Abstract
Fetal-neonatal iron deficiency acutely alters hippocampal biochemistry, neural morphology, and electrophysiology accompanied by a downregulation of brain-derived neurotrophic factor (BDNF). These changes provide a cellular and molecular basis for observed short-term learning and memory impairments. However, the etiology of residual, long-term hippocampal neurotransmission abnormalities and learning impairments after treatment remain unclear. Because BDNF modulates learning and memory, we assessed its expression in 65-d-old formerly iron deficient (FID) male rats that had been iron deficient during the fetal-neonatal period and treated with iron since postnatal day 7. BDNF-III and -IV mRNAs and BDNF protein expression remained down-regulated in FID rats when compared with the always iron-sufficient rats. Expressions of BDNF activity-dependent downstream targets (3-hydroxy-3-methylglutaryl CoA reductase and immediate early genes c-fos, early growth response gene 1 and 2) were reduced in FID rats. In turn, hippocampal expressions of direct targets of early-growth response genes, including hypoxia-inducible factor 1, dual-specificity phosphatase 4, IGF 2, and myelin basic protein were also diminished in FID rats. Collectively, fetal-neonatal iron deficiency lowers hippocampal BDNF expression and function beyond the period of iron deficiency. These findings may underlie the persistence of learning deficits seen after fetal-neonatal iron deficiency.
Similar content being viewed by others
Main
Iron deficiency is a common early-life nutrient deficiency affecting approximately 30–50% pregnancies worldwide, including an estimated 80% of pregnancies in developing countries (1). Late gestational and neonatal iron deficiency in the offspring arises from four maternal conditions during pregnancy: severe iron deficiency anemia, placental vascular insufficiency resulting from maternal hypertension, diabetes mellitus, and cigarette smoking (2–5). In humans, neonatal iron deficiency causes deficits in cognitive function during the period of iron deficiency and poor school performance beyond the period of iron deficiency (6,7). Although certain neurodevelopmental deficits can be corrected with iron treatment, other behavioral and cognitive deficits persist more than 10 y after iron treatment (8). The neural basis of these long-term deficits remains unclear.
Evidence from animal models suggests that fetal, neonatal, and early postnatal iron deficiency influence myelination, monoamine metabolism, and energy metabolism (9–12). Acutely, fetal-neonatal brain iron deficiency results in impaired neuronal morphology, synaptic transmission, and increased susceptibility to infarction in the neonatal hippocampus (13–15). We have described the specific effects of fetal-neonatal iron deficiency on the expression of neurotrophic factors critical for inducing and maintaining hippocampal differentiation and plasticity (16). Hippocampal brain-derived neurotrophic factor (BDNF) expression is down-regulated whereas nerve growth factor (NGF), epidermal growth factor (EGF), and glial-derived neurotrophic factor (GDNF) are up-regulated during iron deficiency in rats (16).
BDNF regulates multiple aspects of hippocampal development and function (17–19). In particular, induction of long-term potentiation (LTP), a cellular phenomenon associated with memory formation, in the rodent hippocampus rapidly increases BDNF transcript levels (20–22). Suppression of BDNF expression and genetic deletion of BDNF lead to impairment of learning and memory (23,24). BDNF is a complex gene with multiple mRNA species, and transcripts III and IV are the most abundant in rat hippocampus (16). BDNF signaling is mediated by tyrosine-receptor kinase B (TrkB) and p75 neurotrophic receptor (p75NTR) (25). There are two known isoforms of TrkB, long (TrkBL) and short (TrkBS). TrkBS lacks the intracellular signaling domain (26). BDNF binding of TrkB promotes neurite outgrowth and synaptic plasticity, in part, through regulation of activity-dependent immediate early genes c-fos, early-growth-response-genes 1 and 2 (Egr1 and Egr2) (27–30). In contrast, BDNF binding of p75NTR facilitates long-term depression and reduces neurite outgrowth (31–33).
Although it is not surprising that multiple neural systems are dysfunctional while the brain is iron deficient, the underlying mechanisms for compromised function long after iron repletion remain unclear. Despite iron treatment beginning in the neonatal period, formerly iron-deficient (FID) adult rats continue to demonstrate deficits on hippocampal-dependent tasks consistent with similar findings in humans (34,35). The behavioral deficits in these rats corroborated the finding of lower LTP expression in a similar model (13). Given its role in modulating synaptic plasticity, we postulated lower BDNF activity in the hippocampus of FID rats. Here, we present evidence that fetal-neonatal iron deficiency continues to downregulate BDNF expression and activity beyond the period of iron deficiency, suggesting a long-term alteration in the programming of BDNF expression. Lower BDNF activity may be an important molecular underpinning for the persistent cognitive deficits in FID rats.
METHODS AND MATERIALS
Animals.
Timed-pregnant Sprague-Dawley rats were purchased from Harlan (Indianapolis, IN). Fetal-neonatal iron deficiency was induced as previously described to achieve a 40% loss of total brain iron at postnatal day (P) 10 (11), a degree of brain iron deficiency equivalent to that seen in newborn humans (3). In this model, the hippocampus remains iron deficient (ID) through P30 (25% loss) and is iron sufficient (IS) by P56 (13). In brief, pregnant dams were maintained on an ID diet (3 mg/kg iron, Rx247497) from gestational day 2 to P7, after which time the nursing dams were given the nonpurified IS control diet (198 mg/kg iron, Rx 241632), both diets were purchased from Harlan Teklad (Madison, WI). IS control animals were given the IS diet throughout the experiment. Litters were culled to 8 pups/litter, all pups were weaned at P21 and fed IS diet for the duration of the experiment. All animal experiments were performed with the approval of the University of Minnesota Institutional Animal Care and Use Committee.
Iron concentration.
Brain iron concentrations were measured as previously described (13). In brief, deeply anesthetized P65 rats (intraperitoneal injection of 100 mg/Kg Beuthanasia) were perfused transcardially with 0.9% NaCl. The hippocampus was dissected and rinsed in saline solution. Hippocampal wet weight was recorded and the tissue was lyophilized for 72 h. Lyophilized tissue was digested with 4:1 nitric/perchloric acid solution for 5 d. Iron concentration was determined by atomic absorption spectroscopy using a standard curve generated from stock iron standards (Sigma Chemical Co.).
Quantitative RT-PCR (qPCR).
P65 male rats were killed by an intraperitoneal injection of Beuthanasia (100 mg/kg). Brains were removed and bisected along the midline. Hippocampus was dissected and flash frozen. Total RNA was isolated from dissected hippocampus using an RNA-isolation kit (Stratagene, La Jolla, CA). Approximately, 4 μg of total RNA was used to generate cDNA using SuperScript III (Invitrogen, Carlsbad, CA) and random hexamer primers, and cDNA was diluted 7-fold. qPCR experiments were performed with 1/2 the manufacturer's recommended volume (Applied Biosystems Inc., Foster City, CA). Data were collected using a MX3000P instrument (Stratagene, La Jolla, CA). Information about the qPCR probes/primers is listed in Table 1.
Immunohistology.
Immunohistochemistry was performed as previously described (16). In brief, 20 μm coronal sections were rehydrated in Tris buffer saline pH 7.6 (TBS). Antigen unmasking was performed by immersing sections in hot (95°C) 10 mM Na-citrate pH 8.6. Sections were permeabilized in TBS + 0.2% Triton X-100, incubated in blocking solution (10 g/L BSA diluted in TBS + 0.1% Tween-20), and then incubated in primary antibody overnight at 4°C. Excess antibody was removed with TBS + 0.1% Tween-20 (TBST) rinses (3X). Sections were retreated with blocking solution and, incubated in fluorescence-labeled secondary antibody overnight at 4°C. Sections were washed with TBST and mounted in aqueous mounting media with DAPI (Vector laboratories, Inc., Burlingame, CA). Antibodies included biotin-conjugated anti-BDNF (5 mg/L) chicken polyclonal (A&D system, Minneapolis, MN), anti-NGF (1:100) rabbit monoclonal (Cell Signaling, Danvers, MA), and anti-p75NTR (1:100) rabbit polyclonal (A gift from Dr. William Engeland, University of Minnesota). Fluorescence-labeled secondary antibodies were used according to the manufacturer's recommendation (Invitrogen, Eugene, OR). Sytox Green (Invitrogen) was used to stain nuclear DNA in some experiments. Confocal images were captured with a Nikon Digital-Eclipse C1 microscope system (Nikon, Japan).
Western blot analysis.
Protein isolation was performed as described previously (36). Approximately, 31 μg of total protein was loaded and separated in 12% and 4–12% gradient SDS-PAGE gels (Invitrogen, Carlsbad, CA) and transferred onto nitrocellulose membranes (Pierce, Rockford, IL). Membranes were blocked in blocking buffer for near infrared fluorescent Western Blotting (Rockland, Gilbertsville, PA) for 1 h at room temperature and incubated overnight at 4°C in primary antibody diluted in Blocking Buffer. Membranes were rinsed in PBS with 0.1% Tween-20 (4X) to remove excess antibody and then incubated in fluorescent secondary antibody diluted in Blocking Buffer with 0.1% Tween-20 and 0.01% SDS at room temperature for 45 min, and rinsed 4X in PBS with 0.1% Tween-20 to remove excess antibody. Membranes were imaged with Odyssey Infrared Imaging System (Li-cor Biosciences, Lincoln, NE) and the integrated intensity of the protein of interest was quantified and normalized to actin. The primary antibodies included anti-BDNF (1:1000) rabbit polyclonal (Abcam, Cambridge, MA), anti-TrkB (1:1000) rabbit monoclonal, anti-NGF (1:1000) rabbit monoclonal, anti-p44/42 MAPK (Thr202/Tyr204) (1:1000) rabbit monoclonal (Cell Signaling, Danvers, MA), anti-p75NTR (1:2000) rabbit polyclonal, and anti-actin (1:5000) mouse monoclonal (Sigma Chemical Co., St. Louis, MO). Secondary antibodies included Alexa Fluor 680 conjugated anti-mouse (1:12500) (Invitrogen, Carlsbad, CA) and Infrared Dye 800 conjugated anti-rabbit antibody (1:12500) (Rockland, Gilbertsville, PA).
Statistical methods.
Data for transcript and protein levels were collected from six male rats for each dietary group. Data were analyzed by a nonparametric Mann-Whitney U test, with significance set at alpha <0.05. Graphs and statistical calculations were performed with GraphPad Prism (GraphPad Software Inc., San Diego, CA).
RESULTS
Hippocampal iron status at P65.
At P65, the mean hippocampal iron concentration of the FID group was not different from the IS control group (IS: 6.79 ± 0.78 μg Fe/g wet weight, n = 7; FID: 6.66 ± 0.84 μg Fe/g wet weight, n = 7; p = 1.00).
Decreased BDNF and TrkB expression in P65 FID hippocampus.
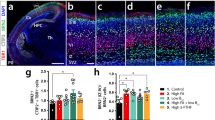
P65 FID rats had lower BDNF-III and -IV mRNA and protein levels compared with control IS rats (Fig. 1A and B). Expression of the total and long form of the BDNF receptor TrkB was lower in the FID group compared with controls (Fig. 1C). The protein level of TrkBL in the FID group was similar to IS control, but TrkBS level was lower (Fig. 1D). TrkBS is expressed at a higher level than TrkBL in the control hippocampus, but not in the FID (Fig. 1D, IS). The levels of p75NTR mRNA (Table 2) and protein (data not shown) were not different between groups. The mRNAs of other growth factors important for neuronal function, including ciliary neurotrophic factor, connective tissue growth factor, EGF, GDNF, and NGF, were not different between control and FID P65 rats (Table 2). Protein levels of NGF were also similar between groups (data not shown). Immunohistologic analysis of hippocampal BDNF, NGF, and p75NTR protein revealed no apparent difference in localization between IS control and FID rats (Fig. 2).
Reduced BNDF and TrkB expression in P65 FID hippocampus. (A) BDNF mRNA levels. (B) BDNF protein levels. (C) Total TrkB and TrkBL mRNA levels. (D) TrkB protein levels. Data are normalized to control (IS) group. Values represent mean ± SEM, n = 4–6. Asterisks denote significant p values. *p < 0.05, **p < 0.01.
Localization of BDNF, NGF, and p75NTR in P65 CA1 hippocampus. (A and B) Similar expression pattern of BDNF (Red) and p75NTR (Green) in IS control (A) and FID (B) hippocampus. Arrows show BDNF+ pyramidal CA1 neurons. Arrowheads indicate p75NTR+ neurites. (C and D) NGF protein (Red) expression in IS control (C) and FID (D) hippocampus. Arrowheads show NGF presence in pyramidal neurons. Nuclei counterstained with Sytox Green. P, pyramidal layer; sr, stratum radiatum. Scale bar = 50 μm.
Reduced BDNF-regulated target gene expression in FID hippocampus.
Transcript levels of several known activity-dependent targets of BDNF signaling were examined to assess BDNF activity in FID hippocampus. These include the 3-hydroxy-3-methylglutaryl CoA reductase (HMGCR) and the immediate early genes c-fos, Egr1 and Egr2 (29,30,37). Consistent with lowered BDNF activity, levels of these target genes were decreased in P65 FID rats (Fig. 3).
Reduced expression of Egr1- and Egr2-regulated target genes in P65 FID hippocampus.
Expression of genes regulated by the transcription factors Egr1 and Egr2 was measured to demonstrate the consequences of lower BDNF activity beyond immediate effectors. These included hypoxia-inducible factor 1α (hif1α) and dual-specificity phosphatase 4 (Dusp4), which are targets of Egr1 (38,39), and IGF 2 (IGF-II) and myelin basic protein (Mbp), which are targets of Egr2 (40,41). Consistent with reduced Egr1 and Egr2 levels, transcript levels of these targets were lowered in P65 FID hippocampus (Fig. 4). Furthermore, phosphorylated extracellular signal-regulated kinases 1 and 2 (ERK1/2) were increased in FID compared with IS rats (Fig. 4C), in line with decreased Dusp4 phosphatase activity in the FID group (Fig. 4B).
Reduced expression of Egr1 and Egr2 target genes in P65 FID hippocampus. (A-C) mRNA levels of Egr1 targets, Hif1α (A) and Dusp4 (B). Increased level of phosphorylated ERK1/2 (P-ERK1/2) in FID hippocampus (C). (D and E) Reduced mRNA levels of IGF-II (D) and Mbp (E) in FID hippocampus compared with IS control. Data are normalized to control (IS) group. Values are mean ± SEM, n = 4–6. Asterisks denote significant p values. *p < 0.05, **p < 0.01.
DISCUSSION
We and others have demonstrated that early iron deficiency results in long-term behavioral and cognitive deficits beyond the period of fetal-neonatal iron deficiency anemia. In particular, fetal-neonatal iron deficiency compromises hippocampal-dependent learning and memory formation in adult FID rats (34). Here, we provide evidence that early iron deficiency results in long-term decreases in BDNF expression and activity without compensatory increases in its receptor TrkB. BDNF and its downstream targets influence a diverse cascade of molecular mediators of synaptic plasticity, which, in turn, form the basis for learning and memory formation (Fig. 5). Reduced hippocampal BDNF expression in FID rats negatively affects this cascade. We speculate that these findings provide molecular bases for the long-term electrophysiologic, morphologic, and ultimately, behavioral abnormalities that persist after fetal-neonatal iron deficiency (8,13,14,34,35,42,43).
BDNF normally induces neuronal HMGCR, the rate-limiting enzyme in cholesterol synthesis that facilitates synaptic vesicle formation (37). Lower HMGCR expression in the FID hippocampus suggests that vesicle formation may be compromised. Combined with lower levels of synaptobrevin I (36), a protein involved in vesicle fusion (44), this finding may contribute to impaired paired-pulse facilitation and reduced synaptic efficacy (LTP) in this model (13). Paired-pulse facilitation and LTP are important indices of neuroelectrophysiologic events during neurotransmission and LTP is widely accepted as a cellular substrate for learning and memory (45,46).
BDNF also normally induces the expression of c-fos, Egr1, and Egr2, activity-dependent immediate early transcription factors that facilitate LTP in the hippocampus (27). Lower expression of c-fos, Egr1, and Egr2 may contribute to reduced plasticity in the hippocampus of FID rats. Lower c-fos expression not only leads to a reduction in expression of genes necessary for LTP (47,48) but also may influence a feed-forward loop, further reducing BDNF activity (49,50).
Reduced expression of Egr1 was accompanied by lower expression of its known target genes, hif1α and Dusp4, in the hippocampus of FID rats. Hif1α is an oxidative-state–dependent transcription factor that regulates chemokine (C-X-C motif) ligand 12 (Cxcl12), an important modulator of synaptic formation (51). Cxcl12 mRNA expression is reduced in FID rats (36). These changes, when combined with similar long-term reductions in postsynaptic density 95 (PSD95) and calmodulin-dependent kinase IIα (CamKIIα), may account for the abnormal dendritic length and branching in FID hippocampal neurons (14,36). DUSP4 (protein) is a dual-specificity phosphatase targeting ERK1 and ERK2, factors phosphorylated during formation of memory (52–54). Reduction of DUSP4 activity could further contribute to impaired synaptic plasticity by dampening neuronal responsiveness to stimulation. Alternatively, sustained ERK1/2 phosphorylation may compensate for already lowered neural plasticity in FID rats.
BDNF-mediated reduction in Egr2 expression resulted in reduced expression of its target genes, Mbp and IGF-II that are important for myelin health and glial contributions to plasticity. Mbp is a complex gene with multiple splice variants, encoding a major component of the myelin sheath of oligodendrocytes. In the adult brain, IGF-II is expressed only in astroglia (55) and regulates myelin-associated protein genes (56). Decreased Mbp and IGF-II expression together may contribute to impairment of myelination and consequently alter neural transmission as seen in P65 FID hippocampus (13). Whole brain Mbp expression is acutely reduced during iron deficiency (57–59); however, the findings in the hippocampus of FID animals provide evidence for a long-term effect of early iron deficiency on the health and function of astrocytes and oligodendrocytes.
The long-term abnormalities in hippocampal function despite iron repletion could be ascribed to changes in structure resulting from lack of iron during critical periods of development or to long-term dysregulation of genes important for experience-dependent plasticity throughout the lifespan. These are not mutually exclusive conditions. We have previously confirmed the former possibility by demonstrating persistently abnormal dendritic structure at P65 in this model (14). Dendritogenesis peaks between P15 and P25 in the hippocampus and iron deficiency during this time period induces substantial alterations in branching and elongation during that time period (14). Complete repletion of hippocampal iron status does not occur in this model until after the end of this critical window and it seems that structural abnormalities induced at the early age remain present in young adulthood.
The current study provides evidence for the second possibility, suggesting a role for iron in long-term programming of hippocampal BDNF and its downstream effectors. The mechanisms mediating the effect are unknown, but may be similar to those involved in the developmental origins of health and disease (60), including epigenetic modifications of genes. Our results emphasize the concept that provision of nutrients alone is not adequate to maintain optimal brain function. Rather, proper regulation of growth factors is also essential to ensure optimal utilization of nutrients. Long-term dysregulation of these growth factors may thus account for persistent abnormal function despite nutrient repletion.
In summary, our findings begin to provide insights into a possible molecular mechanism underpinning long-term cognitive deficits in this model. In addition, this study suggests that interventions that enhance BDNF activity, such as exercise or selective serotonin reuptake inhibitors (61,62), may be useful as therapeutic approaches to treat long-term effects of fetal-neonatal iron deficiency.
Abbreviations
- BDNF:
-
brain-derived neurotrophic factor
- Dusp4:
-
dual specificity phosphatase 4
- Egr1 and 2:
-
early-growth-response-gene 1 and 2
- ERK1 and 2:
-
extracellular-signal regulated kinase 1 and 2
- FID:
-
formerly iron deficient
- Hif1α:
-
hypoxia inducible factor 1α
- HMGCR:
-
3-hydroxy-3-methylglutaryl coenzyme A reductase
- ID:
-
iron deficient
- IS:
-
iron sufficient
- LTP:
-
long-term potentiation
- Mbp:
-
myelin basic protein
- NGF:
-
nerve growth factor
- p75NTR:
-
p75 neurotrophic receptor
- P:
-
postnatal day
- qPCR:
-
quantitative RT-PCR
- TrkB:
-
tyrosine-receptor kinase B
References
Rao R, Georgieff MK 2007 Iron in fetal and neonatal nutrition. Semin Fetal Neonatal Med 12: 54–63
Georgieff MK, Wewerka SW, Nelson CA, Deregnier RA 2002 Iron status at 9 months of infants with low iron stores at birth. J Pediatr 141: 405–409
Petry CD, Eaton MA, Wobken JD, Mills MM, Johnson DE, Georgieff MK 1992 Iron deficiency of liver, heart, and brain in newborn infants of diabetic mothers. J Pediatr 121: 109–114
Chockalingam UM, Murphy E, Ophoven JC, Weisdorf SA, Georgieff MK 1987 Cord transferrin and ferritin values in newborn infants at risk for prenatal uteroplacental insufficiency and chronic hypoxia. J Pediatr 111: 283–286
Sweet DG, Savage G, Tubman TR, Lappin TR, Halliday HL 2001 Study of maternal influences on fetal iron status at term using cord blood transferrin receptors. Arch Dis Child Fetal Neonatal Ed 84: F40–F43
Siddappa AM, Georgieff MK, Wewerka S, Worwa C, Nelson CA, Deregnier RA 2004 Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr Res 55: 1034–1041
Lozoff B, Georgieff MK 2006 Iron deficiency and brain development. Semin Pediatr Neurol 13: 158–165
Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T 2006 Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev 64: S34–S91
Connor JR, Menzies SL 1996 Relationship of iron to oligodendrocytes and myelination. Glia 17: 83–93
Beard J, Erikson KM, Jones BC 2003 Neonatal iron deficiency results in irreversible changes in dopamine function in rats. J Nutr 133: 1174–1179
Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK 2003 Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr 133: 3215–3221
deUngria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK 2000 Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res 48: 169–176
Jorgenson LA, Sun M, O'Connor M, Georgieff MK 2005 Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus 15: 1094–1102
Jorgenson LA, Wobken JD, Georgieff MK 2003 Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci 25: 412–420
Rao R, Tkac I, Townsend EL, Ennis K, Gruetter R, Georgieff MK 2007 Perinatal iron deficiency predisposes the developing rat hippocampus to greater injury from mild to moderate hypoxia-ischemia. J Cereb Blood Flow Metab 27: 729–740
Tran PV, Carlson ES, Fretham SJ, Georgieff MK 2008 Early-life iron deficiency anemia alters neurotrophic factor expression and hippocampal neuron differentiation in male rats. J Nutr 138: 2495–2501
Hall J, Thomas KL, Everitt BJ 2000 Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci 3: 533–535
Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG 2003 Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci 17: 2042–2046
Tapia-Arancibia L, Rage F, Givalois L, Arancibia S 2004 Physiology of BDNF: focus on hypothalamic function. Front Neuroendocrinol 25: 77–107
Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M 1992 Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron 9: 1081–1088
Castren E, Pitkanen M, Sirvio J, Parsadanian A, Lindholm D, Thoenen H, Riekkinen PJ 1993 The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus. Neuroreport 4: 895–898
Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A 2002 Postsynaptic induction of BDNF-mediated long-term potentiation. Science 295: 1729–1734
Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T 1995 Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A 92: 8856–8860
Heldt SA, Stanek L, Chhatwal JP, Ressler KJ 2007 Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry 12: 656–670
Chao MV 2003 Neurotrophins and their receptors: a convergence point for many signaling pathways. Nat Rev Neurosci 4: 299–309
Klein R, Conway D, Parada LF, Barbacid M 1990 The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell 61: 647–656
Alder J, Thakker-Varia S, Bangasser DA, Kuroiwa M, Plummer MR, Shors TJ, Black IB 2003 Brain-derived neurotrophic factor-induced gene expression reveals novel actions of VGF in hippocampal synaptic plasticity. J Neurosci 23: 10800–10808
Koponen E, Lakso M, Castren E 2004 Overexpression of the full-length neurotrophin receptor trkB regulates the expression of plasticity-related genes in mouse brain. Brain Res Mol Brain Res 130: 81–94
Calella AM, Nerlov C, Lopez RG, Sciarretta C, von Bohlen und Halbach O, Bereshchenko O, Minichiello L 2007 Neurotrophin/Trk receptor signaling mediates C/EBPalpha, -beta and NeuroD recruitment to immediate-early gene promoters in neuronal cells and requires C/EBPs to induce immediate-early gene transcription. Neural Develop 2: 4
Rossler OG, Thiel G 2004 Brain-derived neurotrophic factor-, epidermal growth factor-, or A-Raf-induced growth of HaCaT keratinocytes requires extracellular signal-regulated kinase. Am J Physiol Cell Physiol 286: C1118–C1129
Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B 2005 Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci 8: 1069–1077
Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, Zang K, Wang D, Nicoll RA, Lu B, Reichardt LF 2000 The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J Neurosci 20: 6888–6897
Zagrebelsky M, Holz A, Dechant G, Barde YA, Bonhoeffer T, Korte M 2005 The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J Neurosci 25: 9989–9999
Schmidt AT, Waldow KJ, Grove WM, Salinas JA, Georgieff MK 2007 Dissociating the long-term effects of fetal/neonatal iron deficiency on three types of learning in the rat. Behav Neurosci 121: 475–482
Felt BT, Beard JL, Schallert T, Shao J, Aldridge JW, Connor JR, Georgieff MK, Lozoff B 2006 Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res 171: 261–270
Carlson ES, Stead JD, Neal CR, Petryk A, Georgieff MK 2007 Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus 17: 679–691
Suzuki S, Kiyosue K, Hazama S, Ogura A, Kashihara M, Hara T, Koshimizu H, Kojima M 2007 Brain-derived neurotrophic factor regulates cholesterol metabolism for synapse development. J Neurosci 27: 6417–6427
Berasi SP, Huard C, Li D, Shih HH, Sun Y, Zhong W, Paulsen JE, Brown EL, Gimeno RE, Martinez RV 2006 Inhibition of gluconeogenesis through transcriptional activation of EGR1 and DUSP4 by AMP-activated kinase. J Biol Chem 281: 27167–27177
Sperandio S, Fortin J, Sasik R, Robitaille L, Corbeil J, de Belle I 2008 The transcription factor Egr1 regulates the HIF-1alpha gene during hypoxia. Mol Carcinog 48: 38–44
Gillian AL, Svaren J 2004 The Ddx20/DP103 dead box protein represses transcriptional activation by Egr2/Krox-20. J Biol Chem 279: 9056–9063
Jang SW, LeBlanc SE, Roopra A, Wrabetz L, Svaren J 2006 In vivo detection of Egr2 binding to target genes during peripheral nerve myelination. J Neurochem 98: 1678–1687
Felt BT, Lozoff B 1996 Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J Nutr 126: 693–701
McEchron MD, Cheng AY, Liu H, Connor JR, Gilmartin MR 2005 Perinatal nutritional iron deficiency permanently impairs hippocampus-dependent trace fear conditioning in rats. Nutr Neurosci 8: 195–206
Schiavo G, Stenbeck G, Rothman JE, Sollner TH 1997 Binding of the synaptic vesicle v-SNARE, synaptotagmin, to the plasma membrane t-SNARE, SNAP-25, can explain docked vesicles at neurotoxin-treated synapses. Proc Natl Acad Sci U S A 94: 997–1001
Silva AJ 2003 Molecular and cellular cognitive studies of the role of synaptic plasticity in memory. J Neurobiol 54: 224–237
Malenka RC 2003 The long-term potential of LTP. Nat Rev Neurosci 4: 923–926
Miyamoto E 2006 Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. J Pharmacol Sci 100: 433–442
Platenik J, Kuramoto N, Yoneda Y 2000 Molecular mechanisms associated with long-term consolidation of the NMDA signals. Life Sci 67: 335–364
Dong M, Wu Y, Fan Y, Xu M, Zhang J 2006 c-fos modulates brain-derived neurotrophic factor mRNA expression in mouse hippocampal CA3 and dentate gyrus neurons. Neurosci Lett 400: 177–180
Fleischmann A, Hvalby O, Jensen V, Strekalova T, Zacher C, Layer LE, Kvello A, Reschke M, Spanagel R, Sprengel R, Wagner EF, Gass P 2003 Impaired long-term memory and NR2A-type NMDA receptor-dependent synaptic plasticity in mice lacking c-Fos in the CNS. J Neurosci 23: 9116–9122
Klein RS, Rubin JB 2004 Immune and nervous system CXCL12 and CXCR4: parallel roles in patterning and plasticity. Trends Immunol 25: 306–314
Blum S, Moore AN, Adams F, Dash PK 1999 A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci 19: 3535–3544
Guan KL, Butch E 1995 Isolation and characterization of a novel dual specific phosphatase, HVH2, which selectively dephosphorylates the mitogen-activated protein kinase. J Biol Chem 270: 7197–7203
Kelly A, Laroche S, Davis S 2003 Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J Neurosci 23: 5354–5360
Rotwein P, Burgess SK, Milbrandt JD, Krause JE 1988 Differential expression of insulin-like growth factor genes in rat central nervous system. Proc Natl Acad Sci U S A 85: 265–269
Ye P, Li L, Richards RG, DiAugustine RP, D'Ercole AJ 2002 Myelination is altered in insulin-like growth factor-I null mutant mice. J Neurosci 22: 6041–6051
Badaracco ME, Ortiz EH, Soto EF, Connor J, Pasquini JM 2008 Effect of transferrin on hypomyelination induced by iron deficiency. J Neurosci Res 86: 2663–2673
Beard JL, Wiesinger JA, Connor JR 2003 Pre- and postweaning iron deficiency alters myelination in Sprague-Dawley rats. Dev Neurosci 25: 308–315
Clardy SL, Wang X, Zhao W, Liu W, Chase GA, Beard JL, True Felt B, Connor JR 2006 Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. J Neural Transm Suppl 71: 173–196
Gluckman PD, Hanson MA, Cooper C, Thornburg KL 2008 Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359: 61–73
Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW 1996 Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res 726: 49–56
Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW 2000 Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience 101: 305–312
Acknowledgements
We thank Dr. Raghavendra Rao for reviewing this manuscript and Heather McLaughlin for providing editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by NICHD RO1 HD29421 to M.K.G., and NIMH training grant T32MH073129 to P.V.T.
Rights and permissions
About this article
Cite this article
Tran, P., Fretham, S., Carlson, E. et al. Long-Term Reduction of Hippocampal Brain-Derived Neurotrophic Factor Activity After Fetal-Neonatal Iron Deficiency in Adult Rats. Pediatr Res 65, 493–498 (2009). https://doi.org/10.1203/PDR.0b013e31819d90a1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e31819d90a1
This article is cited by
-
Liraglutide Alleviates Cognitive Deficit in db/db Mice: Involvement in Oxidative Stress, Iron Overload, and Ferroptosis
Neurochemical Research (2022)
-
Fetal inflammation induces acute immune tolerance in the neonatal rat hippocampus
Journal of Neuroinflammation (2021)
-
RAPIDIRON: Reducing Anaemia in Pregnancy in India—a 3-arm, randomized-controlled trial comparing the effectiveness of oral iron with single-dose intravenous iron in the treatment of iron deficiency anaemia in pregnant women and reducing low birth weight deliveries
Trials (2021)
-
Iron as a model nutrient for understanding the nutritional origins of neuropsychiatric disease
Pediatric Research (2019)
-
A systematic literature review of the relation between iron status/anemia in pregnancy and offspring neurodevelopment
European Journal of Clinical Nutrition (2019)