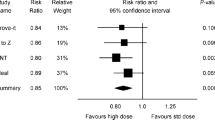
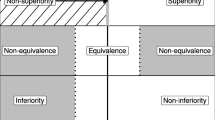
Abstract
Many of the reservations that might attach to the use of meta-analysis generally (for example, regarding publication bias) do not apply in the specific context of drug development. A meta-analysis is, in fact, a highly natural and appropriate way to summarize the results of a drug development program, as has been recognized in the International Conference on Harmonization (ICH) E9 guideline. Since a sponsor will have access to all original data, the data from a set of clinical trials in a drug development program have a very similar (hierarchical) structure to the data from a set of centers in a single multicenter trial. Curiously, however, the controversies over analyzing multicenter trials have often been different from those in the field of meta-analysis. In this paper, the options open to the meta-analyst in drug development are examined and comparisons to approaches used in analyzing multicenter trials are made in an attempt to provide some unifying insights, in particular as regards the handling of models with interactions.
Similar content being viewed by others

References
Senn SJ. Statistical Issues in Drug Development. Chichester, UK: Wiley; 1997.
Petitti DB. Meta-analysis Decision Analysis and Cost-Effectiveness Analysis. Oxford, UK: Oxford; 1994.
Glass GV. Primary, secondary and meta-analysis of research. Ed Res. 1976;5:3–8.
Pearson K. Report on certain enteric fever inoculation statistics. Br Med J. 1904; Nov 5: 1243–1246.
Winkelstein W. The first use of meta-analysis? Am J Epid. 1998;147:717.
Fisher RA. Studies in crop variation. J Agric Sci. 1921;11:107–135.
Healy MJR. Frank Yates, 1902-1994—The work of a statistician. Int Stat Rev. 1995;63:271–288.
Yates F, Cochran WG. The analysis of groups of experiments. J Agr Sci. 1938;28:556–580.
Tippett LHG. The Methods of Statistics. London, UK: Williams and Norgate; 1931.
Fisher RA. Statistical Methods for Research Workers. 4th edition. Edinburgh, UK: Oliver and Boyd; 1932.
Sonneman E. Kombination unabhangiger tests. In: Vollmar, J ed. Biometrie in der chemische pharma-zeutischen Industrie 4. Stuttgart, Germany: Fischer; 1991:91–112.
Peto R. Why do we need systematic overviews of randomized trials? (With discussion) Stat Med. 1987;6:233–240.
Egger M, Davey Smith G. Bias in location and selection of studies. Br Med J. 1998;316:61–66.
Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. J Clin Epid. 2000.
Eysenck H. Meta-analysis and its problems. Br Med J. 1994;309:789–792.
Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses. Lancet. 1998;352:609–613.
Lee PN. Simple methods for checking for possible errors in reported odds ratios, relative risks and confidence intervals. Stat Med. 1999;18:1973–1981.
Stewart LA, Parmar MK. Meta-analysis of the literature or of individual data: is there a difference? Lancet. 1993;341:418–422.
Light RJ. Accumulating evidence from independent studies: what we can win and what we can lose. Stat Med. 1987;6:221–228.
Thompson SG, Pocock, SJ. Can meta-analysis be trusted? Lancet. 1991;338,1127–1130.
Lindbaeck M, Hjortdahl P. How do two meta-analyses of similar data reach opposite conclusions? Br Med J. 1999;318:873.
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Statistical principles for clinical trials. Stat Med. 1999;18:1905–1942.
Richardson W, Bablok, B. Clinical experience with formoterol. In Holgate, ST, Ed. Formoterol: Fast and Long-Lasting Brochodilation. London, UK: Royal Society of Medicine Services; 1992.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Nat Cancer Inst. 1959;22:719–748.
Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomised clinical trials. Stat Med. 1991;31:1665–1677.
Hedges LV, Olkin I. Statistical Methods for Metaanalysis. Orlando, Florida: Academic Press; 1985.
Salsburg, D. Why analysis of variance is inappropriate for multiclinic trials. Cont Clin Trials. 1999;20:453–468.
Senn SJ, Harrell F. On wisdom after the event. J Clin Epidemiol. 1997;50:749–751.
Goldstein H. Multilevel Models in Educational and Social Research. London, UK: Griffin; 1987.
Källén A. Treatment-by-center interaction: what is the issue? Drug Inf J. 1997;31:927–936.
Gallo P. Center-Weighting Issues in Multicenter Clinical Trials. J Biopharm Stats. 2000; 10.
Nelder J. A reformulation of linear models. (With discussion) J R Statist Soc A. 1977;140:48–77.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Cont Clin Trials. 1986;7:177–188.
Hardy RJ, Thompson SG. A likelihood approach to meta-analysis with random effects. Stat Med. 1996;15:619–629.
Brand R, Kragt H. Importance of trends in the interpretation of an overall odds ratio in a meta-anlysis of clinical trials. Stat Med. 1992;11:2077–2082.
Mcintosh M. The population risk as an explanatory variable in research synthesis of clinical trials. Stat Med. 1996;15:1713–1728.
Schmid CH. Exploring heterogeneity in randomized trials via meta-analysis. Drug Inf J. 1999;33:211–224.
Senn SJ. Importance of trends in the interpretation of an overall odds-ratio in a meta-analysis of clinical trials. Stat Med. 1994;13:293–295.
Van Houwelingen H, Senn SJ. Letter to the editor: Investigating underlying risk as a source of hetero-geniety in meta-analysis. Stat Med. 1999;18:110–113.
Van Houwelingen H, Zwinderman K, Stijnen T. A bivariate approach to meta-analysis. Stat Med. 1993;12:2272–2284.
Thompson SG, Smith TC, Sharp SJ. Investigating underlying risk as a source of heterogeneity in metaanalysis. Stat Med. 1997;16:2883–2900.
Thompson SG, Prevost TC, Sharp SJ. Authors’ reply. Stat Med. 1999;18:113–115.
Nelder, J. The statistics of linear models: back to basics. Statist Comp. 1994;4:221–234.
Chuang-Stein C, Tong D. The impact of parameterization on the interpretation of the main-effect term in the presence of an interaction. Drug Inf J. 1996; 30:421–424.
Speed FM, Hocking RR, Hackney OP. Methods of analysis of linear models with unbalanced data. J Am Stat Assoc. 1978;73:105–112.
Jeffreys. Theory of Probability. Oxford, UK: Clarendon Press; 1939.
Fisher RA. Statistical Methods for Research Workers. In Bennett, Ed. Statistical Methods, Experimental Design and Scientific Inference. Oxford, UK: Oxford Science Publications; 1990.
Berry DA. A case for Bayesianism in clinical trials (with discussion). Stat Med. 1993;12:1377–1404.
Smith TC, Spiegelhalter DJ, Parmar MKB. Bayesian meta-analysis of randomized trials using graphical models and BUGS. In: Berry DA and Stangl DK, eds. Bayesian Biostatistics. New York, NY. Marcel Dekker; 1996.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Senn, S. The Many Modes of Meta. Ther Innov Regul Sci 34, 535–549 (2000). https://doi.org/10.1177/009286150003400222
Published:
Issue Date:
DOI: https://doi.org/10.1177/009286150003400222