Abstract
This paper reports a newly fabricated nanophyto-modified wound dressing with microbicidal and anti-adherence properties. Nanofluid-based magnetite doped with eugenol or limonene was used to fabricate modified wound dressings. Nanostructure coated materials were characterized by TEM, XRD, and FT-IR. For the quantitative measurement of biofilm-embedded microbial cells, a culture-based method for viable cell count was used. The optimized textile dressing samples proved to be more resistant to staphylococcal and pseudomonal colonization and biofilm formation compared to the uncoated controls. The functionalized surfaces for wound dressing seems to be a very useful tool for the prevention of wound microbial contamination on viable tissues.
Similar content being viewed by others
Background
Humans are natural hosts for many bacterial species that colonize the skin and mucosa as normal microbiota. However, in certain conditions, some microbes composing our microbiota generically called opportunistic pathogens can cause serious infections mainly by regulating their virulence [1, 2]. Predisposing factors to cutaneous infections include minor trauma, pre-existing skin disease, poor hygiene, and, rarely, impaired host immunity [3]. Based on World Health Organization report in 2011, skin diseases still remain common in many rural communities in developing countries, with serious economic and social consequences, as well as health implications.
As a form of adaptability and evolution, bacteria managed to establish a well-organized behavior into a very efficient assembly, called biofilm. Bacterial biofilm formation is the prevailing microbial lifestyle in natural and man-made environments and occurs on all surface types, including biological surfaces; it can be defined as a community of microorganisms irreversibly attached to a surface, producing extracellular polymeric substances, exhibiting an altered phenotype compared with corresponding planktonic cells, and interacting with each other [4, 5]. One of the most significant clinical aspects is the fact that bacterial biofilms cause chronic infections because they disclose increased tolerance to antibiotics and disinfectants, as well as resisting phagocytosis and other components of the body’s defense system [6]. Approximately, 80% of all human infections are associated with biofilms, and evidence for their role in an ever-growing number of cutaneous disorders is constantly unfolding [7].
In the recent years, researchers aimed to find alternative methods of dealing with infections with biofilm-embedded bacteria, knowing that adherent microbial cells exhibit high antibiotic resistance. One of the most efficient strategies is to interfere with bacterial adherence, the first step in the biofilm formation, by direct blockage of surface receptors [8] or using a non-specific strategy, usually involving compounds with anti-adherence properties [9–11]. Another efficient strategy seems to be the one involving the manipulation of communication processes between bacteria into the biofilm, using different natural or artificially synthetized compounds [12–14]. Bearing in mind that chemically synthetized compounds may be toxic and have usually unpredictable long-term effect on the mammalian host cell, natural compounds exhibiting anti-microbial activity are considered as a more preferred alternative [15, 16]. Essential vegetal oils are natural compounds that have proved to be highly efficient as antimicrobial agents, demonstrating significant anti-adherence and anti-biofilm properties [17, 18]. However, the use of essential oils can be limited by their high volatility and low stability [19].
Magnetic iron oxide nanoparticles have appeared as a well-established technology and an important research field, mainly because of their superparamagnetism properties that allow to be guided with an external magnetic field, [20, 21]. Potential applications in the field of biotechnology and nanomedicine such as biomagnetic separations [22], biosensors [23], carriers for targeted drug delivery [24–28], hyperthermia-producing systems [29], inhibition of biofilm development [30, 31], stabilization of essential oils [32], and contrast agents in magnetic resonance imaging [33, 34] have been proposed. The material surface chemistry and the electronic configuration of the surface complexes have major influences on the reactivity and properties [35].
In this paper, we report preliminary data on new magnetite-based nanostructures used to create nanofluids with both microbicidal and anti-adherence properties, and to evaluate their potential to improve the anti-biofilm properties of a cotton-based material, routinely used for covering cutaneous wounds. The anti-adherence and anti-biofilm properties of this nano-modified wound dressing were assessed in vitro using two strains belonging to bacterial species commonly found in wound infections, i.e., Pseudomonas aeruginosa and Staphylococcus aureus.
Methods
Materials
All chemicals were used as received. FeCl3, FeSO4 · 7H2O, NH3, sodium palmitate (C16), CHCl3, and CH3OH were purchased from Sigma-Aldrich Chemie GmbH (Munich, Germany). The textile wound dressing represented by 1 × 1-cm sections were obtained from the Otolaryngology Department of Coltea Hospital, Bucharest, Romania.
Fabrication of nanostructure
Magnetic iron oxide particles are usually prepared by wet chemical precipitation [36, 37] from aqueous iron salt solutions by means of alkaline media, like NH3. Half gram of sodium palmitate (C16) was solubilized in a known volume of ultrapure water, corresponding to a 1.00% (w/w) solution, under stirring at room temperature. Then, 4 mL of a basic aqueous solution consisting of 28% NH3 was added to C16 dispersion. Thereafter, 100 mL of FeSO4/FeCl3 (molar ratio 2:1) was dropped under permanent stirring up to pH = 8 [38, 39]. The product (Fe3O4@C16) was repeatedly washed with methanol, separated with a strong NdFeB permanent magnet, and subsequently dried in an oven at 40°C, until reaching a constant weight.
Characterization of nanostructure
FT-IR
A Nicolet 6700 Fourier transform infrared spectroscopy (FT-IR) spectrometer (Thermo Nicolet, Madison, WI, USA) connected to the software of the OMNIC operating system (version 7.0 Thermo Nicolet) was used to obtain FT-IR spectra of hybrid materials. The samples were placed in contact with attenuated total reflectance on a multibounce plate of ZnSe crystal at controlled ambient temperature (25°C). FT-IR spectra were collected in the frequency range of 4,000 to 650 cm−1 by co-adding 32 scans and at a resolution of 4 cm−1 with strong apodization. All spectra were ratioed against a background of an air spectrum.
XRD
X-ray diffraction analysis (XRD) was performed on a Shimadzu XRD 6000 diffractometer (Shimadzu Corporation, Kyoto, Japan) at room temperature. In all the cases, CuKα radiation from a Cu X-ray tube (run at 15 mA and 30 kV) was used. The samples were scanned in the Bragg angle 2θ range of 10 to 80.
TEM
The transmission electron microscopy (TEM) images were obtained on finely powdered samples using a Tecnai™ G2 F30 S-TWIN high resolution transmission electron microscope from FEI Company (OR, USA) equipped with EDS and SAED. The microscope was operated in transmission mode at 300 kV with TEM point resolution of 2 Å and line resolution of 1 Å. The fine MNP powder was dispersed into pure ethanol and ultrasonicated for 15 min. After that, diluted sample was put onto a holey carbon-coated copper grid and left to dry before TEM analysis.
DTA-TG
The thermogravimetric (TG) analysis of the biocomposite was assessed with a Shimadzu DTG-TA-50H instrument. Samples were screened to 200 mesh prior to analysis, were placed in alumina crucible, and heated with 10 K · min−1 from room temperature to 800°C, under the flow of 20 mL · min−1 dried synthetic air (80% N2 and 20% O2).
Fabrication of the hybrid phyto-nanostructure
Magnetic nanostructure Fe3O4@C16 (200 mg) was solubilized in 1 mL of chloroform and oriented in magnetic field, and 100 μL analytical standard of eugenol (E) (Sigma-Aldrich) and respectively, limonene (L) (Sigma-Aldrich) were added and mixed until complete evaporation of chloroform was reached. This step was repeated three times for the uniform loading of E and L in the core-shell nanostructure.
Fabrication of the modified wound dressing coated with the phyto-nanostructure
The layer of phyto-nanostructure on the wound dressing material was achieved by submerging the wound dressing pieces (1 × 1 cm) in 5 mL of phyto-nanofluid (Fe3O4@C16/E or Fe3O4@C16/L:chloroform = 1 mg/mL), and the wound dressing pieces have been extemporaneously dried at room temperature. The rapid drying was facilitated by the convenient volatility of chloroform [40]. The phyto-E and phyto-L-nanomodified wound dressing specimens were sterilized by ultraviolet irradiation for 20 min. Figure 1 illustrates the wound dressing with phyto-nanofluid coating.
Schematic representation of the microbial biofilm development on the uncoated and coated wound dressings. (a) wound dressing fiber; (b) biofilm development on the surface of wound dressing fiber; (c) coated wound dressing fiber by the obtained phyto-nanofluid; (d) poorly developed microbial biofilm on the surface of the modified textile material.
Bacterial adherence and biofilm assay by viable cell count method
Overnight bacterial cultures of P. aeruginosa ATCC 27853 and S. aureus ATCC 25923 were diluted in fresh Luria broth (LB) up to a turbidity of 0.5 McFarland (approximately 1 × 108 CFU/mL), and 2 mL of the obtained suspension were seeded in 6 multi-well plates containing the wound dressing specimens previously sterilized by UV irradiation. The plates were incubated for 24 h at 37°C. For the adherence assay, after the incubation time, the materials were gently washed with sterile phosphate buffered saline (PBS) in order to remove the non-adherent bacteria and placed in 2 mL centrifuge tubes containing 1 ml of sterile PBS. The samples were vigorously mixed by vortexing for 1 min and sonicated for 10 s [41]. Serial dilutions obtained from each sample were inoculated on LB agar plates in triplicates, and viable cell counts (VCCs) were assessed after incubation for 24 h at 37°C. For the biofilm assay, the materials containing attached bacteria were washed with sterile PBS and incubated in fresh LB broth for 24 h, 48 h, and 72 h at 37°C. After each incubation period, the samples were gently washed with sterile PBS, mixed by vortexing, and sonicated. Serial dilutions were placed on LB plates in triplicate. After 24 h of incubation at 37°C, VCCs were assessed. The experiment was repeated with three separate occasions.
Statistics
For the statistical interpretation, we have used GraphPadInStat (GraphPad Software, Inc., CA, USA) and Prism softwares (Prism Software Corporation, CA, USA). The results were analyzed and compared using one-way analysis of variance (ANOVA) and Bonferroni Multiple Comparisons Test. P values lower than 0.05 were considered significant.
Results and discussion
Textile industry is a small part of the global research in the emerging areas of nanotechnology, the fibers and textiles industries being in fact the first to have successfully implemented these advances and demonstrated the applications of nanotechnology for consumer usage [42]. Nanotechnologies have been largely used for different biomedical applications.
In our previous papers, we have demonstrated by scanning electron microscopy the ability of Fe3O4@C18 to prevent the fungal adherence of Candida albicans on optimized textile dressing samples coated with functionalized magnetite nanoparticles, as compared to uncoated materials [36]. These functionalized Fe3O4@C18 nanoparticles exhibited also the ability to stabilize, limit the volatilization, and potentiate the fungicidal effect of Salvia officinalis essential oil [43]. On the other hand, limonene and eugenol, the major compounds of essential oils extracted from Anethum graveolen s (56.53%) and Eugenia caryophyllata (92.45%) proved, to exhibit very good antimicrobial properties [28, 44]. In this paper, we report the successful fabrication of two phyto-nanofluids for coating textile wound dressings, based on limonene and eugenol loaded in magnetic nanoparticles, in order to increase their microbicidal and anti-biofilm properties and, thus, combat the cutaneous opportunistic infections.
The obtained nanostructure was characterized by XRD as illustrated in Figure 2, and the results showed that the diffraction patterns and the relative intensities of all diffraction peaks match well with magnetite (based on ICDD 82–1533). Also, the sample has the characteristics of bulk magnetite crystallite phase, and the broad peaks suggest the nanocrystallite nature of magnetite particles [45, 46], the average crystallite size being 10.58 nm (based on Scherrer formula). FT-IR spectrum of the nanostructure exhibits a characteristic broad peak of magnetite at about 533 cm−1 (Fe-O stretching) [47]. The FT-IR analysis also identified the organic coating on the surface of the magnetite nanoparticles (Figure 3). The peaks recorded at about 1,572 and 1,701 cm−1 at FT-IR spectrum of the nanostructure can be assigned to structures of the type COO−M+. The peaks at 2,915 and 2,848 cm−1 were assigned to stretching vibration of C-H (Figure 3). The nanostructure diameter was approximated from the TEM images (as presented in Figure 4), showing that the particles are spherical with an average size of 10 nm which, corroborated with the XRD data, means that the obtained nanoparticles are formed by only one crystallite. The presence of essential oils induces a strong modification of the thermal behavior of the two nanostructured materials (Figure 5). In the case of phyto-E-nanostructurated material, the weight loss increases with about 4.6%, which can be mainly attributed to the eugenol adsorption onto the nanomaterial. The weight loss was surprisingly affected in the phyto-L-nanostructurated material, where the weight loss became even lower than that corresponding to Fe3O4@C16. We explain this anomaly by the fact that limonene and C16 interact by special hydrophobic interactions, and the complex may be partially lost during the drying step.
Due to their widespread, easy manipulation, and low side effects, direct contact wound absorptive natural-based plasters are preferred for wound dressing. Specialized literature reports few studies aimed to improve the quality and antibacterial properties of natural or artificial materials used for wound dressing and covering, but the proposed techniques are mainly based on using artificial, new chemically synthetized compounds [16, 17].
Essential oils represent an alternative for treating microbial infections because they are natural vegetal compounds with lower or no side effects for the host compared with artificially synthetized antimicrobial compounds, representing one of the ecological anti-infectious strategies. However, their effects can be impaired by their great volatility, highlighting the necessity of novel vectoring stabilizing systems. In the recent years, the usage of nanosystems for clinical issues has emerged, mainly because of their reduced structures and their proved characteristics, as antimicrobial activity. Even though nanosystems are considered a novel challenge for medicine, their usage is largely restricted because of their unknown long term effects and sometimes because of their toxicity on eukaryotic cells. During this study, we have investigated the possibility of improving the antimicrobial activity of wound dressings by modifying their surface using a nanofluid to assure the stability and controlled release of some volatile organic compounds isolated from essential oils. Our results obtained on two in vitro monospecific bacterial biofilm models involving cotton-based wound dressers layered with a phyto-nanostructured coating demonstrated that the functionalized textile materials exhibited antimicrobial effects on wound-related pathogens.
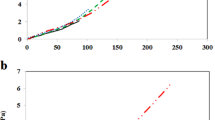
VCCs assessed from mechanically detached biofilm bacteria revealed a slightly different ability of the two modified wound dressings. The results revealed that the nanofluid coating containing L affected both the initial stage of biofilm formation and the development of a mature biofilm, as demonstrated by the lower VCCs obtained at the three harvesting time intervals (i.e., 24 h, 48 h, and 72 h), as comparing with control, uncoated textile materials (P < 0.0001). Even though P. aeruginosa ATCC 27853 grew better, the differences between S. aureus and P. aeruginosa VCC values were not significantly different. The nanofluid exhibiting comparative antibiofilm effects in both models (Figure 5) induced a significantly reduced biofilm development expressed as viable cells in time (P < 0.05). The phyto-E-nano-modified wound dressing model has proved to have also a significant antibiofilm activity, determining a pronounced biofilm inhibition on both S. aureus (Figure 6) and P. aeruginosa (Figure 7) models at all three tested time points (P < 0.0001). The effect of this system seems to be more pronounced on adherence and initial biofilm formation compared to the L-based one, in case of P. aeruginosa.
The logarithmic values of viable cell counts of P. aeruginosa cells. The cells adhered and embedded in biofilms and formed on the wound dressing surface: uncoated vs. nanophyto-L and E-modified. Double asterisk denotes P < 0.01; triple asterisk, P < 0.001. Indicated samples vs. uncoated control based on one way ANOVA test.
For both tested phyto-nanosystems, the most important decrease of VCCs was observed at 72 h, demonstrating the ability of the obtained nanostructure to reduce the volatility of the essential oils and to assure their release in active forms for the entire duration of the experiment. Taken together, our data demonstrate that the obtained phyto-nanofluids are very useful for the stabilization and controlled release of some antimicrobial active compounds, such as the essential oil major compounds with antimicrobial activity, eugenol and limonene. The fabricated nanostructures with an adsorbed shell of L and E compounds are much more efficient in triggering bacterial biofilm disruptions.
Conclusions
In this paper, we report a successful antimicrobial system represented by modified wound dressing coated by a hybrid nanofluid based on magnetite and natural compounds of vegetal origin, i.e., eugenol and limonene, with a great potential of application in wound healing. The functionalized textile material cumulate the anti-adherent properties of magnetite and microbicidal activity of eugenol and limonene, exhibiting significant anti-adherence and anti-biofilm properties against two of the bacterial pathogens most frequently implicated in the etiology of cutaneous wound infections. The tested nanofluid proved to be efficient for stabilizing and controlling the release of volatile natural compounds, thus maximizing their biological activity. The proposed phyto-nanostructures are recommended to be used as a fixed layer on a regular external wound cover. Their topical application at cutaneous level minimizes the risk of toxicity effects normally associated with an implanted device.
References
Alizon S: Virulence evolution and the trade-off hypothesis: history, current state of affairs and the future. J Evol Biol 2009, 22: 245–259. 10.1111/j.1420-9101.2008.01658.x
Brown SP, Cornforth DM, Mideo N: Evolution of virulence in opportunistic pathogens: generalism, plasticity, and control. Trend Microb 2012, 20: 336–342. 10.1016/j.tim.2012.04.005
Norman DC: Factors predisposing to infection. Infect Dis 2009, 1: 11–18.
Donlan RM: Biofilms: microbial life on surfaces. Emerg Infect Dis 2002, 8: 881–890. 10.3201/eid0809.020063
Donlan RM, Costerton JW: Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 2002, 15: 167–193. 10.1128/CMR.15.2.167-193.2002
Høibya N, Bjarnsholta T, Givskovb M, Molinc S, Ciofu O: Antibiotic resistance of bacterial biofilms. Int J Antimicr Agent 2010, 35: 322–332. 10.1016/j.ijantimicag.2009.12.011
Nusbaum AG, Kirsner RS, Charles CA: Biofilms in dermatology. Skin Thearpy Lett 2012, 17: 1–5.
DiMango E, Zar HJ, Bryan R, Prince A: Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J Clin Invest 1995, 96: 2204–2210. 10.1172/JCI118275
Sajjan U, Moreira J, Liu M, Humar A, Chaparro C, Forstner J, Keshavjee S: A novel model to study bacterial adherence to the transplanted airway: inhibition of Burkholderia cepacia adherence to human airway by dextran and xylitol. J Heart Lung Transplant 2004, 23: 1382–1391. 10.1016/j.healun.2003.09.023
Feng W, Garrett H, Speert DP, King M: Improved clearability of cystic fibrosis sputum with dextran treatment in vitro. Am J Respir Crit Care Med 1998, 157: 710–714.
Thomas R, Brooks T: Common oligosaccharide moieties inhibit the adherence of typical and atypical respiratory pathogens. J Med Microbiol 2004, 53: 833–840. 10.1099/jmm.0.45643-0
Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP: Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 2000, 407: 762–764. 10.1038/35037627
Wu H, Song Z, Hentzer M, Andersen JB, Molin S, Givskov M, Hoiby N: Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. J Antimicrob Chemother 2004, 53: 1054–1061. 10.1093/jac/dkh223
Rogan MP, Taggart CC, Greene CM, Murphy PG, O’Neill SJ, McElvaney NG: Loss of microbicidal activity and increased formation of biofilm due to decreased lactoferrin activity in patients with cystic fibrosis. J Infect Dis 2004, 190: 1245–1253. 10.1086/423821
Grumezescu AM, Chifiriuc MC, Marinas I, Saviuc C, Mihaiescu D, Lazar V: Ocimum basilicum and Mentha piperita essential oils influence the antimicrobial susceptibility of Staphylococcus aureus strains. Lett Appl Nano Bio Sci 2012, 1: 14–17.
Marinas I, Grumezescu AM, Saviuc C, Chifiriuc C, Mihaiescu D, Lazar V: Rosmarinus officinalis essential oil as antibiotic potentiator against Staphylococcus aureus. Biointerface Res Appl Chem 2012, 2: 271–276.
Saviuc C, Grumezescu AM, Bleotu C, Holban A, Chifiriuc C, Balaure P, Lazar V: Phenotypical studies for raw and nanosystem embedded Eugenia carryophyllata buds essential oil effect on Pseudomonas aeruginosa and Staphylococcus aureus strains. Biointerface Res Appl Chem 2011, 1: 111.
Coimbra M, Isacchi B, van Bloois L, Torano JS, Ket A, Wu X, Broere F, Metselaar JM, Rijcken CJF, Storm G, Bilia R, Schiffelers RM: Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int J Pharm 2011, 416: 433–442. 10.1016/j.ijpharm.2011.01.056
Saviuc C, Holban AM, Grumezescu AM, Bleotu C, Banu O, Lazar V, Mihaiescu DE, Chifiriuc MC: Testing antifungal activity of some essential oils using flow cytometry. Lett Appl Nanobiosci 2012, 1: 67–71.
Pilloni M, Nicolas J, Marsaud V, Bouchemal K, Frongia F, Scano A, Ennas G, Dubernet C: PEGylation and preliminary biocompatibility evaluation of magnetite–silica nanocomposites obtained by high energy ball milling. Int J Pharm 2010, 401: 103–112. 10.1016/j.ijpharm.2010.09.010
Medeiros SF, Santos AM, Fessi H, Elaissari A: Stimuli-responsive magnetic particles for biomedical applications. Int J Pharm 2011, 403: 139. 10.1016/j.ijpharm.2010.10.011
Manzu D, Ficai A, Voicu G, Vasile BS, Guran C, Andronescu E: Polysulfone based membranes with desired pores characteristics. Mat Plast 2010, 47: 24–27.
Chirea M, Pereira EM, Pereira CM, Silva F: DNA biosensor for the detection of actinomycin D. Biointerface Res Appl Chem 2011, 1: 151–159.
Mihaiescu DE, Horja M, Gheorghe I, Ficai A, Grumezescu AM, Bleotu C, Chifiriuc MC: Water soluble magnetite nanoparticles for antimicrobial drugs delivery. Lett Appl Nano Bio Sci 2012, 1: 45–49.
Grumezescu AM, Saviuc C, Holban A, Hristu R, Stanciu G, Chifiriuc C, Mihaiescu D, Balaure P, Lazar V: Magnetic chitosan for drug targeting and in vitro drug delivery response. Biointerface Res Appl Chem 2011, 1: 160.
Saviuc C, Grumezescu AM, Holban A, Chifiriuc C, Mihaiescu D, Lazar V: Hybrid nanostructurated material for biomedical applications. Biointerface Res Appl Chem 2011, 1: 64.
Wang H, Wang S, Liao Z, Zhao P, Su W, Niu R, Chang J: Folate-targeting magnetic core–shell nanocarriers for selective drug release and imaging. Int J Pharm 2011, 430: 343.
Grumezescu AM, Andronescu E, Ficai A, Bleotu C, Mihaiescu DE, Chifiriuc MC: Synthesis, characterization and in vitro assessment of the magnetic chitosan-carboxymethylcellulose biocomposite interactions with the prokaryotic and eukaryotic cells. Int J Pharm 2012, 436: 771–777. 10.1016/j.ijpharm.2012.07.063
Andronescu E, Ficai M, Voicu G, Ficai D, Maganu M, Ficai A: Synthesis and characterization of collagen/hydroxyapatite: magnetite composite material for bone cancer treatment. J Mat Sci - Mat M 2010, 21: 2237–2242. 10.1007/s10856-010-4076-7
Saviuc C, Grumezescu AM, Chifiriuc MC, Bleotu C, Stanciu G, Hristu R, Mihaiescu D, Lazăr V: In vitro methods for the study of microbial biofilms. Biointerface Res Appl Chem 2011, 1: 031–040.
Grumezescu AM, Chifiriuc MC, Saviuc C, Grumezescu V, Hristu G, Mihaiescu D, Stanciu GA, Andronescu E: Hybrid nanomaterial for stabilizing the antibiofilm activity of Eugenia caryophyllata essential oil. IEEE T Nano Bio Sci 2012, 11(4):360–365.
Saviuc C, Grumezescu AM, Chifiriuc MC, Mihaiescu DE, Hristu R, Stanciu G, Oprea E, Radulescu V, Lazar V: Hybrid nanosystem for stabilizing essential oils in biomedical applications. Digest J Nanomat Biostr 2011, 6: 1657–1666.
Mantle MD: Quantitative magnetic resonance micro-imaging methods for pharmaceutical research. Int J Pharm 2011, 417: 173. 10.1016/j.ijpharm.2010.11.035
Schweiger C, Pietzonka C, Heverhagen J, Kissel T: Novel magnetic iron oxide nanoparticles coated with poly(ethylene imine)-g-poly(ethylene glycol) for potential biomedical application: synthesis, stability, cytotoxicity and MR imaging. Int J Pharm 2011, 408: 130–137. 10.1016/j.ijpharm.2010.12.046
Nel A, Xia T, Madler L, Li N: Toxic potential of materials at the nanolevel. Science 2006, 311: 622–627. 10.1126/science.1114397
Ficai D, Ficai A, Vasile BS, Ficai M, Oprea O, Guran C, Andronescu E: Synthesis of rod-like magnetite by using low magnetic field. Digest J Nanomat Biostr 2011, 6: 943–951.
Grumezescu AM, Andronescu E, Ficai A, Yang CH, Huang KS, Vasile BS, Voicu G, Mihaiescu DE, Bleotu C: Magnetic nanofluid with antitumoral properties. Lett Appl Nano Bio Sci 2012, 1: 56–60.
Andronescu E, Grumezescu AM, Ficai A, Gheorghe I, Chifiriuc M, Mihaiescu DE, Lazar V: In vitro efficacy of antibiotic magnetic dextran microspheres complexes against Staphylococcus aureus and Pseudomonas aeruginosa strains. Biointerface Res Appl Chem 2012, 2: 332–338.
Anghel I, Limban C, Grumezescu AM, Anghel AG, Bleotu C, Chifiriuc MC: In vitro evaluation of anti-pathogenic surface coating nanofluid, obtained by combining Fe3O4/C12 nanostructures and 2-((4-ethylphenoxy) methyl)-N-(substituted-phenylcarbamothioyl)-benzamides. Nanoscale Res Lett 2012, 7: 513. 10.1186/1556-276X-7-513
Saviuc C, Grumezescu AM, Chifiriuc MC, Bleotu C, Stanciu G, Hristu R, Mihaiescu D, Lazar V: In vitro methods for the study of microbial biofilms. Biointerface Res Appl Chem 2011, 1: 31–40.
Anghel I, Grumezescu AM, Andronescu E, Anghel AG, Ficai A, Saviuc C, Grumezescu V, Vasile BS, Chifiriuc MC: Magnetite nanoparticles for functionalized textile dressing to prevent fungal biofilms development. Nanoscale Res Lett 2012, 7: 501. 10.1186/1556-276X-7-501
Singh VK, Sawhney PS, Sachinvala ND, Li G, Pang SS, Condon B, Parachuru R: Applications and future of nanotechnology in textiles. In Beltwide Cotton Conferences: 2006 January 3–6. San Antonio. San Antonio: National Cotton Council; 2006.
Anghel I, Grumezescu I, Andronescu E, Anghel GA, Grumezescu AM, Mihaiescu DE, Chifiriuc MC: Protective effect of magnetite nanoparticle/Salvia officinalis essential oil hybrid nanobiosystem against fungal colonization on the Provox® voice section prosthesis. Digest J Nanomat Biostruct 2012, 7(3):1205–1212.
Grumezescu AM, Ilinca E, Chifiriuc C, Mihaiescu D, Balaure P, Traistaru V, Mihaiescu G: Influence of magnetic MWCNTs on the antimicrobial activity of cephalosporins. Biointerface Res Appl Chem 2011, 1(4):139–144.
Hou YL, Yu HF, Gao S: Solvothermal reduction synthesis and characterization of superparamagnetic magnetite nanoparticles. J Mater Chem 2003, 13: 1983–1987. 10.1039/b305526d
Wang XS, Zhu L, Lu HJ: Surface chemical properties and adsorption of Cu (II) on nanoscale magnetite in aqueous solutions. Desalination 2011, 276: 154–160. 10.1016/j.desal.2011.03.040
Cornell RM, Schwertmann U: The Iron Oxides, Structure, Properties, Reactions, Occurrences and Uses. 2nd edition. Weinheim: Wiley; 2003.
Acknowledgment
AMH was financially supported by the Sectorial Operational Program for Human Resources Development 2007–2013, co-financed by the European Social Fund, under the project number POSDRU/107/1.5/S/80765.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AMH and IA conceived the study, provided the microbial strains, and drafted the manuscript together with AMG and MCC. AMG, AF and MM performed the synthesis and characterization of nanofluid. AMG obtained the essential oil. AMH and AGA performed the biological analyses. EA and VL participated in the design of the study and coordination. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Anghel, I., Holban, A.M., Grumezescu, A.M. et al. Modified wound dressing with phyto-nanostructured coating to prevent staphylococcal and pseudomonal biofilm development. Nanoscale Res Lett 7, 690 (2012). https://doi.org/10.1186/1556-276X-7-690
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1556-276X-7-690