Abstract
Purpose: To test the ability of a reverse transcriptase-PCR (RT-PCR) assay, based on gene expression profiles, to accurately determine the risk of recurrence in patients with node-negative breast cancer who did not receive systemic therapy using formalin-fixed, paraffin-embedded tissue. A secondary objective was to determine whether the quantitative RT-PCR data correlated with immunohistochemistry assay data regarding estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 status.
Patients and Methods: We obtained archival paraffin-embedded tissue from patients with invasive breast cancer but no axillary lymph node involvement who had received no adjuvant systemic therapy and been followed for at least 5 years. RNA was extracted from three 10-μm-thick sections. The expression of 16 cancer-related genes and 5 reference genes was quantified using RT-PCR. A gene expression algorithm was used to calculate a recurrence score for each patient. We then assessed the ability of the test to accurately predict distant recurrence-free survival in this population.
Results: We identified 149 eligible patients. Median age at diagnosis was 59 years; mean tumor diameter was 2 cm; and 69% of tumors were estrogen receptor positive. Median follow-up was 18 years. The 5-year disease-free survival rate for the group was 80%. The 21 gene-based recurrence score was not predictive of distant disease recurrence. However, a high concordance between RT-PCR and immunohistochemical assays for estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 status was noted.
Conclusions: RT-PCR can be done on paraffin-embedded tissue to validate the large numbers of genes associated with breast cancer recurrence. However, further work needs to be done to develop an assay to identify the likelihood of recurrent disease in patients with node-negative breast cancer who do not receive adjuvant tamoxifen or chemotherapy.
A great need exists for better molecular characterization of tumor tissue. This would have several benefits. It would help in the oncology decision-making process, facilitate treatment selection, and ultimately improve patient outcomes. There is a particular need for such information in women with early-stage breast cancer, as evidenced by the fact that there is currently great variety in the treatment prescribed for these women. The serious consequence of this is that some women are likely being undertreated whereas others are being overtreated. The few assays that currently exist to characterize the molecular properties of breast tumors include those that determine estrogen receptor (ER) status, which are routinely done as a standard assessment for hormonal treatment of breast cancer (1, 2). In addition, assays to determine the expression of the human epidermal growth factor receptor 2 (HER-2) were recently developed as prognostic tools and to aid in the selection of certain therapy options, such as treatment with the humanized monoclonal antibody trastuzumab (2–5). Clearly, additional molecular markers are needed to more accurately characterize breast tumors, determine prognosis, and predict the response to various treatments (6).
In the past several years, great advances have been made in the development of sensitive and quantitative gene expression assays using reverse transcriptase-PCR (RT-PCR) and microarray technologies (7, 8). Initial studies have shown that it is possible for these assays to generate a molecular portrait of gene expression in tumor tissue (9, 10). However, fresh frozen tissue, which is not routinely available in standard clinical practice, has been used for most of these assays. Methods that make use of formalin-fixed, paraffin-embedded tissue (FPET) are therefore needed. To bridge this gap, Genomic Health, Inc. (Redwood City, CA) has developed a new RT-PCR assay to assess the expression of ER, progesterone receptor (PR), HER-2, and a variety of other molecular determinants in FPET from breast tumors (11). In this assay, RNA can be isolated from FPET specimens as old as 20 years, and reliable and accurate measurements of the expression of genes of clinical interest are provided. This novel RT-PCR assay was recently used to study gene expression in FPET from patients with early breast cancer in three clinical testing studies (training sets; refs. 12–14). The first two studies were conducted at Providence St. Joseph's Medical Center and Rush-Presbyterian Medical Center and were single-site observational studies that explored the expression of 192 genes in the primary tumor blocks of FPET obtained at the time of the initial diagnosis. The third study was done in collaboration with the National Surgical Adjuvant Breast and Bowel Project (NSABP) and examined FPET from patients enrolled in the tamoxifen-alone arm of the NSABP Study B-20 (15). Univariate Cox survival analysis showed that 41 genes were associated with relapse-free survival in a total of 233 tamoxifen-treated patients. Analysis of the results across all three studies showed that 16 genes were associated with an increased likelihood of breast cancer recurrence or death. Multigene models were then designed on the basis of these univariate results, and the models were analyzed across the three studies. This led to the identification of a single multigene assay consisting of 16 cancer-related genes and 5 reference genes (16). To evaluate the prognostic value of the assay, we tested it in FPET from patients with node-negative breast cancer who did not receive any adjuvant systemic therapy. A secondary objective was to determine whether the quantitative RT-PCR data correlated with immunohistochemistry assay data regarding ER, PR, and HER-2 status.
Patients and Methods
Patient selection. We obtained primary tumor specimens from 149 patients with stage I or IIA breast cancer according to the American Joint Committee on Cancer Classification System (17). All patients underwent definitive surgery at The University of Texas M.D. Anderson Cancer Center between 1978 and 1995. Selection criteria included no evidence of axillary lymph node involvement; no adjuvant systemic therapy; follow up for at least 5 years; and availability of tissue for examination. Patients were excluded if their specimens showed less than 5% invasive tumor, if insufficient RNA was extracted from the specimens, or if the RT-PCR signal for genes was outside the specifications for the assay. The tumors could be ER positive or negative. Pathology slides from the primary tumors from all 149 patients were reviewed by two different pathologists. The histologic grade of the primary tumors was determined according to the modified Black's nuclear grading system and the Bloom-Richardson criteria (grade 1, well differentiated; grade 2, moderately differentiated; and grade 3, poorly differentiated; ref. 18). Standard assays of ER, PR, and HER-2 were done using previously described immunohistochemical methods (19, 20). This study was approved by the Institutional Review Board at M.D. Anderson.
The fact that the patients identified had not received any adjuvant tamoxifen or chemotherapy makes them a highly selected group. Adjuvant chemotherapy for node-negative breast cancer did not become accepted until the early 1990s and at that time the studies were focused on ER-negative tumors or ER-positive tumors larger than 3 cm. In our cohort, most patients underwent surgery before 1990. Of the six cases with a surgery date after 1990, five of the six cases were ER positive by immunohistochemistry and less than 3 cm. The other ER-negative case was a woman who was 76 years of age.
Gene expression analysis. The assay, end points, and analysis plan were specified before initiation of the study. Three 10-μm-thick sections from each primary tumor were sent to Genomic Health for analysis but information on clinical outcome was withheld. RNA was extracted from three sections from each patient as previously described (21). Gene expression levels of 16 cancer-related genes (BAG1, Bcl2, CCNB1, CD68, SCUBE2, CTSL2, EstR1, GRB7, GSTM1, HER2, Ki-67, MYBL2, PR, STK15, STMY3, and SURV) and 5 reference genes (β-actin, GAPDH, GUS, RPLPO, and TFRC) were quantitatively analyzed by using RT-PCR. The reference genes are known to be relatively invariant in breast cancer as well as under various sample and process conditions, making them useful for normalizing gene expression values for variation in RNA quantity or quality. RT-PCR was done in triplicate with RNA input at 2 ng per reaction, yielding cycle threshold (CT) measurements for each study gene. The CT measurements were then normalized relative to the five reference genes. Reference gene-normalized expression levels typically range from 0 to 15, with an increase of one unit generally reflecting a 2-fold increase in RNA quantity.
After completion of the assays, gene expression and clinical data were exchanged electronically through a secure data transfer website. After successful transfer of locked data, the codes were shared to link the laboratory and clinical data for independent statistical analyses by Genomic Health and M.D. Anderson statisticians.
Statistical analysis. A recurrence score (RS) on a scale from 0 to 100 was derived from an algorithm based on the reference-normalized expression measurements, as previously described (16). The proliferation group had the largest coefficient in the algorithm, followed by the ER group and the HER-2 group, respectively. The cutoff points between the low-, intermediate-, and high-risk recurrence groups were predefined as follows: low risk of recurrence, RS < 18; intermediate risk of recurrence, RS ≥ 18 < 31; and high risk of recurrence, RS ≥ 31. The cutoff points of 18 and 31 were selected based on an analysis of primarily the tamoxifen-treated patients in NSABP Study B-20, with the desire for the distant recurrence rate at 10 years for the low-risk group to be <10%.
The primary objective was to determine whether there was a significant relationship between the risk of distant recurrence-free survival and the RS as measured by the multigene RT-PCR assay using the prespecified genes and RS algorithm. Distant recurrence-free survival was defined as the time (in years) from surgery to first distant recurrence. In this assessment, patients were divided into three recurrence risk groups (low, intermediate, and high) using the prespecified RS algorithm and cutoff points. We tested differences in distant recurrence-free survival vis-à-vis the RS groups using the Fleming and Harrington log-rank test and the Cox proportional hazards model with the covariates age and tumor size based on goodness-of-fit tests (22).
Concordance between RT-PCR and immunohistochemistry results in terms of ER, PR, and HER-2 gene expression was evaluated using Cohen's κ statistics (23). Classifications of ER, PR, and HER-2 status as shown by gene expression measurement were based on pre-established cutoff points for reference-normalized gene expression measurements for ER, PR, and HER-2, respectively. Specifically, cutoff points for ER, PR, and HER-2 gene expression levels, as established on the basis of analyses of clinical results from prior studies involving populations of patients consisting of those both positive and negative for the ER, PR, and HER-2 genes, were 7.0, 6.0, and 11.5 (relative to the reference genes; log 2), respectively. We also analyzed the gene clustering patterns of the 21 genes.
Results
A total of 4,268 patients were diagnosed with breast cancer at M.D. Anderson between 1978 and 1995. Of these, 220 patients met the initial selection criteria for this study. Sufficient tissue was not available in 42 (19%) patients. The amount of RNA extracted from 4 (2%) specimens was not sufficient, and the RT-PCR profile was not within the specifications in 3 (1%) patients. Further, the RT-PCR signal for reference genes was low in 22 (10%) patients. This left us with a total of 149 (68%) evaluable patients for this study. Table 1 summarizes the characteristics of these 149 patients by age, menopausal status, race, tumor size, nuclear grade, and HER-2, ER, and PR status. Forty-five (30%) tumors had a high nuclear grade (poorly differentiated). Sixty-nine percent of tumors were ER positive, and 17% showed evidence of HER-2 overexpression. The median follow up was 18 years.
Patient characteristics (n = 149)
| Characteristic . | Value . | |
|---|---|---|
| Age | ||
| Mean | 58.0 | |
| 25%, median, 75% | 51.0, 59.0, 67.0 | |
| SD | 11.5 | |
| Menopausal status | ||
| Premenopausal | 122 (81.9%) | |
| Postmenopausal | 27 (18.1%) | |
| Race | ||
| Caucasian | 126 (84.6%) | |
| Hispanic | 8 (5.4%) | |
| Asian | 5 (3.4%) | |
| Black | 10 (6.7%) | |
| Histologic tumor diameter (cm) | ||
| Mean | 2.3 | |
| 25%, median, 75% | 1.5, 2.0, 2.8 | |
| SD | 1.1 | |
| Nuclear grade | ||
| 1 (well differentiated) | 18 (12.1%) | |
| 2 (moderately well differentiated) | 86 (57.7%) | |
| 3 (poorly differentiated) | 45 (30.2%) | |
| HER-2 | ||
| Negative (score, <3+) | 124 (83.2%) | |
| Positive (score, 3+) | 25 (16.8%) | |
| ER | ||
| Negative | 46 (30.9%) | |
| Positive | 103 (69.1%) | |
| PR | ||
| Negative | 99 (66.4%) | |
| Positive | 50 (33.6%) | |
| Characteristic . | Value . | |
|---|---|---|
| Age | ||
| Mean | 58.0 | |
| 25%, median, 75% | 51.0, 59.0, 67.0 | |
| SD | 11.5 | |
| Menopausal status | ||
| Premenopausal | 122 (81.9%) | |
| Postmenopausal | 27 (18.1%) | |
| Race | ||
| Caucasian | 126 (84.6%) | |
| Hispanic | 8 (5.4%) | |
| Asian | 5 (3.4%) | |
| Black | 10 (6.7%) | |
| Histologic tumor diameter (cm) | ||
| Mean | 2.3 | |
| 25%, median, 75% | 1.5, 2.0, 2.8 | |
| SD | 1.1 | |
| Nuclear grade | ||
| 1 (well differentiated) | 18 (12.1%) | |
| 2 (moderately well differentiated) | 86 (57.7%) | |
| 3 (poorly differentiated) | 45 (30.2%) | |
| HER-2 | ||
| Negative (score, <3+) | 124 (83.2%) | |
| Positive (score, 3+) | 25 (16.8%) | |
| ER | ||
| Negative | 46 (30.9%) | |
| Positive | 103 (69.1%) | |
| PR | ||
| Negative | 99 (66.4%) | |
| Positive | 50 (33.6%) | |
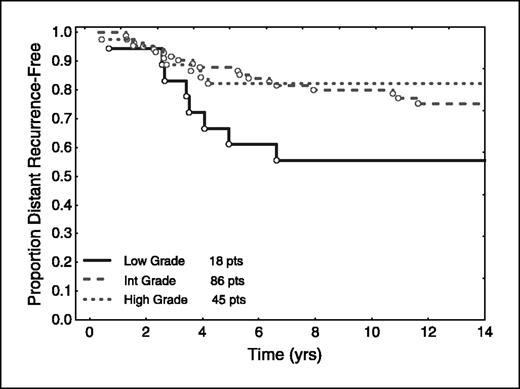
In this group of patients not treated with adjuvant hormonal therapy, ER, PR, and HER-2 had no prognostic value. Interestingly, nuclear grade was significantly correlated with prognosis (P = 0.02), but not in the expected direction (Fig. 1). Patients with well-differentiated tumors (low nuclear grade) had a worse survival compared with patients whose tumors were either moderately well differentiated or poorly differentiated. The Proliferation Group Score (based on the expression by RT-PCR of the five proliferation genes) correlated with nuclear grade (P < 0.001), and expression of the individual gene, Ki-67, by RT-PCR correlated with nuclear grade (P < 0.001; Fig. 2).
We found no significant correlation between age, tumor size, or RS and distant recurrence-free survival. There was also no significant difference between the low-, intermediate-, and high-risk groups in terms of either the RS or 10-year distant recurrence-free survival (Fig. 3).
We evaluated the correlation of the RT-PCR assay with known standard prognostic markers in FPET. Specifically, the relationship between RT-PCR and immunohistochemistry for ER, PR, and HER2 was examined. The concordance between RT-PCR and immunohistochemistry results in terms of the PR status was moderate (κ 0.48). The concordance between RT-PCR and immunohistochemistry results for ER status was high (Fig. 4A) and was somewhat lower for HER2 (Fig. 4B). For HER2 measurement by RT-PCR, we used a logistic model, treating immunohistochemistry as a quantal response (1 if immunohistochemistry 3+ and 0 otherwise). The resulting model fit displayed in Fig. 5 clearly separates immunohistochemistry 3+ cases by RT-PCR, indicating a significant (P < 0.0001) degree of correlation between immunohistochemistry and RT-PCR. The cutoff point for HER-2 positivity was prespecified as 11.5 based on prior studies. Using the prespecified cutoff point of 11.5, the sensitivity and specificity of the test for HER2 by RT-PCR are 84% and 89%, respectively. With regard to alternative cutoff points, using a cutoff point of 12, specificity increases to 95% at a cost of decreasing sensitivity to 68%, whereas using a cutoff point of 11, sensitivity remains at 84% at a cost of decreasing specificity to 77%. Additional studies are planned to obtain additional information concerning measurement of ER, PR, and HER2 by RT-PCR.
Correlation of ER expression (A, top) and HER-2 expression (B, bottom) shown by RT-PCR and immunohistochemistry (IHC). Y axis, expression of the mRNA measured by RT-PCR. Each circle represents one patient. Solid circles, patients whose tumors were shown to be positive by immunohistochemistry.
Correlation of ER expression (A, top) and HER-2 expression (B, bottom) shown by RT-PCR and immunohistochemistry (IHC). Y axis, expression of the mRNA measured by RT-PCR. Each circle represents one patient. Solid circles, patients whose tumors were shown to be positive by immunohistochemistry.
Logistic response model for HER-2 RT-PCR measurement (HER-2 3+ by immunohistochemistry shown in solid circles).
Logistic response model for HER-2 RT-PCR measurement (HER-2 3+ by immunohistochemistry shown in solid circles).
Figure 6 shows clustering of genes into ER, HER-2, and proliferation groups, as expected. Unsupervised hierarchical clustering analysis of gene expression showed that patients clustered into either an ER-negative or an ER-positive group (data not shown).
Unsupervised clustering of 16 cancer-related genes. Expected clusters of genes are observed.
Unsupervised clustering of 16 cancer-related genes. Expected clusters of genes are observed.
Discussion
In this study, we found no association between the RS and distant recurrence-free survival in 149 patients with node-negative breast cancer who did not receive adjuvant tamoxifen or chemotherapy followed up for a median time of 18 years. The gene expression correlations were consistent with expectations, and there was concordance between mRNA and protein expression in terms of the ER, PR, and HER-2 status.
In contrast, Paik et al. (14, 16) noted a strong correlation between the RS and distant recurrence-free survival in 668 patients with node-negative, ER-positive breast cancer treated with adjuvant tamoxifen for 5 years on protocol NSABP B-14. The discrepancy between the findings of this study and ours could be due to the fact that our patient cohort differed in a number of ways from the population in the NSABP B-14 study. First, our cohort was from a single institution and there is an admitted selection bias (i.e., referral to a tertiary cancer center, decision not to use systemic therapy). Therefore, the generalizability of our findings should be interpreted accordingly. Second, in contrast to the NSABP study, in which only patients with ER-positive breast cancer who were treated with tamoxifen were enrolled, our study included both ER-positive patients (who were not treated with tamoxifen) and ER-negative patients. Because the multigene assay was derived largely from studies in which many patients were treated with tamoxifen, it is possible that much of the contribution the ER group genes make to the prediction of recurrence is, in fact, a reflection of the prediction of response to tamoxifen. To address this important issue, Paik and colleagues evaluated the 21-gene RT-PCR assay in tissue from patients receiving placebo on the NSABP B-14 trial. This study showed that the RS has prognostic value in untreated patients. Furthermore, when the tamoxifen-treated patients were compared with the placebo group, patients with low or intermediate RS had large improvements in disease-free survival if treated with tamoxifen, whereas patients with high RS had a smaller benefit, if any, from tamoxifen (24). Interestingly, data from the NSABP B-20 trial showed that patients with high RS may benefit from adjuvant methotrexate/fluorouracil-based chemotherapy, whereas in patients with low RS, adjuvant chemotherapy did not add to tamoxifen (24).
Another potential confounding factor in our study is the unexpected association between high nuclear grade and improved outcome. This could also have reduced the performance of the RS in this selected cohort of patients because proliferation genes are important in the RS model and generally correlate with nuclear grade, as was shown in this study (Fig. 2).
In conclusion, RT-PCR analysis of FPET is feasible and the results correlate with known prognostic markers measured by immunohistochemistry. However, the prognostic role of the RS has not been established for node-negative breast cancer patients who have not received adjuvant tamoxifen treatment or chemotherapy, and new larger studies are needed.
Grant support: Genomic Health, Inc. (Redwood City, CA) and Career Development Award from the National Cancer Institute (K23 CA82119; F.J. Esteva).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Note: This study was presented in part at the 26th Annual San Antonio Breast Cancer Symposium, December 4, 2003, San Antonio, Texas.
Acknowledgments
We thank the Nellie B. Connally Breast Cancer Research Fund for supporting the Breast Cancer Tumor Bank at The University of Texas M.D. Anderson Cancer Center. We also acknowledge the contributions of Rick Baehner (University of California San Francisco) and Claire Alexander, Marti Haskins, Daniel Klaus, Mylan Pho, Mei-Lan Liu, Debjani Dutta, Anhthu Nguyen, Jennie Jeong, and Raymond Chao at Genomic Health.