Abstract
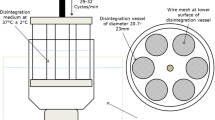
The aim of this study was to investigate how beaker size, basket assembly, use of disk, and immersion medium impact the disintegration time of dietary supplements. The disintegration times were determined for five tablet and two capsule products. A two-station disintegration tester was used with Apparatus A or Apparatus B as described in the United States Pharmacopeia (USP) chapters, <701> and <2040>. Two beakers complying with the harmonized specifications were used, one with a volume of 1,000 mL and one with a 1,500-mL volume. The disintegration data were analyzed using ANOVA for the following factors: beaker size, equipment (App A and B) and condition (with/without disk). Two tablet products were not sensitive to any changes in the test conditions or equipment configurations. One product was only partially sensitive to the test conditions. The other products showed impact on the disintegration time for all test conditions. The results revealed that these tablet products might pass or fail current USP disintegration requirements depending on the equipment configuration. Similar results were obtained for the two investigated capsule formulations. One product might fail current USP disintegration requirements if the large beaker was used, but might pass the disintegration requirements when the small beaker was used. Hydroxy propyl methyl cellulose capsules were mostly influenced if sodium instead of a potassium buffer was used as the immersion medium. The results demonstrate that the current harmonized ICH specifications for the disintegration test are insufficient to make the disintegration test into reliable test for dietary supplements.
Similar content being viewed by others
REFERENCES
Gupta A, Hunt RL, Shah RB, Sayeed VA, Khan MA. Disintegration of highly soluble immediate release tablets: a surrogate for dissolution. AAPS PharmSciTech. 2009;10(2):495–9.
Han JH, Gallery, J. A risk based approach to in vitro performance testing: a case study on the use of dissolution vs. disintegration for liquid filled gelatin capsules. American Pharmaceutical Review. 2006;9(6):152–157.
Narazaki R, Harada T, Takami N, Kato Y, Ohwaki T. A new method for disintegration studies of rapid disintegrating tablet. Chem Pharm Bull (Tokyo). 2004;52(6):704–7.
Shukla D, Chakraborty S, Singh S, Mishra B. Mouth dissolving tablets ii: an overview of evaluation techniques. Sci Pharm. 2009;77(2):327–41.
Park JH, Holman KM, Bish GA, Krieger DG, Ramlose DS, Herman CJ, et al. An alternative to the USP disintegration test for orally disintegrating tablets. Pharm Technol. 2008;32(8):54–58.
Tong C, Lozano R, Mao Y, Mirza T, Löbenberg R, Nickerson B, et al. The value of in vitro dissolution in drug development. A position paper from the AAPS in vitro release and dissolution focus group. Pharm Technol. 2009;33(4):52–64.
The United States Pharmacopeial Convention, Rockville, MD: USP 32: The United States Pharmacopeia Convention; 2009. Chapter <701>. 262–263 p.
The United States Pharmacopeial Convention, Rockville, MD: USP 32: United States Pharmacopeia Convention; 2009. Chapter <2040>. 782–784 p.
Donauer N, Lobenberg R. A mini review of scientific and pharmacopeial requirements for the disintegration test. Int J Pharm. 2007;345(1–2):2–8.
The United States Pharmacopeial Convention, Rockville, MD: USP 24: The United states Pharmacopeia Convention; 2000. Chapter <701>. 1941 p.
Schmid K, Lobenberg R. Influence of the changed USP specifications on disintegration test performance. Dissolution Technologies. 2010;16, 18, 49:6–10.
International Conference on Harmonisation. Draft guidance on Q4B evaluation and recommendation of pharmacopoeial texts for use in the International Conference on Harmonisation Regions; Annex on disintegration test general chapter; availability. Notice Fed Regist. 2008;73(151):45466–7.
Kamba M, Seta Y, Takeda N, Hamaura T, Kusai A, Nakane H, et al. Measurement of agitation force in dissolution test and mechanical destructive force in disintegration test. Int J Pharm. 2003;250(1):99–109.
Acknowledgements
The authors want to thank the United States Pharmacopeia Convention and Sotax for their support for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editor: Susan D'Souza
Rights and permissions
About this article
Cite this article
Almukainzi, M., Salehi, M., Araci Bou-Chacra, N. et al. Investigation of the Performance of the Disintegration Test for Dietary Supplements. AAPS J 12, 602–607 (2010). https://doi.org/10.1208/s12248-010-9221-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-010-9221-1