Abstract
Studies have shown the important roles of several regulatory and proinflammatory cytokines in insulin-dependent diabetes mellitus (IDDM). CC-chemokine receptors CCR2 and CCR5 bind chemokines that are involved in the trafficking of leukocytes in both basal and inflammatory states. A common 32-bp deletion mutation in the CCR5 gene (CCR5Δ32) and a G-to-A nucleotide substitution in the CCR2 at position 190 (CCR2-64I) have recently been described. In the present study, we have determined the frequency of the CCR5Δ32 and CCR2-64I alleles in children with IDDM [n = 115; age 1-14 (9.3 ± 4.3) y] and in nondiabetic subjects [n = 280; age 1-14 (8.5 ± 4.5) y]. The CCR5Δ32 allele frequencies were 0.117 in children with IDDM and 0.111 in nondiabetic subjects, indicating that the deletion allele has no association with IDDM. The CCR2-64I allele frequency in children with IDDM was 0.226, which differed significantly from the allele frequency in controls (0.114, p = 0.001). The role of this mutation in IDDM cannot be explained yet, but, because CCR2 mediates the chemotaxis of CD4+ and CD8+ T cells to areas of inflammation and because these cells play important roles in insulitis, a mutation in the CCR2 gene may contribute to the susceptibility to the disease. Alternatively, the 64I allele could be a marker of a linked mutation through linkage disequilibrium. According to these results, the CCR2 gene may be a new candidate for the susceptibility locus of IDDM. However, because no IDDM locus has been identified near 3p21 until now, further investigations are needed to confirm this statement.
Similar content being viewed by others
Main
IDDM in animal models is T cell mediated and requires the participation of both CD8+ class I MHC-restricted and CD4+ class II MHC-restricted T cells(1). Studies have shown the important roles of several regulatory and proinflammatory cytokines(2). Chemotactic cytokines, or chemokines, are small signaling proteins that are deeply involved in the physiology of acute and chronic inflammatory processes, as well as in the pathologic dysregulations of these processes, by attraction and stimulation of specific subsets of leukocytes(3). Chemokines are 70-90 amino acids in length and are subdivided into two gene families (CC and CXC chemokines) depending on the relative position of the first two conserved cysteines(4). The CXC chemokines predominantly activate neutrophils, whereas the CC chemokines generally activate monocytes, lymphocytes, basophils, and eosinophils(4). Recent studies have shown that the actions of chemokines are mediated by subfamilies of G-protein-coupled receptors(5). Several human CCR have been cloned and characterized recently(6). The genes for the receptors are dispersed in small clusters of genes that have related function and sequence. The genes for CCR2 and CCR5 have been mapped to human chromosome 3p21(7,8). CCR5, which is expressed in monocytes, macrophages, and primary T cells, binds, among others, to RANTES (regulated on activation normal T cell expressed)(8). RANTES is a chemoattractant for monocytes, memory T cells, and eosinophils and induces the release of histamine by basophils. CCR2 binds to monocyte chemotactic protein-1, which is highly effective on CD4+ and CD8+ T cells(9). IL-2 enhances the CCR2 expression and chemotactic responsiveness of CD4+ T cells(10). A common 32-bp deletion mutation in the CCR5 gene (CCR5Δ32), which causes truncation and loss of CCR5 receptors on lymphoid cell surfaces, has recently been described(11). In the CCR2 receptor, a G-to-A nucleotide substitution has been detected at position 190 that substitutes the amino acid residue valine to isoleucine (CCR2-64I), a conservative change located within the first transmembrane domain of the CCR2 receptor(12). The CCR2 and CCR5 loci are tightly linked (17.5 kb apart) on chromosome 3 and the mutant alleles are in strong, perhaps complete, linkage disequilibrium with each other. This means that CCR5Δ32 invariably occurs on a haplotype that is wild-type CCR2, whereas CCR2-64I occurs on a haplotype that contains wild-type CCR5. The CCR5Δ32 mutation is common only in individuals of Caucasian origin, whereas the CCR2-64I mutation is present in all ethnic groups(12,13).
As part of an ongoing study of genetic polymorphisms in children with IDDM(14), we have determined the frequency of the CCR5Δ32 and CCR2-64I alleles in children with IDDM and in nondiabetic subjects.
METHODS
Subjects. One hundred fifteen children with IDDM [age 1-14 (9.3 ± 4.3) y] were investigated. The mean age at onset of IDDM was 5.2 y (range 0.5-13 y). All patients were Hungarian and were treated in the Heim Pál Pediatric Hospital, Budapest, or in the 3rd Department of Internal Medicine, Semmelweis University of Budapest.
We have also determined the frequency of the two mutations in nondiabetic Hungarian children [n = 280, age 1-14 (8.5 ± 4.5) y]. The nondiabetic children were randomly selected from patients in the Heim Pál Pediatric Hospital.
All human studies were approved by an Institutional Review Board.
Protocols. Total genomic DNA was extracted from white blood cells by the method of Miller(15).
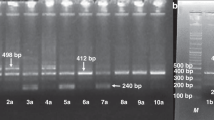
Genotyping of CCR5 was performed by DNA amplification by PCR by use of the CCR5-specific primer pair (F: 5′-CTT CAT TAC ACC TGC AGC TCT CA-3′; R: 5′-CAC AGC CCT GTG CCT CTT CTT CTC A-3′) that flanks the 32-bp deletion, separated in 4% agarose gel, and stained with ethidium bromide. The CCR2-64I mutation was determined with a PCR-RFLP assay using a BsaBI site introduced into the PCR primer next to the C-T transition. Amplification with the primers F: 5′-TTG TGG GCA ACA TG a TGG-3′, which has a cytosine substituted with an adenine (in lower case), and R: 5′- GAG CCC ACA ATG GGA GAG TA-3′ generated a 128-bp product. Digestion with BsaBI yields 110 and 18-bp fragments when an isoleucine is present instead of valine at position 64. The products were separated in 4% agarose gel and stained with ethidium bromide(12).
Statistical methods. Allele frequencies were calculated by allele counting. Hardy-Weinberg equilibrium was tested by using a χ2 goodness-of-fit test. Fischer's exact test was used to test for differences in allele distributions between the two groups.
RESULTS
The results are presented in Table 1. There were no significant differences in the frequency of CCR5Δ32 mutation between IDDM and healthy children. The CCR2-64I allele frequency in children with IDDM was 0.226, which differed significantly from the allele frequency in controls (0.114, p = 0.001). The results overall were in Hardy-Weinberg equilibrium.
DISCUSSION
The recently characterized CCR5 has rapidly become the object of interest because it has been found to be the major coreceptor on CD4+ cells for primary M-tropic HIV-1 strains(11). The mutant CCR5 allele, which carries the 32-bp deletion, is not expressed on the cell surface. Until now, no obvious physiologic defect has been found associated with the absence of functional CCR5. In the present study, we have found no differences in the frequency of CCR5Δ32 mutations between IDDM children and nondiabetic individuals, indicating that there is no association of the deletion with IDDM.
CCR2 can serve as a minor coreceptor for some strains of HIV-1 in the initial stages of infection. It has not yet been proven whether the 64I mutation impairs the function of the receptor(12) or is just a neutral polymorphism(16). The CCR2-64I allele frequency was significantly higher in children with IDDM than in the control group. The importance of this mutation in IDDM cannot be explained yet, but, because CCR2 mediates the chemotaxis of CD4+ and CD8+ T cells to areas of inflammation(9) and because these cells play important roles in insulitis, a mutation in the CCR2 gene may contribute to the susceptibility of contracting the disease. In animal models, efficient transfer of diabetes is achieved by spleen CD4+ T cells from diabetic and prediabetic donors(17). CD4+ T cells recognize antigens presented by class II major histocompatibility molecules that fail to be expressed by β cells. Thus CD4+ T cells are likely to act through either the release of soluble mediators (e.g. chemokines) or non-T-cellular effectors (e.g. activated macrophages, natural killer cells). Both macrophages and natural killer cells express CCR2 on their surfaces and migrate in response to monocyte chemotactic protein-1(3). The CCR2-64I mutation may influence both the expression and/or the response of the receptor and may contribute to the alteration of the immune system.
An alternative explanation is also possible: The 64I allele may be a marker of a linked mutation through linkage disequilibrium.
According to these results, the CCR2 gene may be a new candidate for the susceptibility locus of IDDM. Because no IDDM locus has been identified near 3p21 until now, further investigations using larger IDDM populations, animals, or cell cultures are urgently needed.
Abbreviations
- CCR5:
-
CC-chemokine receptor 5
- CCR2:
-
CC-chemokine receptor 2
- IDDM:
-
insulin-dependent diabetes mellitus
References
Wicker LS, Todd JA, Peterson LB 1995 Genetic control of autoimmune diabetes in NOD mouse. Annu Rev Immunol 13: 179–200
Lenschow DJ, Ho SC, Sattar H, Rhee L, Gray G, Nabavi N, Herold KC, Bluestone JA 1995 Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J Exp Med 181: 1145–1155
Baggiolini M 1998 Chemokines and leukocyte traffic. Nature 392: 565–568
Baggiolini M, Walz A, Kunkel SL 1994 Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv Immunol 55: 97–179
Mackay CR 1996 Chemokine receptors and T cell chemotaxis. J Exp Med 184: 799–802
Murphy PM 1996 Chemokine receptors: structure, function, and role in microbial pathogenesis. Cytokine Growth Fact Rev 7: 47–64
Daugherty BL, Springer MS 1997 The beta-chemokine receptor genes CCR1 (CMKBR1), CCR2 (CMKBR2), and CCR3 (CMKBR3) cluster within 285 kb on human chromosome 3p21. Genomics. 41: 294–295
Samson M, Labbe O, Mollereau C, Vassart G, Parmentier M 1996 Molecular cloning and functional expression of a new human CC-chemokine receptor gene. Biochemistry 35: 3362–3367
Baggiolini M, Dewald B, Moser B 1997 Human chemokines: an update. Annu Rev Immunol 15: 685–691
Loetscher P, Seitz M, Baggiolini M, Moser B 1996 Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J Exp Med 184: 569–577
Dean M, Carrington M, Winkler C, Huttley GA, Smith MW, Allikmets R, Goedert JJ, Buchbinder SS, Vittinghof E, Gomperts E, Donfield S, Vlahov D, Kaslow R, Saah A, Rinaldo C, Detels R 1996 Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science 273: 1856–1862
Smith MW, Dean M, Carrington M, Winkler C, Huttley GA, Lomb DA, Goedert JJ, O'Brien TR, Jacobson LP, Kaslow R, Buchbinder S, Vittinghof E, Vlahov D, Hoots K, Hilgartner MW 1997 Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Science 277: 959–965
Martinson JJ, Chapman NH, Rees DC, Liu YT, Clegg JB 1997 Global distribution of the CCR5 gene 32-basepair deletion. Nat Gen 16: 100–103
Szalai C, Császár A, Czinner A, Bihari-Varga M, Romics L 1997 Apolipoprotein A-IV and E polymorphisms in children with IDDM. Diabetes Care 20: 1926–1927
Miller SA, Dykes DD, Polesky HF 1988 A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16: 1215
Michael NL, Louie LG, Rohrbaugh AL, Schultz KA, Dayhoff DE, Wang CE, Sheppard HW 1997 The role of CCR5 and CCR2 polymorphism in HIV-1 transmission and disease progression. Nat Med 3: 1160–1162
Christianson SW, Shultz LD, Leither EH 1993 Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T cells from diabetic versus prediabetic NOD NON-Thy-1a donors. Diabetes 42: 44–55
Author information
Authors and Affiliations
Additional information
Supported by grants OTKA T-016111 and T-13225 from the Hungarian Scientific Academy and ETT T-07726/93 from the Hungarian Health Ministry.
Rights and permissions
About this article
Cite this article
Szalai, C., Császár, A., Czinner, A. et al. Chemokine Receptor CCR2 and CCR5 Polymorphisms in Children with Insulin-Dependent Diabetes Mellitus. Pediatr Res 46, 82–84 (1999). https://doi.org/10.1203/00006450-199907000-00014
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199907000-00014
This article is cited by
-
DNA methylation and cardiovascular disease in humans: a systematic review and database of known CpG methylation sites
Clinical Epigenetics (2023)
-
Treg cells in pancreatic lymph nodes: the possible role in diabetogenesis and β cell regeneration in a T1D model
Cellular & Molecular Immunology (2012)
-
The Consequence of a Founder Effect: CCR5-∆32, CCR2-64I and SDF1-3’A Polymorphism in Vlach Gypsy Population in Hungary
Pathology & Oncology Research (2012)