Abstract
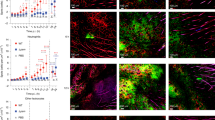
Inflammation is a defensive process by which the body responds to both localized and systemic tissue damage by the induction of innate and adaptive immunity. Literature from human and animal studies links inappropriate in utero inflammation to preterm parturition and fetal injury. The pathways by which such inflammation may cause labor, however, are not fully understood. Any proinflammatory agonist in the amniotic fluid will contact the fetal skin, in its entirety, but a potential role of the fetal skin in the pathways to labor have not previously been explored. We hypothesized that the fetal skin would respond robustly to the presence of intra-amniotic lipopolysaccharide (LPS) in our ovine model of in utero inflammation. In vitro and in utero exposure of fetal ovine keratinocytes or fetal skin to Escherichia coli LPS reliably induced significant increases in interleukin 1β (IL-1β), IL-6, tumor necrosis factor α (TNF-α), and IL-8 expression. We demonstrate that, in utero, this expression requires direct exposure with LPS suggesting that the inflammation is triggered directly in the skin itself, rather than as a secondary response to a systemic stimuli and that inflammation involves Toll-like receptor (TLR) regulation and neutrophil chemotaxis in concordance with an acute inflammatory reaction. We show that this response involves multiple inflammatory mediators, TLR regulation, and localized inflammatory cell influx characteristic of an acute inflammatory reaction. These novel data strongly suggests that the fetal skin acts as an important mediator of the fetal inflammatory response and as such may contribute to preterm birth.
Similar content being viewed by others
References
Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–1507.
Tracy SK, Tracy MB, Dean J, Laws P, Sullivan E. Spontaneous preterm birth of liveborn infants in women at low risk in Australia over 10 years: a population-based study. BJOG. 2007;114(6):731–735.
Hack M, Fanaroff AA. Outcomes of children of extremely low birthweight and gestational age in the 1990s. Semin Neonatol. 2000;5(2):89–106.
Lemons JA, Bauer CR, Oh W, et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network January 1995 through December 1996 NICHD Neonatal Research Network. Pediatrics. 2001;107(1):E1.
Roseboom TJ, Van der Meulen JHP, Ravelli ACJ, Osmond C, Barker DJP, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Twin Res. 2001;4(5):293–298.
Barker DJP, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. Br Med J. 1990;301(6746):259–262.
Dorner G, Plagemann A. Perinatal hyperinsulinism as possible predisposing factor for diabetes mellitus obesity and enhanced cardiovascular risk in later life. Horm Metab Res. 1994;26(5):213–221.
Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Semin Fetal Neonatal Med. 2009;14(1):2–7.
Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79(1):50–57.
Gomez R, Romero R, Ghezzi F, Bo HY, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179(1):194–202.
Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations other markers of inflammation and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol. 2006;195(3):803–808.
Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84.
Kemp MW, Saito M, Newnham JP, Nitsos I, Okamura K, Kallapur SGG. Preterm birth infection and inflammation advances from the study of animal models. Reprod Sci. 2010;17(7):619–628.
Kaga N, Katsuki Y, Obata M, Shibutani Y. Repeated administration of low-dose lipopolysaccharide induces preterm delivery in mice: a model for human preterm parturition and for assessment of the therapeutic ability of drugs against preterm delivery. Am J Obstet Gynecol. 1996;174(2):754–759.
Adams-Waldorf KM, Persing D, Novy MJ, Sadowsky DW, Gravett MG. Pretreatment with toll-like receptor 4 antagonist inhibits lipopolysaccharide-induced preterm uterine contractility cytokines and prostaglandins in rhesus monkeys. Reprod Sci. 2008;15(2):121–127.
Newnham JP, Moss TJ, Kramer BW, Nitsos I, Ikegami M, Jobe AH. The fetal maturational and inflammatory responses to different routes of endotoxin infusion in sheep. Am J Obstet Gynecol. 2002;186(5):1062–1068.
Moss TJM, Nitsos I, Newnham JP, Ikegami M, Jobe AH. Chorioamnionitis induced by subchorionic endotoxin infusion in sheep. Am J Obstet Gynecol. 2003;189(6):1771–1776.
Moss TJM, Nitsos I, Ikegami M, Jobe AH, Newnham JP. Experimental intrauterine ureaplasma infection in sheep. Am J Obstet Gynecol. 2005;192(4):1179–1186.
Kallapur SG, Jobe AH, Ball MK, et al. Pulmonary and systemic endotoxin tolerance in preterm fetal sheep exposed to chorioamnionitis. J Immunol. 2007;179(12):8491–8499.
Holbrook KA, Odland GF. The fine structure of developing human epidermis: light scanning and transmission electron microscopy of the periderm. J Invest Dermatol. 1975;65(1):16–38.
Sugita K, Kabashima K, Atarashi K, Shimauchi T, Kobayashi M, Tokura Y. Innate immunity mediated by epidermal keratinocytes promotes acquired immunity involving Langerhans cells and T cells in the skin. Clin Exp Immunol. 2007;147(1):176–183.
Miller LS, Modlin RL. Toll-like receptors in the skin. Semin Immunopathol. 2007;29(1):15–26.
Kim YM, Romero R, Chaiworapongsa T, Espinoza J, Mor G, Kim CJ. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology. 2006;49(5):506–514.
Jobe AH, Newnham JP, Willet KE. Effects of antenatal endotoxin and glucocorticoids on the lungs of preterm lambs. Am J Obstet Gynecol. 2000;182(2):401–408.
Kemp MW, Edwards B, Burgess M, et al. Syncoilin isoform organization and differential expression in murine striated muscle. J Struct Biol. 2009;165(3):196–203.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408.
Chang J-S, Russell GC, Jann O, Glass EJ, Werling D, Haig DM. Molecular cloning and characterization of Toll-like receptors 1-10 in sheep. Vet Immunol Immunopathol. 2009;127(1–2):94–105.
Sow FB, Gallup JM, Meyerholz DK, Ackermann MR. Gene profiling studies in the neonatal ovine lung show enhancing effects of VEGF on the immune response. Dev Comp Immunol. 2009;33(6):761–771.
Arnaout MA. Leukocyte adhesion molecules deficiency: its structural basis pathophysiology and implications for modulating the inflammatory response. Immunol Rev. 1990;114:145–180.
Song PI, Park Y-M, Abraham T, et al. Human keratinocytes express functional CD14 and toll-like receptor 4. J Invest Dermatol. 2002;119(2):424–432.
Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity inflammation and cancer. Trends Mol Med. 2008;14(3):109–119.
Yoshimura T, Matsushima K, Oenheim JJ, Leonard EJ. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL 1). J Immunol. 1987;139(3):788–793.
Djeu JY, Matsushima K, Oenheim JJ, Shiotsuki K, Blanchard DK. Functional activation of human neutrophils by recombinant monocyte-derived neutrophil chemotactic factor/IL-8. J Immunol. 1990;144(6):2205–2210.
Schroder J-M, Mrowietz U, Morita E, Christophers E. Purification and partial biochemical characterization of a human monocyte-derived neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol. 1987;139(10):3474–3483.
Schroder J-M. The monocyte-derived neutrophil activating peptide (NAP/interleukin 8) stimulates human neutrophil arachidonate-5-lipoxygenase but not the release of cellular arachidonate. J Exp Med. 1989;170(3):847–863.
Lu Y-C, Yeh W-C, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145–151.
Honda K, Yanai H, Mizutani T. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signalling. Proc Natl Acad Sci U S A. 2004;101(43):15416–15421.
Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-β. Nat Immunol. 2008;9(4):361–368.
Raicevic G, Rouas R, Najar M, et al. Inflammation modifies the pattern and the function of Toll-like receptors expressed by human mesenchymal stromal cells. Hum Immunol. 2010;71(3):235–244.
Narendran V, Visscher MO, Abril I, Hendrix SW, Hoath SB. Biomarkers of epidermal innate immunity in premature and full-term infants. Pediatr Res. 2010;67(4):382–386.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kemp, M.W., Saito, M., Nitsos, I. et al. Exposure to In Utero Lipopolysaccharide Induces Inflammation in the Fetal Ovine Skin. Reprod. Sci. 18, 88–98 (2011). https://doi.org/10.1177/1933719110380470
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719110380470