-
PDF
- Split View
-
Views
-
Cite
Cite
Nicole Focks, Christoph Benning, wrinkled1: A Novel, Low-Seed-Oil Mutant of Arabidopsis with a Deficiency in the Seed-Specific Regulation of Carbohydrate Metabolism, Plant Physiology, Volume 118, Issue 1, September 1998, Pages 91–101, https://doi.org/10.1104/pp.118.1.91
Close - Share Icon Share
Abstract
During oil deposition in developing seeds of Arabidopsis, photosynthate is imported in the form of carbohydrates into the embryo and converted to triacylglycerols. To identify genes essential for this process and to investigate the molecular basis for the developmental regulation of oil accumulation, mutants producing wrinkled, incompletely filled seeds were isolated. A novel mutant locus, wrinkled1 (wri1), which maps to the bottom of chromosome 3 and causes an 80% reduction in seed oil content, was identified. Wild-type and homozygouswri1 mutant plantlets or mature plants were indistinguishable. However, developing homozygous wri1seeds were impaired in the incorporation of sucrose and glucose into triacylglycerols, but incorporated pyruvate and acetate at an increased rate. Because the activities of several glycolytic enzymes, in particular hexokinase and pyrophosphate-dependent phosphofructokinase, are reduced in developing homozygouswri1 seeds, it is suggested that WRI1 is involved in the developmental regulation of carbohydrate metabolism during seed filling.
Following morphogenesis, developing embryos of Arabidopsis accumulate lipids in the form of triacylglycerols as the major carbon and energy reserves, which are then used for germination and growth of the young seedling. The triacylglycerols are stored in oil bodies that occupy close to 60% of the cell volume of the cotyledons in mature embryos (Mansfield and Briarty, 1992). In this respect, Arabidopsis resembles the closely related crop plant canola, which is an important source of commercial seed oil. Many aspects of fatty acid biosynthesis and modification in developing seeds are well established (Miquel and Browse, 1994; Ohlrogge and Browse, 1995;Harwood, 1996), and much of our knowledge is derived from a large number of mutants of the model plant Arabidopsis (Browse and Somerville, 1994). Furthermore, schemes have been developed (and some have already been successfully implemented) to modify the fatty acid composition of seed oils by genetic engineering (Kinney, 1994;Ohlrogge, 1994; Töpfer et al., 1995). Nevertheless, little is known about the molecular basis for the developmental regulation of triacylglycerol biosynthesis in developing oilseeds (Ohlrogge and Jaworski, 1997). A number of mutants of Arabidopsis, includingfus3, lec1, and tag1, that do not accumulate triacylglycerols in their seeds to the same extent as the wild type have been isolated (Bäumlein et al., 1994; Meinke et al., 1994; Katavic et al., 1995). FUS3 and LEC1presumably encode more general regulators of late embryo development (Parcy et al., 1997) rather than factors specifically governing triacylglycerol biosynthesis. On the contrary, TAG1 has been proposed to encode a diacylglycerol acyltransferase (Katavic, 1995), but may also be involved in regulatory aspects of triacylglycerol biosynthesis, based on the complex phenotype of developingtag1 mutant seeds.
Another poorly understood aspect of developing oilseeds is the conversion of carbohydrates provided by photosynthesis into precursors of fatty acid biosynthesis. In canola the photoassimilate Suc is produced primarily by the silique wall during the seed-filling stage and is imported into the embryo, where it is cleaved by Suc synthase into UDP-Glc and Fru (King et al., 1997). The precise route(s) of conversion of these two metabolites is less clear. At least two glycolytic pathways, one cytosolic and the other plastidic, are operational in leaf tissues (Plaxton, 1996), and experiments with plastids isolated from developing embryos of canola suggest that a complete glycolytic pathway is also present in these plastids (Kang and Rawsthorne, 1994). Of the different substrates supplied to plastids from developing seeds, pyruvate and Glc-6-P supported the highest rates of fatty acid biosynthesis. In the same study it was also shown that plastids of developing seeds contain high pyruvate decarboxylase activity, which is required for the conversion of pyruvate to acetyl-CoA, which is carboxylated to malonyl-CoA, the precursor for fatty acid biosynthesis. Glc-6-P was the most efficient substrate for the biosynthesis of starch that intermittently accumulates in plastids of canola during seed development (Kang and Rawsthorne, 1994). Furthermore, Glc-6-P is thought to be metabolized via the oxidative pentose phosphate pathway, a process that apparently provides NADPH for fatty acid biosynthesis (Kang and Rawsthorne, 1996).
Although canola seems to be more suitable for the physiological approach described above, its close relative Arabidopsis is the better genetic model organism. To dissect the pathway(s) of carbohydrate metabolism and to identify genes essential for the regulation of carbon partitioning and triacylglycerol biosynthesis in developing oilseeds, we began to search for genetic mutants of Arabidopsis that would produce seeds with reduced oil content. In an attempt to identify the metabolic defect in these mutants, we determined the amounts of triacylglycerols, proteins, and carbohydrates, as well as the activity of enzymes involved in carbohydrate metabolism in developing seeds of Arabidopsis.
MATERIALS AND METHODS
Plant Material
Seeds of Arabidopsis ecotypes Columbia (Col-2) or Landsberg erecta (Ler) were surface sterilized according to the method of Estelle and Somerville (1987) and germinated on Murashige-Skoog medium (Murashige and Skoog, 1962) solidified with 1% agarose. The plates were transferred to growth chambers after a cold treatment of 1 to 3 d at 4°C in the dark, and incubated at 20°C/15°C day/night temperatures under a 16-h/8-h light/dark regime (100 μmol m−2 s−1). After approximately 2 weeks, the plantlets were transferred to soil (Einheitserde type P: Einheitserde type T:sand [4:2:3], Gebrüder Patzer, Sinntal-Jossa, Germany) and grown under the same conditions as before. For determination of growth, aerial parts of six different plants per line were weighed every 5 d for up to 50 d following the transfer to soil. To harvest siliques of defined developmental stages, individual flowers were tagged using colored threads on the day of flowering. Only primary shoots were used; secondary shoots were removed.
Mutant Screening and Genetic Mapping
A previously described mutagenized M2population of Col-2 was used for the mutant screening (Dörmann et al., 1995). Putative low-seed-oil mutants were preselected either by visual examination of M2 seeds for wrinkledness or according to density by centrifugation of M2seeds in a mixture of 1-bromohexan (density 1.176) and 1,6-dibromohexan (density 1.589). For this purpose, about 8,000 seeds each of the 20 independent M2 batches were suspended in 1 mL of this mixture in a 1.5-mL reaction vessel and centrifuged for 5 min at 16,000g. The ratio of the two solvents was empirically adjusted to give a density such that about 5% of the seeds collected at the bottom of the tube. The bulk of the seeds floating on top was discarded. To remove the organic solvent, the seeds were washed three times in light silicon oil, which is inert but miscible with the two organic solvents. The seeds were surface sterilized and germinated on Murashige-Skoog medium supplemented with 1% Suc as described above.
Prior to detailed analysis selected wri mutants were backcrossed three times to the Col-2 wild type. For mapping purposes the wri1–1 mutant was crossed with the Ler wild type. Wrinkled F2 seeds were visually selected and germinated. Homozygous mutant plants were confirmed by examining F3 seeds for wrinkledness and low-oil content. DNA was isolated from individual F2 plants according to the method of Edwards et al. (1991) and used as a template for PCR-based markers, either cut, amplified polymorphic sequences (Konieczny and Ausubel, 1993) or simple-sequence-length polymorphisms (Bell and Ecker, 1994).
Lipid and Protein Analysis
For triacylglycerol quantification, 10 seeds were ground in a 1.5-mL polypropylene test tube with a glass rod, and lipids were extracted in 50 μL of chloroform:methanol:formic acid (10:10:1, v/v). Following the extraction with 12.5 μL of 1 m KCl and 0.2m H3PO4 and separation of the organic and aqueous phases by centrifugation at 16,000g for 5 min, the lipids in the lower phase were separated on a silica TLC plate (Si 250 PA, J.T. Baker, Philipsburg, NJ) developed with hexane:diethylether:acetic acid (60:40:1, v/v). Lipids were visualized by staining with iodine vapor. For quantification of triacylglycerols by GLC of the corresponding fatty acyl methyl esters, 10 seeds were ground directly in a glass reaction tube and incubated in 1 mL of 1 n methanolic HCl at 80°C for 2 h. Fatty acyl methyl esters were extracted into 1 mL of hexane following the addition of 1 mL of 0.9% (w/v) NaCl. Myristic acid was used as an internal standard and GLC was performed as described previously (Rossak et al., 1997).
For quantification of total seed protein, 20 seeds were homogenized in 250 μL of acetone in a 1.5-mL polypropylene test tube. Following centrifugation at 16,000g, the supernatant was discarded and the vacuum-dried pellet was resuspended in 250 μL of extraction buffer containing 50 mm Tris-HCl, pH 8.0, 250 mm NaCl, 1 mm EDTA, and 1% (w/v) SDS. Following incubation for 2 h at 25°C, the homogenate was centrifuged at 16,000g for 5 min and 200 mL of the supernatant was used for protein measurements employing the Lowry DC protein assay (Bio-Rad). Protein in enzyme extracts of developing seeds was quantified using the Bradford assay (Bio-Rad). In both assays γ-globulin was used for calibration.
Carbohydrate Analysis
For the extraction of soluble sugars and starch, 50 seeds were homogenized in 500 μL of 80% (v/v) ethanol in a 1.5-mL polypropylene test tube and incubated at 70°C for 90 min. Following centrifugation at 16,000g for 5 min, the supernatant was transferred to a new test tube. The pellet was extracted twice with 500 μL of 80% ethanol. The solvent of the combined supernatants was evaporated at room temperature under a vacuum. The residue was dissolved in 50 μL of water, representing the soluble carbohydrate fraction. The pellet left from the ethanol extraction, which contained the insoluble carbohydrates including starch, was homogenized in 200 μL of 0.2n KOH, and the suspension was incubated at 95°C for 1 h to dissolve the starch. Following the addition of 35 μL of 1n acetic acid and centrifugation for 5 min at 16,000g, the supernatant was used for starch quantification.
To quantify soluble sugars, 10 μL of the sugar extract was added to 990 μL of reaction buffer containing 100 mm imidazole, pH 6.9, 5 mm MgCl2, 2 mmNADP, 1 mm ATP, and 2 units mL−1 of Glc-6-P dehydrogenase. For enzymatic determination of Glc, Fru, and Suc, 4.5 units of hexokinase, 1 unit of phosphoglucoisomerase, and 2 μL of a saturated fructosidase solution were added in succession. The production of NADPH was followed photometrically at a wavelength of 340 nm. Similarly, starch was assayed in 30 μL of the insoluble carbohydrate fraction with a kit from Boehringer Mannheim.
Feeding of Labeled Precursors
For incorporation studies, 20 Arabidopsis seeds of each of the developmental stages were removed from two tagged siliques and transferred to 100 μL of 100 mm Hepes buffer, pH 7.4. The seed coat was not removed. The following 14C-labeled compounds were added in the concentrations and specific activities indicated: [U-14C]Suc (DuPont/NEN), 34 mm, 2.5 GBq mol−1;d-[U-14C]Glc (Amersham), 34 mm, 2.5 GBq mol−1; [2-14C]pyruvate (DuPont/NEN), 1 mm, 25 GBq mol−1; or [1-14C]acetate (Amersham), 1 mm, 90 GBq mol−1. If not otherwise mentioned in the text, the seeds were incubated under gentle rocking for 18 h in the light (100 μmol m−2 s−1). Following incubation, the seeds were washed with 800 μL of water. Triacylglycerols were extracted and separated by TLC as described above. Silica material containing the triacylglycerols was transferred from the TLC plate into scintillation cocktail and radioactivity was determined by scintillation counting.
Preparation of Protein Extracts and Enzyme Assays
To determine the activity of different enzymes in developing embryos, approximately 400 seeds from 12 to 15 siliques taken 9 to 11 d after flowering (if not otherwise indicated) were transferred into 100 μL of chilled (4°C) extraction buffer containing 50 mm Hepes-KOH, pH 7.4, 5 mmMgCl2, 1 mm EDTA, 1 mmEGTA, 1 mm DTT, 2 mm benzamidine, 2 mm ε-amino-n-caproic acid, 0.5 mmPMSF, 0.1% (w/v) fatty acid-free BSA, 10% (v/v) glycerol, and 0.1% (w/v) Triton X-100 (Geigenberger and Stitt, 1993). The subsequent manipulations were at 4°C. Seeds were homogenized in 1.5-mL polypropylene test tubes using a motor-driven mortar. Following the addition of 400 μL of chilled buffer, gentle rocking for 10 min, and centrifugation at 16,000g for 10 min at 4°C, the supernatant was desalted on NAP-5 columns (Pharmacia) equilibrated with the extraction buffer. The proteins were eluted with 1 mL of extraction buffer, and 200-μL aliquots were frozen in liquid nitrogen prior to storage at −70°C.
The following enzymes were assayed as previously described: hexokinase (EC 2.7.1.1) and fructokinase (EC 2.7.1.4) according to the method ofRenz et al. (1993); phosphoglucoisomerase (EC 5.3.1.9), ATP-dependent 6-phosphofructokinase (EC 2.7.1.11), pyrophosphate-dependent 6-phosphofructokinase (EC 2.7.1.90), Fru-1,6-bisphosphate aldolase (EC 4.1.2.13), triose phosphate isomerase (EC 5.3.1.1), glyceral-3-P dehydrogenase (EC 1.2.1.12), phosphoglycerate kinase (EC 2.7.2.3), phosphoglycerate mutase (EC 5.4.2.1), enolase (EC 4.2.1.11), and pyruvate kinase (EC 2.7.1.40) according to the method of Burrell et al. (1994); and UDP-Glc-pyrophosphorylase (EC 2.7.7.9) according to the method of Zrenner et al. (1995). In the pyruvate kinase assay, the reaction buffer was modified to a pH of 7.25. Different amounts of extract were used, depending on the enzyme activity, to give a linear reaction for over 5 min. All assays were performed in a double-beam spectral photometer (model Uvicon 930, Kontron Instruments, Milano, Italy) equipped with a cell changer (model 900, Kontron).
RESULTS
Isolation of wri Mutants
For the isolation of low-oil-seed mutants, one has to take into consideration that tissues of different genetic constitution are involved in the photoassimilation and import of carbohydrates and their conversion into oil. In Arabidopsis, photoassimilates are produced primarily by maternal tissues, but the biosynthesis of seed oil is a function of embryonic tissues. Accordingly, with the possible exception of apoplastic carbohydrate-modifying enzymes such as invertases or carbohydrate transporters in the adjacent maternal tissues, the genome of the embryo encodes the enzymes required for the conversion of carbohydrates into triacylglycerols. Thus, screening of M2 seeds permits the identification of embryos that carry a homozygous recessive mutation specifically affecting the accumulation of oil.
Following selfing, the corresponding homozygous M2 plants should exclusively produce seeds with low-oil content. To avoid the redundant isolation of embryo-lethal mutants with defects in many different aspects of development (Müller, 1963; Meinke, 1991), we did not examine developing M2 seeds still attached to the M1 plants, but screened bulked M2 seeds and assumed that those with low-oil content were still able to germinate in the presence of an external carbon source. To identify M2 seeds with low-oil content, we used two selection procedures, one based on the assumption that seeds with reduced storage material accumulation are not completely filled and look wrinkled, and a second that assumed that seeds with reduced oil content should be slightly more dense due to the low specific gravity of oil. For this purpose we centrifuged bulked M2 seeds in a mixture of 1-bromohexan and 1,6-dibromohexan, adjusting the ratio of both solvents to an overall density such that approximately 5% of the seeds collected at the bottom of the tube, whereas 95% floated on top of the solvent. Afterward, the toxic solvent was removed by repeated suspension in light silicone oil. We used this nonaqueous system to avoid changes in seed density due to the uptake of water during the procedure.
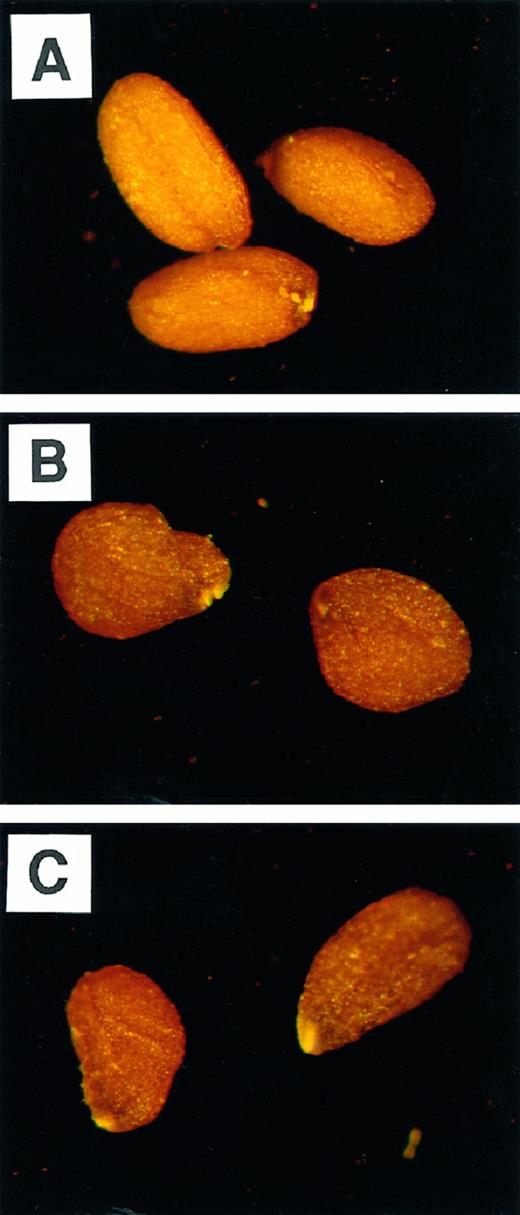
Appearance of wild-type (A),wri1–1 (B), and wri1–2 (C) mature seeds.
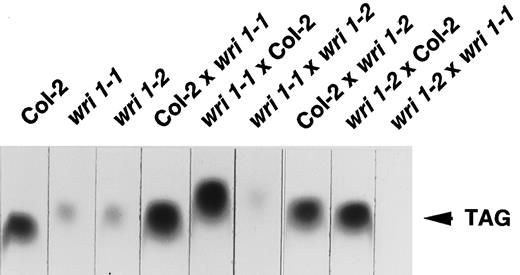
TLC of lipid extracts from mature seeds of wild type, homozygous mutant wri1–1 andwri1–2 seeds, and F1 seeds derived from reciprocal crosses between wild-type (Col-2) and mutant plants as indicated. Only the upper portion of the TLC plate is shown. Triacylglycerols (TAG) were visualized by exposure to iodine vapor.
Inheritance of the Low-Oil, Wrinkled Seed Trait and Mapping ofwri1–1
Unfortunately, a number of unfavorable environmental factors, including poor watering, aphid infections, or temperature increases in the greenhouse during seed setting, can cause Arabidopsis to produce wrinkled seeds. Furthermore, during the course of this study we discovered that Col-2 wild-type plants often produce a few slightly wrinkled seeds. Thus, to sort out false-positive putativewri mutants, we had to apply a rigorous genetic analysis. Contrary to many of the other putative wri mutant lines, the phenotype of wri1–1 and wri1–2 could be easily followed through multiple rounds of backcrosses. As shown in Figure 2, F1 seeds derived from backcrosses contained oil amounts comparable to those of the wild type. The segregation of wrinkled seeds in different F2 populations following reciprocal crosses with wild-type plants of ecotypes Col-2 and Ler is shown in Table I. Visual examination revealed the fraction of wrinkled seeds to be between 25% and 18%, close to the 3:1 ratio expected for the segregation of a single recessive nuclear mutation. However, presumably due to the low germination rate (20%–50%, depending on the storage time) of homozygous wri1 seeds, the recovery of homozygouswri1 F2 plants producing exclusively wrinkled seeds was less than 10%.
Segregation of F2 seeds according to seed shape determined by visual examination under a dissecting microscope
| Cross . | F2 Seed Shape . | |||
|---|---|---|---|---|
| Female . | . | Male . | Filled . | Wrinkled . |
| Col-2 | × | wri 1–1 | 505 (75) | 164 (25) |
| wri 1–1 | × | Ler | 2501 (82) | 542 (18) |
| wri 1–2 | × | Col-2 | 1761 (82) | 388 (18) |
| wri 1–2 | × | Ler | 721 (80) | 177 (20) |
| Cross . | F2 Seed Shape . | |||
|---|---|---|---|---|
| Female . | . | Male . | Filled . | Wrinkled . |
| Col-2 | × | wri 1–1 | 505 (75) | 164 (25) |
| wri 1–1 | × | Ler | 2501 (82) | 542 (18) |
| wri 1–2 | × | Col-2 | 1761 (82) | 388 (18) |
| wri 1–2 | × | Ler | 721 (80) | 177 (20) |
Values shown in parentheses are percentage of total.
Segregation of F2 seeds according to seed shape determined by visual examination under a dissecting microscope
| Cross . | F2 Seed Shape . | |||
|---|---|---|---|---|
| Female . | . | Male . | Filled . | Wrinkled . |
| Col-2 | × | wri 1–1 | 505 (75) | 164 (25) |
| wri 1–1 | × | Ler | 2501 (82) | 542 (18) |
| wri 1–2 | × | Col-2 | 1761 (82) | 388 (18) |
| wri 1–2 | × | Ler | 721 (80) | 177 (20) |
| Cross . | F2 Seed Shape . | |||
|---|---|---|---|---|
| Female . | . | Male . | Filled . | Wrinkled . |
| Col-2 | × | wri 1–1 | 505 (75) | 164 (25) |
| wri 1–1 | × | Ler | 2501 (82) | 542 (18) |
| wri 1–2 | × | Col-2 | 1761 (82) | 388 (18) |
| wri 1–2 | × | Ler | 721 (80) | 177 (20) |
Values shown in parentheses are percentage of total.
Crosses between homozygous wri1–1 and wri1–2plants produced only seeds with low oil contents (Fig. 2), indicating the lack of complementation and suggesting that the two lines harbor different mutant alleles of wri1. From a cross betweenwri1–1 and the Ler wild type, we collected 49 F2 plants homozygous for wri1–1 as confirmed by analysis of F3 seeds. These F2 plants provided the basis for a mapping population that was tested with 16 different PCR-based DNA markers mapping at locations spread over the complete genome. Of all of the markers tested, we observed tight linkage of wri1–1 to nga707 placed at 110.1 cM on chromosome number three according to the map posted by the Arabidopsis Genome Center at the University of Pennsylvania (http://cbil.humge.upenn.edu/atgc/images/chr3 map.gif; as of February, 1998) and less tight linkage to AthGAPab placed at 77.1 cM. Based on our data, the calculated map distance betweenwri1–1 and nga707 was 6.4 ± 2.6 cM and betweenwri1–1 and AthGAPab, 43.6 ± 9.7 cM.
The Primary Phenotype of wri1 Mutants
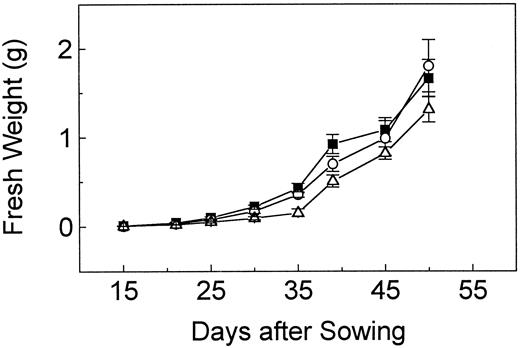
Growth of wild-type (▪), wri1–1(○), and wri1–2 (▵) mutant plants in soil. For each time point, the fresh weights of aerial parts from six plants were averaged and the ses are indicated.
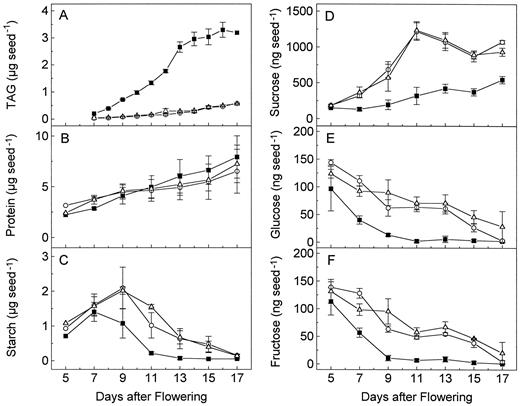
Time courses of triacylglycerol (TAG), protein, and carbohydrate accumulation in developing seeds of the wild type (▪), wri1–1 (○), and wri1–2 (▵) mutant. A, Triacylglycerols; B, total protein; C, starch; D, Suc; E, Glc; F, Fru. Each time point represents duplicate (A–C) or triplicate (D–F) measurements and ses are indicated.
Fatty acid composition of mature wild type and mutant wri 1–1 and wri 1–2 seeds
| Fatty Acid . | Composition . | ||
|---|---|---|---|
| Col-2 (wild type) . | wri 1–1 . | wri 1–2 . | |
| mol % | |||
| 16:0 | 11.3 ± 1.0 | 10.2 ± 1.7 | 11.3 ± 1.2 |
| 18:0 | 2.8 ± 0.1 | 2.5 ± 0.2 | 2.2 ± 0.2 |
| 18:1 | 23.4 ± 3.2 | 13.2 ± 4.8 | 11.9 ± 3.7 |
| 18:2 | 25.7 ± 0.8 | 18.7 ± 0.9 | 19.9 ± 0.0 |
| 18:3 | 10.2 ± 1.8 | 15.5 ± 2.7 | 17.2 ± 2.8 |
| 20:0 | 2.5 ± 0.3 | 4.8 ± 0.3 | 4.6 ± 1.0 |
| 20:1 | 21.0 ± 2.0 | 18.8 ± 4.0 | 16.5 ± 4.9 |
| 22:0 | 0.4 ± 0.0 | 1.9 ± 0.1 | 1.7 ± 0.3 |
| 22:1 | 2.7 ± 0.1 | 14.8 ± 0.6 | 15.0 ± 1.4 |
| Fatty Acid . | Composition . | ||
|---|---|---|---|
| Col-2 (wild type) . | wri 1–1 . | wri 1–2 . | |
| mol % | |||
| 16:0 | 11.3 ± 1.0 | 10.2 ± 1.7 | 11.3 ± 1.2 |
| 18:0 | 2.8 ± 0.1 | 2.5 ± 0.2 | 2.2 ± 0.2 |
| 18:1 | 23.4 ± 3.2 | 13.2 ± 4.8 | 11.9 ± 3.7 |
| 18:2 | 25.7 ± 0.8 | 18.7 ± 0.9 | 19.9 ± 0.0 |
| 18:3 | 10.2 ± 1.8 | 15.5 ± 2.7 | 17.2 ± 2.8 |
| 20:0 | 2.5 ± 0.3 | 4.8 ± 0.3 | 4.6 ± 1.0 |
| 20:1 | 21.0 ± 2.0 | 18.8 ± 4.0 | 16.5 ± 4.9 |
| 22:0 | 0.4 ± 0.0 | 1.9 ± 0.1 | 1.7 ± 0.3 |
| 22:1 | 2.7 ± 0.1 | 14.8 ± 0.6 | 15.0 ± 1.4 |
Values are ±se; n = 3.
Fatty acid composition of mature wild type and mutant wri 1–1 and wri 1–2 seeds
| Fatty Acid . | Composition . | ||
|---|---|---|---|
| Col-2 (wild type) . | wri 1–1 . | wri 1–2 . | |
| mol % | |||
| 16:0 | 11.3 ± 1.0 | 10.2 ± 1.7 | 11.3 ± 1.2 |
| 18:0 | 2.8 ± 0.1 | 2.5 ± 0.2 | 2.2 ± 0.2 |
| 18:1 | 23.4 ± 3.2 | 13.2 ± 4.8 | 11.9 ± 3.7 |
| 18:2 | 25.7 ± 0.8 | 18.7 ± 0.9 | 19.9 ± 0.0 |
| 18:3 | 10.2 ± 1.8 | 15.5 ± 2.7 | 17.2 ± 2.8 |
| 20:0 | 2.5 ± 0.3 | 4.8 ± 0.3 | 4.6 ± 1.0 |
| 20:1 | 21.0 ± 2.0 | 18.8 ± 4.0 | 16.5 ± 4.9 |
| 22:0 | 0.4 ± 0.0 | 1.9 ± 0.1 | 1.7 ± 0.3 |
| 22:1 | 2.7 ± 0.1 | 14.8 ± 0.6 | 15.0 ± 1.4 |
| Fatty Acid . | Composition . | ||
|---|---|---|---|
| Col-2 (wild type) . | wri 1–1 . | wri 1–2 . | |
| mol % | |||
| 16:0 | 11.3 ± 1.0 | 10.2 ± 1.7 | 11.3 ± 1.2 |
| 18:0 | 2.8 ± 0.1 | 2.5 ± 0.2 | 2.2 ± 0.2 |
| 18:1 | 23.4 ± 3.2 | 13.2 ± 4.8 | 11.9 ± 3.7 |
| 18:2 | 25.7 ± 0.8 | 18.7 ± 0.9 | 19.9 ± 0.0 |
| 18:3 | 10.2 ± 1.8 | 15.5 ± 2.7 | 17.2 ± 2.8 |
| 20:0 | 2.5 ± 0.3 | 4.8 ± 0.3 | 4.6 ± 1.0 |
| 20:1 | 21.0 ± 2.0 | 18.8 ± 4.0 | 16.5 ± 4.9 |
| 22:0 | 0.4 ± 0.0 | 1.9 ± 0.1 | 1.7 ± 0.3 |
| 22:1 | 2.7 ± 0.1 | 14.8 ± 0.6 | 15.0 ± 1.4 |
Values are ±se; n = 3.
As shown in Figure 4B, there were only subtle differences in the time courses of protein accumulation during seed development between the mutants and the wild type. It appears that young mutant seeds contain more protein, whereas mature mutant seeds contain slightly less than wild-type seeds. Because the amount of total protein was determined, we confirmed by PAGE of the extracts that the bulk of the protein in mature seeds is represented by the different 12S and 2S storage proteins (data not shown).
Soon after flowering, wild-type seeds of Arabidopsis accumulate starch that is degraded during maturation of the seeds (Fig. 4C). This starch is present in the plastids of embryo cells but also in seed coat cells, as was confirmed by microscopy following starch-specific iodine staining (data not shown). The maximal amount of starch was observed on d 7 after flowering. Higher amounts of starch intermittently accumulated in wri1 mutant seeds with a maximum on d 9, but the same low amounts of starch were observed in mature seeds of the wild type and the wri1 mutants. Furthermore, the amounts of soluble sugars, including Suc, Glc, and Fru, were elevated throughout development of wri1 mutant seeds (Fig. 4, D–F). At d 11 after flowering, the relative amounts of all three soluble sugars were more than 5-fold increased in the mutant seeds. Mature mutant seeds contained approximately twice as much Suc. Unlike triacylglycerols, Suc is dissolved in water that is partially lost during maturation of the seed. The resulting reduction in seed volume in combination with a low elasticity of the seed coat, may be the cause for the wrinkled appearance of the mutant seeds (Fig. 1). By a similar reasoning, the appearance of wrinkled pea seeds, which was originally studied by Mendel, has been explained (Wang and Hedley, 1991).
Incorporation of Different Precursors into Triacylglycerols
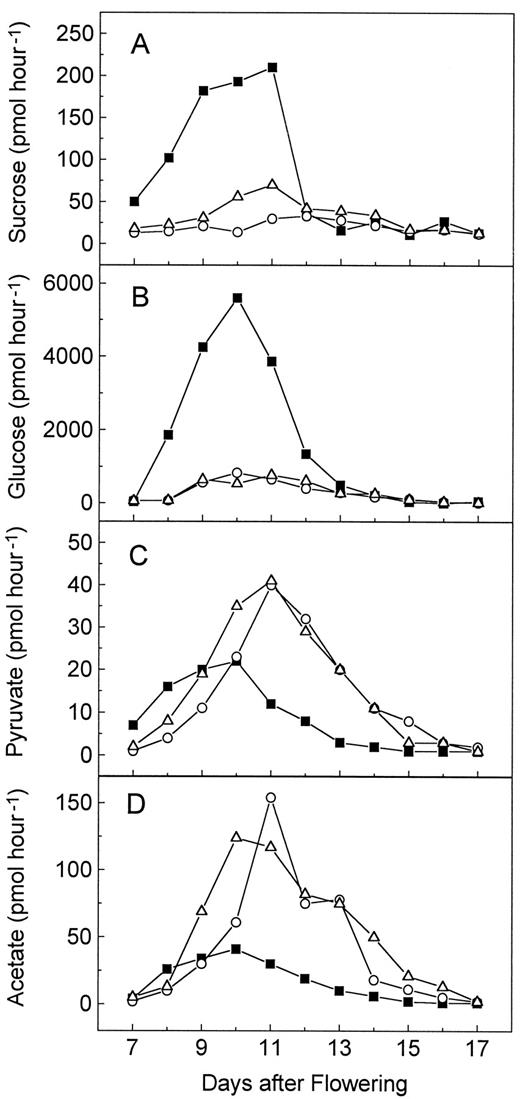
Incorporation of different precursors into triacylglycerol by developing embryos of the wild type (▪),wri1–1 (○), and wri1–2 mutant (▵). Tested compounds were Suc (A), Glc (B), pyruvate (C), and acetate (D). The rates are expressed as picomoles of precursor converted to triacylglycerol per 20 seeds per hour. Single representative experiments are shown.
Following the course of triacylglycerol accumulation during development (Fig. 4A), the incorporation of each of the compounds into triacylglycerols reached a maximum at d 11 after flowering. Thewri1 mutant seeds showed a drastically decreased incorporation of the carbohydrates Suc and Glc (Fig. 5, A and B) and a similarly drastic increase in the incorporation of the precursors of fatty acid biosynthesis, acetate and pyruvate (Fig. 5, C and D). In the case of the two carbohydrates, one could assume that the labeled sugar molecules were diluted in the mutant seeds due to increased soluble sugar pools (Fig. 4, D and E). However, the developmental profile of the differences in soluble sugar content between wild-type and mutant seeds did not match the profile for the incorporation of labeled carbohydrates, with a sharp maximum at around d 11 (Fig. 5, A and B). Therefore, the results are in agreement with a block in the conversion of carbohydrates into precursors of fatty acid biosynthesis such as pyruvate or acetate.
The increased incorporation of pyruvate and acetate into triacylglycerols by the mutant seeds suggested that fatty acid biosynthesis and triacylglycerol assembly are not affected in the mutant. Furthermore, we assume that the pools of precursors for fatty acid biosynthesis may be decreased in the mutant seeds as a possible cause for the increased incorporation of these two precursors. However, the determination of pyruvate or acetyl-CoA pools in the limited amount of tissue provided by developing seeds of Arabidopsis is currently beyond our technical abilities.
Activity of Different Carbohydrate-Metabolizing Enzymes
The accumulation of carbohydrates in developing seeds of the twowri1 mutants (Fig. 4) and the results of the incorporation experiments (Fig. 5) suggested that the primary metabolic defect is in the conversion of carbohydrates into precursors of fatty acid biosynthesis. To identify the biochemical lesion in the wri1mutants, we determined the activity of different glycolytic enzymes in developing seeds collected during the peak of metabolic activity as indicated by the precursor incorporation rates (9–11 d after flowering). In addition, we measured the activity of UDP-Glc pyrophosphorylase, which catalyzes the conversion of UDP-Glc to Glc-1-P. The activities were normalized on the basis of the total protein content of the extracts, which did not differ between the mutants and the wild type (Fig. 4B). The results of these experiments are summarized in Table III. It is immediately apparent that several glycolytic enzymes are affected in the wri1 mutants. In particular, the activity of hexokinase was reduced to approximately 17% and that of pyrophosphate-dependent phosphofructokinase to approximately 38% in the wri1mutants.
Activities of different enzymes involved in Glc metabolism in the wild type and in wri1 mutants
| Enzyme . | Enzyme Activity . | ||
|---|---|---|---|
| Col-2 (wild type) . | wri 1–1 . | wri 1–2 . | |
| nmol min−1 mg−1 protein | |||
| Hexokinase | 2.4 ± 0.5 | 0.4 ± 0.1 | 0.3 ± 0.1 |
| Fructokinase | 3.7 ± 0.8 | 2.3 ± 0.7 | 2.4 ± 0.2 |
| Phosphoglucose isomerase | 35.8 ± 0.9 | 31.5 ± 0.4 | 35.1 ± 3.0 |
| ATP-dependent phosphofructokinase | 3.4 ± 0.5 | 3.2 ± 0.5 | 2.6 ± 0.3 |
| PPi-dependent phosphofructokinase | 1.3 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 |
| Aldolase | 3.5 ± 0.5 | 2.1 ± 0.3 | 1.9 ± 0.1 |
| Triose phosphate isomerase | 1571 ± 86.7 | 1382 ± 120.5 | 1494 ± 88.7 |
| GAPDH | 81.8 ± 4.2 | 79.9 ± 5.2 | 73.3 ± 3.0 |
| Phosphoglycerate kinase | 202.3 ± 14.9 | 164.7 ± 6.9 | 176.5 ± 8.2 |
| Phosphoglycerate mutase | 79.1 ± 13.4 | 52.5 ± 14.4 | 46.6 ± 14.7 |
| Enolase | 15.4 ± 1.7 | 9.3 ± 1.2 | 9.1 ± 0.4 |
| Pyruvate kinase | 13.0 ± 2.6 | 8.6 ± 1.7 | 7.5 ± 1.2 |
| UDP-Glc pyrophosphorylase | 164.7 ± 5.8 | 174.2 ± 5.9 | 182.6 ± 8.6 |
| Enzyme . | Enzyme Activity . | ||
|---|---|---|---|
| Col-2 (wild type) . | wri 1–1 . | wri 1–2 . | |
| nmol min−1 mg−1 protein | |||
| Hexokinase | 2.4 ± 0.5 | 0.4 ± 0.1 | 0.3 ± 0.1 |
| Fructokinase | 3.7 ± 0.8 | 2.3 ± 0.7 | 2.4 ± 0.2 |
| Phosphoglucose isomerase | 35.8 ± 0.9 | 31.5 ± 0.4 | 35.1 ± 3.0 |
| ATP-dependent phosphofructokinase | 3.4 ± 0.5 | 3.2 ± 0.5 | 2.6 ± 0.3 |
| PPi-dependent phosphofructokinase | 1.3 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 |
| Aldolase | 3.5 ± 0.5 | 2.1 ± 0.3 | 1.9 ± 0.1 |
| Triose phosphate isomerase | 1571 ± 86.7 | 1382 ± 120.5 | 1494 ± 88.7 |
| GAPDH | 81.8 ± 4.2 | 79.9 ± 5.2 | 73.3 ± 3.0 |
| Phosphoglycerate kinase | 202.3 ± 14.9 | 164.7 ± 6.9 | 176.5 ± 8.2 |
| Phosphoglycerate mutase | 79.1 ± 13.4 | 52.5 ± 14.4 | 46.6 ± 14.7 |
| Enolase | 15.4 ± 1.7 | 9.3 ± 1.2 | 9.1 ± 0.4 |
| Pyruvate kinase | 13.0 ± 2.6 | 8.6 ± 1.7 | 7.5 ± 1.2 |
| UDP-Glc pyrophosphorylase | 164.7 ± 5.8 | 174.2 ± 5.9 | 182.6 ± 8.6 |
Values are ± se; n = 3.
Activities of different enzymes involved in Glc metabolism in the wild type and in wri1 mutants
| Enzyme . | Enzyme Activity . | ||
|---|---|---|---|
| Col-2 (wild type) . | wri 1–1 . | wri 1–2 . | |
| nmol min−1 mg−1 protein | |||
| Hexokinase | 2.4 ± 0.5 | 0.4 ± 0.1 | 0.3 ± 0.1 |
| Fructokinase | 3.7 ± 0.8 | 2.3 ± 0.7 | 2.4 ± 0.2 |
| Phosphoglucose isomerase | 35.8 ± 0.9 | 31.5 ± 0.4 | 35.1 ± 3.0 |
| ATP-dependent phosphofructokinase | 3.4 ± 0.5 | 3.2 ± 0.5 | 2.6 ± 0.3 |
| PPi-dependent phosphofructokinase | 1.3 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 |
| Aldolase | 3.5 ± 0.5 | 2.1 ± 0.3 | 1.9 ± 0.1 |
| Triose phosphate isomerase | 1571 ± 86.7 | 1382 ± 120.5 | 1494 ± 88.7 |
| GAPDH | 81.8 ± 4.2 | 79.9 ± 5.2 | 73.3 ± 3.0 |
| Phosphoglycerate kinase | 202.3 ± 14.9 | 164.7 ± 6.9 | 176.5 ± 8.2 |
| Phosphoglycerate mutase | 79.1 ± 13.4 | 52.5 ± 14.4 | 46.6 ± 14.7 |
| Enolase | 15.4 ± 1.7 | 9.3 ± 1.2 | 9.1 ± 0.4 |
| Pyruvate kinase | 13.0 ± 2.6 | 8.6 ± 1.7 | 7.5 ± 1.2 |
| UDP-Glc pyrophosphorylase | 164.7 ± 5.8 | 174.2 ± 5.9 | 182.6 ± 8.6 |
| Enzyme . | Enzyme Activity . | ||
|---|---|---|---|
| Col-2 (wild type) . | wri 1–1 . | wri 1–2 . | |
| nmol min−1 mg−1 protein | |||
| Hexokinase | 2.4 ± 0.5 | 0.4 ± 0.1 | 0.3 ± 0.1 |
| Fructokinase | 3.7 ± 0.8 | 2.3 ± 0.7 | 2.4 ± 0.2 |
| Phosphoglucose isomerase | 35.8 ± 0.9 | 31.5 ± 0.4 | 35.1 ± 3.0 |
| ATP-dependent phosphofructokinase | 3.4 ± 0.5 | 3.2 ± 0.5 | 2.6 ± 0.3 |
| PPi-dependent phosphofructokinase | 1.3 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 |
| Aldolase | 3.5 ± 0.5 | 2.1 ± 0.3 | 1.9 ± 0.1 |
| Triose phosphate isomerase | 1571 ± 86.7 | 1382 ± 120.5 | 1494 ± 88.7 |
| GAPDH | 81.8 ± 4.2 | 79.9 ± 5.2 | 73.3 ± 3.0 |
| Phosphoglycerate kinase | 202.3 ± 14.9 | 164.7 ± 6.9 | 176.5 ± 8.2 |
| Phosphoglycerate mutase | 79.1 ± 13.4 | 52.5 ± 14.4 | 46.6 ± 14.7 |
| Enolase | 15.4 ± 1.7 | 9.3 ± 1.2 | 9.1 ± 0.4 |
| Pyruvate kinase | 13.0 ± 2.6 | 8.6 ± 1.7 | 7.5 ± 1.2 |
| UDP-Glc pyrophosphorylase | 164.7 ± 5.8 | 174.2 ± 5.9 | 182.6 ± 8.6 |
Values are ± se; n = 3.
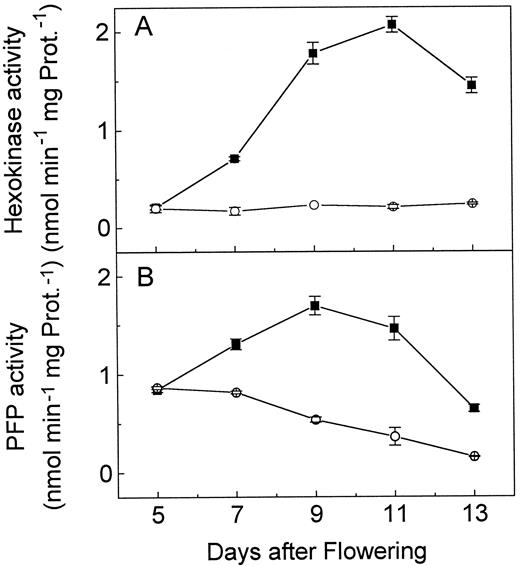
Activity of hexokinase (A) and pyrophosphate-dependent phosphofructokinase (PFP) (B) in developing embryos of the wild type (▪) and the wri1–1 (○) mutant. Measurements were done in triplicate and ses are indicated. Prot., Protein.
Similarly, the activity of pyrophosphate-dependent phosphofructokinase increased in the wild type during seed development but decreased in thewri1 mutant. These results suggest that basic activities of both enzymes are maintained, but that the seed-specific developmental regulation of their activity is abolished in the wri1mutants. In an attempt to provide corroborating evidence for this hypothesis, we examined the activity of these two enzymes in leaves of the wild type and the wri1 mutants. The activity of hexokinase in leaves of the wild type and the mutants was identical within the statistical limitations (0.9 nmol min−1 mg−1 protein), and the activity of pyrophosphate-dependent phosphofructokinase was below the detection limit in both.
DISCUSSION
The wri1 Phenotype Is Seed Specific
Employing a simple screening procedure based on density centrifugation and visual examination of M2seeds, wri1, a novel mutant locus of Arabidopsis that causes wrinkledness of the seed and a strong reduction in the accumulation of seed oil, has been identified. No differences between the wild-type and mutant plants were observed, in particular with respect to the number of siliques or seeds, a factor that could affect seed filling. In addition, genetic analysis revealed that the genotype of the embryo but not that of the maternal tissues determines the observed seed phenotype. Thus, a deficiency in maternal tissues causing a limitation in the supply of photosynthate to the embryo can be ruled out as the cause for the low-oil content of homozygous wri1 seeds.
Carbohydrate Metabolism Is Affected in the wri1Mutant
Several lines of evidence suggest that the wri1 mutant is not directly affected in fatty acid or triacylglycerol biosynthesis, but is affected in the conversion of Glc into fatty acid precursors. First, the amounts of hexoses and Suc are increased in developingwri1 mutant seeds. Second, labeled Suc or Glc supplied to developing seeds are incorporated at reduced rates into triacylglycerols by the mutant. However, the incorporation of labeled pyruvate and acetate is increased in developing wri1 mutant seeds. Third, the total activity of several glycolytic enzymes is decreased in developing wri1 seeds. In principle, glycolytic pathways in the plastid or the cytosol could contribute precursors to fatty acid biosynthesis in developing oilseeds. The reduction in the activity of several glycolytic enzymes raises the question of whether one particular set of glycolytic enzymes in one of the two subcellular compartments is preferentially affected in the wri1 mutant.
Although no definitive answer can be provided at this time, we could demonstrate that the activity of pyrophosphate-dependent phosphofructokinase, an enzyme exclusively present in the cytosol (Plaxton, 1996), is strongly reduced in the wri1 mutant. This would imply a reduction in carbon flow through cytosolic glycolysis if one assumes that pyruvate-dependent phosphofructokinase catalyzes a net glycolytic reaction in developing seeds of Arabidopsis, as has been postulated for developing potato tubers and tobacco sink leaves (Hajirezaei et al., 1994; Paul et al., 1995). The observation that pyruvate, the end product of glycolysis, can be taken up and be incorporated at high rates into fatty acids by isolated plastids of developing seeds of canola or castor (Smith et al., 1992;Kang and Rawsthorne, 1994) suggests that carbon flow from carbohydrates to triacylglycerols may involve extraplastidic glycolysis. Therefore, a deficiency in cytoplastic glycolysis in the wri1 mutant would be in agreement with reduced amounts of seed oil.
The Role of Seed Starch
Starch intermittently accumulates to higher amounts in developingwri1 mutant seeds compared with the wild type (Fig. 4C). Because the same low amounts of starch are present in mature mutant and wild-type seeds, one has to conclude that wri1 plastids are capable of metabolizing starch. However, given the low amount of oil in mature wri1 embryos, the carbon flow through starch can apparently only supply a fraction of the precursors required for triacylglycerol biosynthesis in the wri1 mutant. Whether this is due to a deficiency in glycolysis in the wri1 mutant or is an indication of a generally low rate of conversion of starch into precursors of triacylglycerol biosynthesis in Arabidopsis can currently not be distinguished. However, in canola the intermittent starch amount is thought to be insufficient to support triacylglycerol biosynthesis (King et al., 1997).
Corroborating evidence has been provided by the expression of bacterial ADP-Glc pyrophosphorylase in developing seeds of canola that led to an increased amount of starch and a decrease in oil content (Boddupalli et al., 1995), suggesting that the flow of carbon from photoassimilates to triacylglycerols does not go through starch, at least during the later stages of development. Apparently, the pathways for the biosynthesis of starch and triacylglycerols compete and starch biosynthesis is normally repressed at the middle stage of development in canola seed. In thewri1 mutant seeds, the amount of starch is at least transiently increased, presumably because excess carbon is funneled into starch biosynthesis instead of triacylglycerols.
The reduction in oil content in spite of the accumulation of starch in developing wri1 mutant seeds would be consistent with a reduction in glycolytic flux in the cytosol. Most likely, Suc imported into the embryo is converted to UDP-Glc and Fru by cytosolic Suc synthase, and UDP-Glc is further metabolized to Glc-1-P by the action of cytosolic UDP-Glc pyrophosphorylase and is subsequently converted to Glc-6-P by phosphoglucomutase. Instead of entering glycolysis in the cytosol, Glc-6-P is imported into the plastid and preferentially incorporated into starch, as has been observed for isolated plastids of canola (Kang and Rawsthorne, 1994), or is metabolized via the oxidative pentose phosphate pathway (Kang and Rawsthorne, 1996). Accordingly, the activity of UDP-Glc pyrophosphorylase was not decreased, but slightly increased in wri1 mutant seeds (Table III). Furthermore, this pathway does not involve hexokinase, the enzyme most severely decreased in activity in the wri1 mutant.
The Primary Defect in the wri1 Mutant
The reduced activity of several glycolytic enzymes raises the question for the possible primary defect in the wri1mutant. Either a structural gene for one of the glycolytic enzymes, e.g. hexokinase, is mutated, leading to an altered glycolytic flux with secondary consequences for the activity of the other glycolytic enzymes, or a developmental regulator of carbohydrate metabolism specific to seeds is affected.
Recently, it has been shown that hexokinase has dual functions in Arabidopsis (Jang et al., 1997): It catalyzes the phosphorylation of hexoses and also acts as a sugar sensor, repressing the expression of different genes encoding photosynthetic proteins in the presence of high sugar concentrations. Although sugar sensing and sugar-mediated regulation of gene expression are intensely studied phenomena in plants (Koch, 1996; Smeekens and Rook, 1997), it is not known whether hexokinase of Arabidopsis as the possible sugar sensor may affect (stimulate) the expression of genes encoding glycolytic enzymes following the import of sugars into developing embryos. If this were the case, a defect in the structural gene for a seed-specific hexokinase in the wri1 mutant would explain the observed loss in hexokinase activity and the changes in overall glycolytic activity in developing wri1 seeds. However, ifWRI1 indeed represents a structural gene for hexokinase, it must be different from ATHXK1 and ATHXK2described as sugar-sensor genes (Jang et al., 1997), because all three genes map to different chromosomes of Arabidopsis and, therefore,wri1 cannot be a mutant allele of one of the two known hexokinase genes.
The alternative suggestion, that WRI1 encodes not hexokinase but a gene product primarily involved in the developmental regulation of glycolytic activity in seeds during storage compound accumulation, would be consistent with the altered developmental activity profile of hexokinase and pyrophosphate-dependent phosphofructokinase in thewri1 mutant (Fig. 6). In this context it should be pointed out that the developmental activity profiles for hexokinase (Fig. 6) and for the incorporation of Glc (Fig. 5) differ during the later stages of development in the wild type. Although Glc incorporation declines sharply after d 10, hexokinase activity remains fairly high. This observation suggests that, at least during later stages of seed development, factors other than hexokinase may be limiting for the conversion of carbohydrates into triacylglycerols. Because the morphogenesis of the embryo and the seedling of the wri1mutant is not affected, it seems unlikely that WRI1 encodes a general regulator of late embryo development such as LEC1or FUS3 (Parcy et al., 1997). The clearly definedwri1 phenotype restricted to metabolism would suggest that it encodes a regulator specifically governing the carbon flow from carbohydrates to fatty acids during seed filling.
Consequences for Fatty Acid Biosynthesis and Modification
Based only on the low oil content of the wri1 seeds and the change in fatty acid composition of the remaining oil (Table II), one could conclude that WRI1 is more directly involved in triacylglycerol biosynthesis than has been suggested above. However, isolated developing wri1 seeds are clearly capable of incorporating pyruvate and acetate at high rates into triacylglycerols, and the change in fatty acid composition of the remaining triacylglycerols may simply be a consequence of reduced carbon flow into fatty acid biosynthesis. Apparently, the relative amounts of the end product of fatty acid desaturation, 18:3, and of fatty acid elongation, 22:1, are strongly increased, whereas the relative amount of the precursor of both reaction sequences, 18:1, is most strongly decreased. This observation also implies that no feedback regulation occurs in developing wri1 seeds to adjust the degree of unsaturation and elongation of the fatty acid substituents of triacylglycerols to the decrease of carbon flow into this pathway, and that, presumably, different regulators control the activity of enzymes involved in carbohydrate and lipid metabolism or the expression of the respective genes during seed development.
CONCLUSIONS
In summary, a novel mutant of Arabidopsis has been isolated with a deficiency in seed carbohydrate metabolism that leads to a reduction in seed-oil accumulation. This mutant identifies a genetic locus designated wri1, which encodes either a regulatory protein governing carbohydrate metabolism during seed development or a novel hexokinase that may act as sugar sensor in developing seeds, controlling the activity or expression of other glycolytic enzymes. Given the high rate of photosynthate import during seed filling, regulation of seed-specific carbohydrate metabolism by sugar sensing seems to be a reasonable mechanism with which to control the flux of carbon into starch or oil in developing seeds. To gain further insight into the function of the WRI1 gene product and into the regulation of carbon flow in developing oilseeds, genes encoding seed-specific glycolytic enzymes have to be identified and their expression compared in the wild type and the wri1 mutant. Ultimately, the WRI1 locus has to be isolated and characterized to understand the molecular basis for the complex phenotype of the wri1 mutant and to exploit the possibility of using the respective gene for the modification of carbon partitioning in developing oilseeds by genetic engineering. The availability of the wri1 mutant provides the basis for a positional cloning approach.
ACKNOWLEDGMENTS
We would like to thank Chris Somerville for his contributions during the early phase of this work, which was initiated in his laboratory at the Michigan State University-Department of Energy Plant Research Laboratory.
LITERATURE CITED
Boddupalli SS, Stark DM, Barry GF, Kishore GM (1995) Effect of overexpressing ADPGlc pyrophosphorylase on the oil biosynthesis in canola. Biochemistry Molecular Biology Plant Fatty Acids Glycerolipids Symposium, June 1–4, 1995, South Lake Tahoe, CA. InJB Ohlrogge, JG Jaworski, eds, National Plant Lipid Cooperative, Abstract P-102
Miquel M, Browse J (1994) Lipid biosynthesis in developing seeds.In H Kigel, G Galili, eds, Seed Development and Germination. Marcel Dekker, New York, pp 169–193
Author notes
This work was financially supported in part by the Deutsche Forschungsgemeinschaft (grant nos. Be 1591/2-1 and Be 1591/2-2) and by the Bundesminister für Bildung und Forschung (grant no. 0311024).
Corresponding author; e-mail benning@pilot.msu.edu; fax 1–517–353–9334.