymphatic cell or similar appearing cells in this region may be a precursor cell for formation of new Leydig cells [38–41]. In place of Leydig cells, however, feminized Dhh-null animals possess undifferentiated cells that are fibroblastic in nature. Their presence in large numbers may result from a failure of differentiation of interstitial mesenchymal cells or peritubular cells into Leydig cells; however, our studies of Dhh-null testis prior to 20 days of age reveal that many of the interstitial fibroblastic cells are present prior to the expected time of formation of adult-type Leydig cells. If parietal lymphatic endothelial cells respond to Dhh directly, as indicated by the presence of ptc-LacZ expression in prepubertal testes, or indirectly from their interaction with myoid cells, Dhh may be an essential molecule that regulates the differentiation of Leydig cells from parietal lymphatic peritubular cells. Then, one might ask, why are adult-type Leydig cells present in masculinized animals? Perhaps other Hh proteins (Sonic or Indian) secreted from distant sources may act to induce some differentiation of Leydig cells in the masculinized Dhh-null animals. Whatever the mechanism, there is likely a genetic basis for the phenotypic variation described here, similar to that reported by Bitgood and coworkers [8].
In the feminized phenotype, the relatively few Leydig cells that were present were identified as fetal in type based on their distinct morphology. The ultrastructure of fetal-type Leydig cells, as herein shown in the mouse, is very different from adult-type Leydig cells, and thus the two are readily distinguishable. There is a controversy as to whether fetal Leydig cells persist into adulthood, with evidence for some species that they degenerate, and from the same or other species that they persist [17, 35]. The presence of fetal-type Leydig cells in adult feminized Dhh-null animals is evidence that fetal Leydig cells have the capability to remain into adulthood, as has been suggested [41–43]. If fetal-type Leydig cells are present in the normal adult testis, they may not be readily detected because they lie among a much larger population of adult-type Leydig cells.
How does the lack of Dhh affect other tissues where Dhh is normally expressed, and how do these effects compare to those described here for the testis? In the embryo, Dhh is expressed in the endocardium of the atrioventricular canal and truncus arteriosus, Schwann cells of sensory and motor nerves, and Sertoli cells of the testis [7]. Although no defective phenotype of the endocardium has been reported for the Dhh-null mice, the abnormalities seen in the peripheral nerves bear similarities to those reported here for the testis. In the peripheral nerves of the Dhh-null mice, the cross-sectional profiles were misshapen and flattened instead of round [44], similar to the distorted shapes of the seminiferous tubules in the testis (present paper). The perineurial cells of the nerve sheath were relatively fewer in number, appeared to be immature, and did not deposit a complete basal lamina, strikingly similar to the abnormalities seen in the myoid cells in the testis. The next cellular layer in each structure was also adversely affected; the epineurial cells were less compacted and did not construct a proper collagen sheath around the nerve, and the parietal lymphatic cells in the testis were absent and did not give rise to adult-type Leydig cells.
As proposed by Parmantier and coworkers for the peripheral nerve [44], Dhh from Schwann cells is not required to signal the nearby mesenchymal cells to form a primitive sheath around the nerve, but Dhh is necessary to induce those cells, once arranged, to differentiate into perineurial cells, form tight junctions, and deposit basal lamina. Likewise in the rudimentary testis, Dhh from Sertoli cells is not needed to recruit mesenchymal cells that migrate into the gonad from the embryonic mesonephros and take up residence around the seminiferous cords [45, 46]; however, Dhh does appear to be required for those cells to mature as myoid and parietal lymphatic cells, to deposit a proper basal lamina, and to form adult-type Leydig cells. There is a description of the migration of mesenchymal stromal cells from embryonic hind limb buds into primordial testes in vitro. The cells from the hind limb buds formed seminiferous cords and deposited basal lamina [47]. Based on the present studies, we speculate that differentiation of the cells from the limb bud may have been due to the action of Dhh from Sertoli cells. Although other Hh proteins are involved in mesenchymal-epithelial interactions in nontesticular tissues [48–51], Dhh in peripheral nerve and testis appears to be responsible for inducing mesenchymal cells to differentiate and contribute to an infrastructure that involves deposition of basal lamina.
In summary, we have described the Dhh-null mouse testis at periods from birth into adulthood and have shown several features of testicular development that go astray, leading to a testis that is small and is virtually devoid of spermatogenesis. Masculinized and feminized phenotypes were described. Masculinized testes showed an incomplete layer of parietal lymphatic endothelial cells, whereas the feminized phenotype lacked both adult-type Leydig cells and a complete layer of parietal lymphatic endothelial cells. The feminized males also showed an immature myoid cell as well as defects in or the absence of a basal lamina that may have been responsible for the extracordal germ cells that were present in newborn animals. Peritubular myoid cells and prepubertal parietal lymphatic endothelial cells were shown to express ptc, the receptor for Dhh. We conclude that the primary defect in the Dhh-null mouse is the dysfunction of the peritubular cells; myoid cells in the adult and perhaps all peritubular cells of the developing animal. We believe that the other defects that follow, including the disruption of spermatogenesis, are the result of dysfunctional peritubular cells.
Acknowledgments
The authors thank Dr. David Bumcrot and Dr. Benoit St.-Jacques for critical review of this manuscript. We gratefully acknowledge Jean Flanagan, Jean-Claude Sova, and Sara Leiker for excellent care of the animals, and John Lydon, Ricardo Sanchez, Angie Raymer, and Ying Li for histological work and help with creating the figures.