Abstract
Even brief interruption of cardiac compressions significantly reduces critical coronary perfusion pressure during cardiopulmonary resuscitation (CPR). End-tidal CO2 (ETCO2) monitoring may provide a continuous noninvasive method of assessing return of spontaneous circulation (ROSC) without stopping to auscultate for heart rate (HR). However, the ETCO2 value that correlates with an audible HR is unknown. Our objective was to determine the threshold ETCO2 that is associated with ROSC after asphyxia-induced asystole. Neonatal swine (n = 46) were progressively asphyxiated until asystole occurred. Resuscitation followed current neonatal guidelines with initial ventilation with 100% O2 followed by cardiac compressions followed by epinephrine for continued asystole. HR was auscultated every 30 s, and ETCO2 was continuously recorded. A receiver operator curve was generated using the calculated sensitivity and specificity for various ETCO2 values, where a positive test was defined as the presence of HR >60 bpm by auscultation. An ETCO2 cut-off value of 14 mm Hg is the most sensitive ETCO2 value with the least false positives. When using ETCO2 to guide uninterrupted CPR in this model of asphyxia-induced asystole, auscultative confirmation of return of an adequate HR should be performed when ETCO2 ≥14 mm Hg is achieved. Correlation during human neonatal CPR needs further investigation.
Similar content being viewed by others
Main
Although the need for neonatal cardiopulmonary resuscitation (CPR) in the delivery room is rare (1,2), morbidity and mortality rates are extremely high for newborns requiring CPR (3). During CPR, it is critical that adequate coronary perfusion should be achieved to establish return of spontaneous circulation (ROSC) (4–7). Adult clinical data and experimental models of ventricular fibrillation-induced cardiac arrest demonstrate that even brief interruption of compressions significantly reduces blood flow and coronary perfusion pressure and reduces ROSC and survival (8–10). No information is available from neonatal models of asphyxia-induced cardiac arrest.
Current adult CPR guidelines recommend a ratio of 30 compressions to 2 breaths for medical providers to limit interruptions of compressions (11) and even uninterrupted compression-only CPR for lay-providers in the field (12). Pediatric life support guidelines for medical providers emphasize a chest compression/ventilation ratio of 15:2 for all children except neonates (11). American Academy of Pediatrics/American Heart Association Neonatal Resuscitation Program (NRP) guidelines recommend a ratio of 3 compressions to 1 breath and pausing every 30 s to auscultate for return of a heart rate (HR) (13). Such frequent interruptions of compressions may limit the ability to achieve an adequate coronary perfusion pressure for ROSC.
End-tidal CO2 (ETCO2) monitoring is a noninvasive tool that has been shown to predict and demonstrate ROSC during experimental and human adult cardiac arrest (14–18). Carbon dioxide is produced by cellular metabolism and is subsequently transported by the venous system to the right heart, where it is pumped into the lungs and diffuses into the exhaled air where it can be measured as ETCO2 (19). Thus, CO2 production, alveolar ventilation, and pulmonary perfusion interact to determine ETCO2. When CO2 production and cardiac output are stable, ETCO2 changes are typically due to changes in alveolar ventilation. During CPR, if ventilation is held constant and CO2 production is assumed to be very low and thus constant, excretion of CO2 through the lungs depends on pulmonary perfusion and therefore relates to cardiac output (14,20–22).
We hypothesized that ETCO2 monitoring might provide a continuous noninvasive method of assessing ROSC without interrupting compressions during neonatal CPR. As a first step, we needed to determine whether there is a consistent threshold ETCO2 that is associated with return of an audible HR >60 bpm after asphyxia-induced asystole. The infrequent and unexpected need for neonatal CPR coupled with the difficulty of obtaining informed consent for such studies has impeded the design and completion of rigorous delivery room CPR studies. Thus, we designed an animal study to determine the threshold ETCO2 that is associated with ROSC during CPR in asphyxiated, asystolic neonatal piglets whose cardiopulmonary compromise parallels that of asphyxiated infants who require CPR at birth.
METHODS
This investigation was approved by the Institutional Review Board for Animal Research at The University of Texas Southwestern Medical Center at Dallas.
Surgical preparation.
A convenience sample of 46 American domestic swine (mean ± SD: weight, 2.2 ± 0.6 kg; postnatal age, 8 ± 4 d) were studied. All piglets were administered ketamine (20 mg/kg i.m.) as premedication and then were instrumented under pentobarbital anesthesia of 20 mg/kg i.v. bolus followed by 10 mg/kg/h with additional boluses as needed to prevent spontaneous breathing. All surgical sites were infiltrated with 1% xylocaine (Steris Laboratories Inc., Phoenix, AZ). A tracheotomy was performed with placement of a 3.5-mm endotracheal tube. Piglets were ventilated (Harvard Apparatus Rodent Respirator, model 680; Millis, MA) with 70% nitrous oxide and 30% O2 using rates of 60 breaths/min and tidal volume adjusted to achieve arterial partial pressure of CO2 (PaCO2) in the mid-40s mm Hg range during the stabilization period. Catheters were positioned by aseptic technique in the right and left external jugular vein, the left internal jugular vein, and the left common carotid artery and left femoral artery. After catheter placement, the inspired gas was changed to 70% nitrogen and 30% O2. The piglet's body temperature was maintained between 38 and 39°C, using a thermal blanket wrapped around the body and circulating warm water (40–45°C) through the blanket.
Experimental protocol (Fig. 1).
After instrumentation, animals were allowed to stabilize for 60 min before acquisition of baseline measurements. Asphyxia was induced by changing ventilatory gases to 7.5% CO2 and 5.3% O2, and the ventilator rate was reduced by 10 breaths/min for every 15 min until asystole occurred. Asystole was defined as mean arterial pressure = 0 mm Hg on the continuous blood pressure tracing and confirmed by auscultation of an absent HR. Resuscitation was implemented by a four-member NRP-trained resuscitation team. One team member was assigned to each of the following roles: 1) positive pressure ventilation (PPV) with a self-inflating bag, 2) manual chest compressions, 3) blood sampling and administration of i.v. medications, and 4) code supervisor, who coordinated the timing and sequence of resuscitation interventions as defined in the protocol (Table S1, http://links.lww.com/PDR/A67). Once asystole occurred, the initial steps of neonatal resuscitation (positioning, suctioning, and stimulation) were simulated for 30 s. Asphyxia was reversed by initiating resuscitation using PPV via a self-inflating anesthesia bag with 100% inspired O2. Resuscitation continued with the initiation of manual cardiac compressions. Depth of compressions and compressor fatigue were assessed by real-time evaluation of the aortic compression pressures as displayed on the monitor. Our goals were to achieve an aortic compression pressure around 50–60 mm Hg. Compressions were followed by 0.01–0.03 mg/kg i.v. epinephrine doses in 3-min intervals in accordance with NRP guidelines until ROSC (defined as a HR ≥60 bpm) occurred. If there was no ROSC after 15 min of resuscitation, resuscitation efforts ceased. If ROSC was achieved, the piglet was maintained on the ventilator for 2 h without additional medications or interventions before euthanasia (using 200 mg/kg pentobarbital) unless death occurred early.
Measurements.
HR and arterial blood pressure were recorded on either a 2-channel recorder (220; Gould, Oxnard, CA) or a Power Lab data acquisition system (model 16/30; AD Instruments, CO Springs, CO). Other measures, including O2 saturation, HR, ETCO2, and minute ventilation were recorded with a CO2SMO Plus Respiratory Profile Monitor (Novametrix, Wallingford, CT). These vital signs and respiratory parameters were continuously monitored. Blood chemistries (arterial blood gas, hematocrit, lactate, and glucose) were obtained at control and subsequently every 15 min during asphyxia, every 3 min during the resuscitation phase, and every 30 min during the postresuscitation phase. The arterial pH, Pco2, and Po2 were analyzed by an Instrumentation Laboratory Micro gas analyzer (15 μL of blood). The microhematocrit method was used to determine hematocrit. Serum glucose concentrations were measured using an Accu-Chek Advantage glucometer (Roche Diagnostics, Indianapolis, IN). The plasma concentration of lactic acid was determined by a quantitative enzymatic determination assay (Sigma Chemical Co., St. Louis, MO).
Statistical analysis.
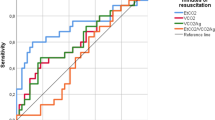
Data analysis was completed using Sigma Stat 11.0 (SPSS, Chicago, IL). The results are reported as the mean ± SD. Nonparametric analyses were used when indicated. A p-value ≤0.05 was considered statistically significant. A receiver operator characteristic (ROC) curve (a plot of the true positives against the false positives for different test cut-off points) was generated for various ETCO2 values where a positive test was defined as an ETCO2 value that was associated with return of a HR >60 bpm. The optimal ETCO2 cut-off value on the curve was selected based on a combination of a priori criteria including 1) significant area under the curve compared with a 45° line of equality, 2) optimum sensitivity and specificity values, 3) maximum perpendicular distance (d) above the 45° line of equality, and 4) the highest proportion of correct predictions (accuracy).
RESULTS
At baseline before initiation of asphyxia, piglets had HR, pH, PaCO2, ETCO2, lactate, and glucose levels similar to healthy term neonates (Table 1). The mean time from initiation of asphyxia to asystole was 48 ± 13 min. As expected with asphyxiation, by the time asystole occurred, the piglets developed a severe mixed respiratory and metabolic acidosis similar to infants who require CPR in the delivery room (3). ETCO2 levels had climbed concordant with the respiratory acidemia.
The actual mean (±SD) aortic compression (systolic) and relaxation (diastolic) pressures attained with this experimental neonatal CPR model were the following: 1) 1 min after initiation of CPR, the mean systolic compression pressure was 56 ± 8 mm Hg, with a diastolic pressure of 4 ± 2 mm Hg; 2) Immediately after the first dose of epinephrine, the mean systolic compression pressure remained 60 ± 11 mm Hg, with a diastolic pressure of 5 ± 3 mm Hg; 3) Immediately before ROSC, mean systolic compression pressure increased to 95 ± 9 mm Hg, with increased diastolic pressures of 19 ± 3 mm Hg.
A representative blood pressure/HR/ETCO2 tracing is shown in Figure 2. Immediately before cardiac arrest, the high ETCO2 values (due to asphyxia) drifted down as pulmonary blood flow became increasingly limited. After 30 s of PPV, the remaining CO2 was ventilated off of the lung, and ETCO2 decreased to near zero. When a small amount of pulmonary blood flow was reestablished by initiation of cardiac compressions, a slight increase in ETCO2 was detected. A sudden increase in ETCO2 was observed in all animals after ROSC (usually after epinephrine administration) as pulmonary blood flow quickly improved with the resumption of the pumping action of the heart. ROSC was achieved in 42 of 46 piglets.
Representative tracing of ETCO2 and mean arterial pressure (MAP) during CPR. After initial PPV, ETCO2 values decreased from asphyxia values to 6 mm Hg and then gradually increased as blood pressure increased with initiation of CPR. ETCO2 values decreased during each 10 s pause to auscultate for HR, reflecting loss of blood flow with interruption of cardiac compressions. After epinephrine administration, blood pressure improved and ETCO2 increased to 15 mm Hg, at which point ROSC occurred and an audible HR was detected. The hatch marks along the bottom of each frame represent 1 s.
The sensitivity, specificity, false positive, false negative, positive likelihood ratio, and % correct prediction for return of an audible HR >60 bpm for a range of ETCO2 values are seen in Table 2. The receiver operator curve is seen in Figure 3, and area under the curve statistics was significant at 0.94 (95% CI 0.88–1.00). An ETCO2 cut-off >14 mm Hg had a sensitivity of 93%, specificity of 81%, positive likelihood ratio of 5, and 88% accuracy at predicting return of HR >60 bpm. The distance d calculated from the ROC analysis for the ETCO2 critical value of 14 mm Hg or higher was 0.524.
DISCUSSION
In this neonatal model of asphyxia-induced asystole, increasing ETCO2 values seem to correlate with ROSC and an audible HR >60 bpm. Given the consistent threshold >14 mm Hg at which ETCO2 correlates with return of an adequate HR, it may be useful to guide uninterrupted neonatal CPR. An ETCO2 cut-off >14 mm Hg seems most useful for determining when to interrupt CPR to auscultate for a HR by providing the lowest combination of false positives and false negatives and the best fit with optimal calculated distance to an ideal curve using ROC analysis with a sensitivity of 93%, 81% specificity, and a positive likelihood ratio of 5.
The current NRP recommendation to interrupt cardiac compressions every 30 s for a 6 s auscultation pause to check for ROSC (13) may not be optimal. In an adult animal model, such breaks in cardiac compressions further delay reestablishment of adequate coronary perfusion pressure, which is very dependent on ongoing uninterrupted compressions (23). NRP also recommends checking for the presence or absence of a palpable pulse during the resuscitative effort to assess the adequacy of artificial perfusion during cardiac compressions (13); however, coronary perfusion pressure (which is calculated as the aortic diastolic blood pressure − the right atrial diastolic blood pressure) is not impacted by the difference in systolic and diastolic pressures represented by a palpable pulse but rather by the aortic diastolic pressure itself (24). Thus, monitoring ETCO2 trends during CPR would allow uninterrupted cardiac compressions and might provide a better indicator of the effectiveness of perfusion during compression administration.
ETCO2 is a measure of the Pco2 at the end of an exhaled breath and is mainly determined by alveolar ventilation, pulmonary perfusion (right cardiac output), and CO2 production due to metabolism. During acutely low cardiac output states as in cardiac arrest, decreased pulmonary blood flow becomes the primary determinant of ETCO2, resulting in low values (25,26). The concept of change in ETCO2 reflecting the changes in pulmonary blood flow in the presence of constant cardiac compressions and ventilation has been used to assess circulatory status during cardiac arrest and resuscitation in adults (17). In experimental models of ventricular fibrillation-induced cardiac arrest, ETCO2 concentration during ongoing CPR correlates with cardiac output, coronary perfusion pressure, and successful resuscitation from cardiac arrest (27,28).
Experimental animal adult studies of atraumatic cardiac arrest have reported increasing ETCO2 to be also associated with ROSC (21). In such models, an ETCO2 threshold of 15 mm Hg predicted ROSC with a positive predictive value 91% and negative predictive value of 91% (25).
In contrast to adult models of ventricular fibrillation, animal models of brief asphyxial pediatric cardiac arrest reported initially elevated ETCO2 levels reflective of the high alveolar Pco2 present at the time of asphyxial arrest. This was followed by a subsequent decrease in Pco2 once ventilation was initiated (22,29) as the Pco2 present in the lung at the time of arrest was ventilated off and no further CO2 was brought to the lung due to arrested perfusion. In our neonatal model of asphyxia-induced asystole, we observed similar findings with high initial ETCO2 at the time of asystole that decreased after 30 s of adequate PPV. This pattern of ETCO2 changes is different from that observed in ventricular fibrillation arrest, because Pco2 levels are typically normal at the time of cardiac arrest from ventricular fibrillation as opposed to very elevated at the time of asphyxial cardiac arrest. No previous study of asphyxial cardiac arrest has determined ETCO2 values that correlate with return of an audible HR >60 bpm, which is the current clinical goal for stopping cardiac compressions after asphyxia-induced asystole in neonates.
A strength of this translational study is the use of a piglet asphyxia model that closely mimics delivery room events with gradual onset of severe asphyxia leading to asystole. The presence of a dedicated focused clinical resuscitation team with current NRP training, along with designated roles during the resuscitation, a supervisor leading the code and a recorder for accuracy of documentation, makes this piglet asphyxia model an ideally controlled mega code environment. We attempted to control for depth of compressions and compressor fatigue by real-time assessment and adjustment of the pulse pressures generated by the compressor. This controlled setting allowed us to generate an ROC curve related to the predictive values of ETCO2, with minimum confounding variables.
The following limitations should be considered before general application of ETCO2 guidance in future clinical neonatal resuscitation trials. The current model is one where the animals have already undergone fetal to neonatal transition and in addition are sedated/anesthetized. The findings are still relevant despite this limitation, because the distribution of cardiac output in the fetus and posttransitional neonate during asphyxial episodes are qualitatively similar (30–32). In addition, responsiveness and reactivity of the cerebral circulation to factors that modulate cerebral blood flow such as hypoxia qualitatively remain intact under barbiturate anesthesia (33,34). Another limitation is that manual ventilation and chest compressions could cause ETCO2 to fluctuate with the effort of compression and rate of ventilation (20,35), so uniform compressions need to be delivered for ETCO2 to be used as a predictor of ROSC. In addition, acute and chronic illness with comorbidities can result in a ventilation/perfusion mismatch, which can limit the accuracy of ETCO2 (36). This is unlikely to be a problem in the post transitional neonatal piglet model currently used in this study. The effect of resuscitation medications such as epinephrine needs to be carefully recorded and should also be taken into consideration. According to adult studies, NaHCO3 can transiently increase ETCO2, whereas epinephrine can lead to decreased levels (37). Finally, this model is based on HR assessment in term animals and does not include evaluation for pseudo-pulseless electrical activity, where there is no clinically palpable pulse but presence of blood flow. The model does not address issues of prematurity or low birth weight.
In conclusion, our study using this piglet model of asystole due to asphyxia demonstrates that capnometry can be used as a predictor of ROSC, and may be a useful substitute for frequent pauses in cardiac compressions to auscultate HR during neonatal CPR. Further investigation is needed to determine whether uninterrupted ETCO2-guided CPR can improve time to ROSC and short- and long-term outcomes after neonatal resuscitation.
Abbreviations
- CPR:
-
cardiopulmonary resuscitation
- d :
-
distance
- ETCO2:
-
end-tidal CO2
- HR:
-
heart rate
- NRP:
-
Neonatal Resuscitation Program
- PPV:
-
positive pressure ventilation
- ROC:
-
receiver operator characteristic
- ROSC:
-
return of spontaneous circulation
REFERENCES
Perlman JM, Risser R 1995 Cardiopulmonary resuscitation in the delivery room. Arch Pediatr Adolesc Med 149: 20–25
Wyckoff MH, Perlman JM, Laptook AR 2005 Use of volume expansion during delivery room resuscitation in near-term and term infants. Pediatrics 115: 950–955
Barber CA, Wyckoff MH 2006 Endotracheal versus intravenous epinephrine during neonatal cardiopulmonary resuscitation in the delivery room. Pediatrics 118: 1028–1034
Ralston SH, Voorhees WD, Babbs CF 1984 Intrapulmonary epinephrine during prolonged cardiopulmonary resuscitation: improved regional flow and resuscitation in dogs. Ann Emerg Med 13: 79–86
Sanders AB, Ewy GA, Taft TV 1984 Prognostic and therapeutic importance of the aortic diastolic pressure in resuscitation from cardiac arrest. Crit Care Med 12: 871–873
Niemann JT, Criley JM, Rosborough JP, Niskanen RA, Alferness C 1985 Predictive indices of successful cardiac resuscitation. Ann Emerg Med 14: 521–528
Halperin HR, Tsitlik JE, Guerci AD, Mellits ED, Levin HR, Shi A-Y, Chandra N, Weisfeldt ML 1986 Determinants of blood flow to vital organs during cardiopulmonary resuscitation in dogs. Circulation 73: 539–550
Kern KB, Hilwig RW, Berg RA, Ewy GA 1998 Efficacy of chest compression-only BLS CPR in the presence of an occluded airway. Resuscitation 39: 179–188
Berg RA, Sanders AB, Kern KB, Hilwig RW, Heidenreich JW, Porter ME, Ewy GA 2001 Adverse effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation 104: 2465–2470
Kern KB, Hilwig RW, Berg RA, Sanders AB, Ewy GA 2002 Importance of continuous chest compressions during cardiopulmonary resuscitation: improved outcome during a simulated single lay-rescuer scenario. Circulation 105: 645–649
ECC Committee Subcommittees and Task Forces of the American Heart Association 2005 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 3. Overview of CPR. Circulation 112: IV-12–IV-18
Sayre MR, Berg RA, Cave DM, Page RL, Potts J, White RD 2008 Hands-only (compression-only) cardiopulmonary resuscitation: a call to action for bystander response to adults who experience out-of-hospital sudden cardiac arrest: a science advisory for the public from the American Heart Association Emergency Cardiovascular Care Committee. Circulation 117: 2162–2167
Kattwinkel J 2006 Textbook of Neonatal Resuscitation. 5th ed. American Academy of Pediatrics/American Heart Association, Elk Grove, IL, pp, 10–20
Weil MH, Bisera J, Trevino RP, Rackow EC 1985 Cardiac output and end-tidal carbon dioxide. Crit Care Med 13: 907–909
Trevino RP, Bisera J, Weil MH, Rackow EC, Grundler WG 1985 End-tidal CO2 as a guide to successful cardiopulmonary resuscitation: a preliminary report. Crit Care Med 13: 910–911
Garnett AR, Ornato JP, Gonzalez ER, Johnson EB 1987 End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. JAMA 257: 512–515
Falk JL, Rackow EC, Weil MH 1988 End-tidal carbon dioxide concentration during cardiopulmonary resuscitation. N Engl J Med 318: 607–611
Kern KB, Sanders AB, Voorhees WD, Babbs CF, Tacker WA, Ewy GA 1989 Changes in expired end-tidal carbon dioxide during cardiopulmonary resuscitation in dogs: a prognostic guide for resuscitation efforts. J Am Coll Cardiol 13: 1184–1189
Bhende MS 2001 End-tidal carbon dioxide monitoring in pediatrics—concepts and technology. J Postgrad Med 47: 153–156
Sanders AB, Kern KB, Otto CW, Milander MM, Ewy GA 1989 End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. JAMA 262: 1347–1351
Idris AH, Staples ED, O'Brien DJ, Melker RJ, Rush WJ, Del Duca KD, Falk JL 1994 End-tidal carbon dioxide during extremely low cardiac output. Ann Emerg Med 23: 568–572
Bhende MS, Karasic DG, Karasic RB 1996 End-tidal carbon dioxide changes during cardiopulmonary resuscitation after experimental asphyxial cardiac arrest. Am J Emerg Med 14: 349–350
Ewy GA, Zuercher M, Hilwig RW, Sanders AB, Berg RA, Otto CW, Hayes MM, Kern KB 2007 Improved neurological outcome with continuous chest compressions compared with 30:2 compressions-to-ventilations cardiopulmonary resuscitation in a realistic swine model of out-of-hospital cardiac arrest. Circulation 116: 2525–2530
Wyckoff MH, Berg RA 2008 Optimizing chest compressions during delivery room resuscitation. Semin Fetal Neonatal Med 13: 410–415
Callaham M, Barton C 1990 Prediction of outcome of cardiopulmonary resuscitation from end-tidal carbon dioxide concentration. Crit Care Med 18: 358–362
Domsky M, Wilson RF, Heins J 1995 Intraoperative end-tidal carbon dioxide values and derived calculations correlated with outcome: prognosis and capnography. Crit Care Med 23: 1497–1503
Sanders AB, Ewy GA, Bragg S, Atlas M, Kern KB 1985 Expired PCO2 as a prognostic indicator of successful resuscitation from cardiac arrest. Ann Emerg Med 14: 948–952
Gudipati CV, Weil MH, Bisera J, Deshmukh HG, Rackow EC 1988 Expired carbon dioxide: a noninvasive monitor of cardiopulmonary resuscitation. Circulation 77: 234–239
Berg RA, Henry C, Otto CW, Sanders AB, Kern KB, Hilwig RW, Ewy GA 1996 Initial end-tidal CO2 is markedly elevated during cardiopulmonary resuscitation after asphyxial cardiac arrest. Pediatr Emerg Care 12: 245–248
Behrman RE, Lees MH, Peterson EN, De Lannoy CW, Seeds AE 1970 Distribution of the circulation in the normal and asphyxiated fetal primate. Am J Obstet Gynecol 108: 956–969
Cohn HE, Sacks EJ, Heymann MA, Rudolph AM 1974 Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol 120: 817–824
Leffler CW, Busija DW, Beasley DG, Fletcher AM, Green RS 1986 Effects of indomethacin on cardiac output distribution in normal and asphyxiated piglets. Prostaglandins 31: 183–190
Hohimer AR, Bissonnette JM 1989 Effects of cephalic hypotension, hypertension, and barbiturates on fetal cerebral flood flow and metabolism. Am J Obstet Gynecol 161: 1344–1351
Donegan JH, Traystman RJ, Koehler RC, Jones MD Jr, Rogers MC 1985 Cerebrovascular hypoxic and autoregulatory responses during reduced brain metabolism. Am J Physiol 249: H421–H429
Steedman DJ, Robertson CE 1990 Measurement of end-tidal carbon dioxide concentration during cardiopulmonary resuscitation. Arch Emerg Med 7: 129–134
LaValle TL, Perry AG 1995 Capnography: assessing end-tidal CO2 levels. Dimens Crit Care Nurs 14: 70–77
Callaham M, Barton C, Matthay M 1992 Effect of epinephrine on the ability of end-tidal carbon dioxide readings to predict initial resuscitation from cardiac arrest. Crit Care Med 20: 337–343
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by an American Academy of Pediatrics Neonatal Resuscitation Program Research Grant. In addition, L.F.C. and L.H. are supported by grant KL2RR024983, titled, “North and Central Texas Clinical and Translational Science Initiative” from the National Center for Research Resources (NCRR, NIH).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.pedresearch.org).
Rights and permissions
About this article
Cite this article
Chalak, L., Barber, C., Hynan, L. et al. End-Tidal CO2 Detection of an Audible Heart Rate During Neonatal Cardiopulmonary Resuscitation After Asystole in Asphyxiated Piglets. Pediatr Res 69, 401–405 (2011). https://doi.org/10.1203/PDR.0b013e3182125f7f
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3182125f7f
This article is cited by
-
Volumetric capnography and return of spontaneous circulation in an experimental model of pediatric asphyxial cardiac arrest
Scientific Reports (2023)
-
Versorgung und Reanimation des Neugeborenen nach der Geburt
Notfall + Rettungsmedizin (2021)
-
Continuous capnography monitoring during resuscitation in a transitional large mammalian model of asphyxial cardiac arrest
Pediatric Research (2017)
-
Die Versorgung und Reanimation des Neugeborenen
Notfall + Rettungsmedizin (2015)