-
PDF
- Split View
-
Views
-
Cite
Cite
Raymond Liu, Margaretta Page, Karla Solheim, Sherry Fox, Susan M. Chang, Quality of life in adults with brain tumors: Current knowledge and future directions, Neuro-Oncology, Volume 11, Issue 3, June 2009, Pages 330–339, https://doi.org/10.1215/15228517-2008-093
Close - Share Icon Share
Abstract
Quality of life is an important area of clinical neurooncology that is increasingly relevant as survivorship increases and as patients experience potential morbidities associated with new therapies. This review of quality-of-life studies in the brain tumor population aims to summarize what is currently known about quality of life in patients with both low-grade and high-grade tumors and suggest how we may use this knowledge to direct future research. To date, reports on quality of life have been primarily qualitative and focused on specific symptoms such as fatigue, sleep disorders, and cognitive dysfunction, as well as some symptom clusters. However, the increasing interest in exploring quality of life as a primary end point for cancer therapy has established a need for prospective, controlled studies to assess baseline and serial quality-of-life parameters in brain tumor patients in order to plan and evaluate appropriate and timely interventions for their symptoms.
Quality of life (QOL) is a concept that encompasses the multidimensional well-being of a person and reflects an individual's overall satisfaction with life. QOL is a broad term that involves several dimensions, including physical or functional status, emotional well-being, and social well-being.1
Patients with primary brain tumors face serious challenges to their QOL. They have difficulties with general symptoms such as headache, anorexia, nausea, seizures, and insomnia. These patients also face symptoms secondary to focal neurologic deterioration, including motor deficits, personality changes, cognitive deficits, aphasia, or visual field defects.2,3
Despite these many challenges, there are few well-tested interventions to improve QOL and no established systematic way to study it in these patients. Few adequately controlled or powered studies have addressed QOL, and clinical guidelines are limited on how to manage symptoms in primary brain tumor patients. This review summarizes what is currently known about the QOL of adult primary brain tumor patients, the challenges to QOL research, and future directions for QOL research in brain tumor patients.
QOL in Brain Tumor Patients: Current Knowledge
The overall symptom burden and disability for glioma patients are significant, especially in those with high-grade or recurrent disease.3,4 Malignant glioma patients score significantly lower in all domains of functioning compared to age-matched and sex-matched healthy controls and have lower social functioning and more problems with vision, motor functions, communication, headaches, and seizures than do matched, non-small-cell lung cancer patients.5 Patients with high-grade tumors do not appear to differ in QOL between those with grade III and grade IV tumors,3,6–8 although perceived QOL in patients with grade III tumors may be better.9 The difference in QOL may be less dependent on the grade of tumor and more dependent on whether the tumor is stable or progressive. For example, one study found that patients with malignant gliomas with low QOL at baseline tended to deteriorate over time.10
Patients with low-grade glioma also have a significant symptom burden.11 In one study, 45% of patients with low-grade glioma reported low overall QOL, with fewer than half able to carry out normal activities without restriction. Fatigue, sleep disturbance, and pain were the most frequent symptom complaints, but patients also had difficulties with other realms of QOL, including difficulties with cognitive and emotional dimensions.12 Compared to control groups of patients with non-CNS cancers and healthy patients, low-grade glioma patients specifically report more fatigue, cognitive dysfunction, and altered mood states.11,13–15 The prognostic significance of symptom burden in low-grade glioma patients is unclear because studies have shown contradictory findings.16,17
Specific Symptoms
Research addressing specific symptoms that affect QOL has focused on fatigue, sleep, pain, seizures, mood disturbance, and cognitive function. This research has been mostly descriptive, and most studies examine a heterogeneous group of patients receiving different therapies at different stages of their illness.
Fatigue appears to be the most significant symptom facing patients with high-grade gliomas3,6 and may be more significant a problem compared to patients with low-grade tumors.18 In patients with recurrent malignant gliomas, the incidence of fatigue may approach 89%–94%.3 In a study evaluating patients with high-grade gliomas enrolled in three phase II protocols, significant fatigue was found in one-third of patients and independently predicted poorer overall survival.6 In that study, Eastern Cooperative Oncology Group performance status was correlated with overall QOL, excessive daytime somnolence, and increased fatigue. For low-grade glioma patients, one small study showed that of all QOL variables tested, fatigue had the strongest relationship with overall QOL.12
Sleep disturbance is also a common problem in primary brain tumor patients.12,19 For example, one study evaluating the incidence of major depressive disorder in a neurooncology clinic showed some type of sleep disturbance in 31.6%–52% of the patients evaluated.19 Other patient series suggest that high-grade glioma patients may have an even higher prevalence of sleep disturbance.3,20
Pain is another symptom that glioma patients often face.21 Headache is the most common type of pain, which more than 50% of patients with high-grade gliomas experience.3 Unfortunately, studies to date have not examined the type, location, or intensity of headache pain, the distress it caused, or its overall relationship to QOL in brain tumor patients.
Seizures in glioma patients are associated with deterioration in multiple cognitive domains.13 Low-grade glioma patients may have a higher incidence of epileptic seizures than do high-grade glioma patients, and in one study, antiepileptic therapy was able to control seizures only in approximately 50% of patients.13 The presence of seizures has not been shown to be a prognostic factor in some studies17 and was a positive prognostic factor in other studies when no other symptoms were present.16,22,23
Studies on mood disturbance in brain tumor patients have reported varying results, noting depression in anywhere between 7.9% and 90% of cases8,19,24–26 and significant anxiety in 29%–60% of cases.8,25,27 The use of formal criteria from the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, was employed in only a few studies to study depression.19,26 Of note, in some cohorts of primary brain tumor patients, depression was the most important independent predictor of QOL28,29 and has been shown to have an adverse impact on survival.26,30,31 Depression may also be undertreated. For example, in one study, only 60% of patients reported by their physicians to be depressed received anti depressants.26
Depression and anxiety may both have biologic foundations in brain tumor patients. In particular, measurable serotonin binding sites and strong peripheral benzodiazepine receptor expression were present in the tumors of rats implanted with gliomas.32 Expression of certain serotonin receptors has also been found in human fetal astrocytes and glioma cell lines.33 The biologic connections between tumor and symptoms could suggest that symptoms in some cases may not only be prognostic, but may be a surrogate marker for disease.
Cognitive functioning has also been studied in brain tumor patients and has been recently reviewed.34 Malignant glioma patients, in particular, deal with a significant burden in terms of cognitive dysfunction, with as many as 49% experiencing cognitive impairment.5 In smaller trials, there is evidence that cognitive dysfunction is present even prior to treatment.35 There is an unmet need for early neurocognitive evaluation and intervention. In addition, the prevalence of neurocognitive dysfunction has implications for decision making and informed consent.
Evaluation of cognitive function also has prognostic value.9,36,37 For example, one study of recurrent malignant gliomas showed that cognitive deterioration may precede radiographic evidence of progression by almost 6 weeks.36 A simple screening examination such as the Mini Mental Status Examination (MMSE) can have prognostic significance in glioma patients.38–40 However, while the MMSE is feasible and convenient to use in brain tumor patients, it has not consistently been shown to detect cognitive decline in brain tumor patients.6 Moreover, the test has been validated for dementia screening but does not have a high degree of sensitivity for other cognitive domains.41 New neuropsychologic tests need to be validated in this population, because many of the standard tests lack sensitivity, especially at the lower end of the impaired range.42 In fact, attempts are being made in large cooperative trials to standardize the measurement of cognitive status. Numerous brain studies being conducted by cooperatives such as the Radiation Therapy Oncology Group are using well-known tests of neurocognitive function, such as the Hopkins Verbal Learning Test, the Trail Making Test, and the Controlled Word Association Test.
In summary, brain tumor patients deal with a significant symptom burden. The main areas of concern are in fatigue, sleep, pain, seizures, mood, and cognitive function. Malignant glioma patients are particularly affected by fatigue, whereas low-grade glioma patients have a large symptom burden from seizures. Further research is necessary not only to better define this symptom burden, but also to examine individual symptoms within specific populations.
Long-Term Symptoms: Survivorship
The current literature on survivorship in brain tumor patients consists of small, mostly retrospective, noncontrolled studies. Moreover, they follow patients over varying amounts of time.43 Patients in survivorship studies are also highly selected, as they tend to be young, have high pretreatment performance status, and often have had a gross total resection of their tumor. Prospective studies evaluating survivorship would be ideal, but these studies pose special difficulties because compliance with self-reported questionnaires declines over time, with the sickest patients unable to complete follow-up. For example, one study following neuropsychologic impairments in adults with brain tumors showed heterogeneous results, with only 5 of 49 patients completing the third follow-up assessment.44
Despite the many limitations on survivorship studies, there is some indication that long-term survivors may have acceptable long-term QOL. When compared to a heterogeneous group of inpatients with chronic neurologic diseases, patients with malignant brain tumors had comparable levels of QOL, and 73% of them were able to continue or resume previous work activity.45 Other studies have found that between 44% and 60% of patients were able to go back to work, although mostly on a part-time basis.46 In a study of interstitial implantation of brachytherapy, survivors of recurrences were generally able to maintain their functional status, as measured by KPS.47
The most serious challenge survivors of brain tumors face may be cognitive dysfunction, and this is especially true for patients with malignant glioma.42,43,48–50 Cognitive decline was found in 50% of glioblastoma patients surviving up to 6 years.48 Cognitive deficits have been noted on formal testing of supposedly symptom-free survivors of malignant brain tumors, suggesting that cognitive deficits may be present even in those patients without overt evidence of impairment.51 However, malignant glioma survivors may still report experiencing good overall QOL despite suffering from cognitive impairments.46
Survivors of malignant gliomas also deal with significant mood disturbance. A small study examining 10 patients with glioblastoma surviving for more than 5 years found that depression or anxiety affected a significant proportion of the patients (4 of the 10 patients).50 Larger prospective studies are needed to better describe the long-term QOL of brain tumor patients. The urgent need for research in this field is driven by the growing population of brain tumor survivors.
A Common Etiology to Symptoms: Symptom Clusters
Although symptoms have been studied separately, they are often interrelated and may in some cases have a common etiology. The interrelationship between symptoms has been studied in the symptom cluster literature.52,53 In general cancer patients, anxiety, depression, pain, fatigue, and sleep disturbance have been consistently studied as potential components of symptom clusters, and studies have found relationships among these symptoms in various configurations.53 Research specifically concerning symptom clusters in primary brain tumor patients has been extremely limited. In a high-grade glioma study, depression, fatigue, sleep disturbance, and cognitive impairment formed a symptom cluster that explained 29% of the variance in QOL, and depression, fatigue, sleep disturbance, cognitive impairment, and pain formed a symptom cluster that explained 62% of the variance in functional status.54
Cognitive function has been correlated to increased fatigue and depression in newly diagnosed malignant glioma patients.6 Performance status was also strongly correlated with cognitive function, as measured by the MMSE, in one study evaluating two large, prospective, randomized trials of newly diagnosed malignant glioma patients.55 Fatigue, in turn, may be related to other aspects of QOL and was found to have negative effects on all components of QOL in glioblastoma patients, with the exception of nutritional well-being.56
The etiologies of specific clusters of symptoms are generally unknown. Expression of proinflammatory cytokines such as tumor necrosis factor-α, interleukin-1, and interleukin-6 has been proposed as one mechanism for common symptoms.52,53 Support for this hypothesis is provided by studies that show an elevation in fatigue, anxiety, sleep disturbance, and pain sensitivity when these cytokines are introduced into animal models.52,53 Increased levels of these cytokines are also seen in depressed patients and cancer patients with fatigue.52,53 Although there is some evidence that cytokines contribute to symptom clusters, it is very possible that other explanations exist, or that clustered symptoms do not share a common etiology.53 Other biologic mechanisms for symptom clusters are being studied as well. For example, the symptom cluster of fatigue, appetite loss, and sleep disruption may result from interference by epidermal growth factor receptor (EGFR) ligands with the hypothalamic modulation of circadian rhythms, as EGFR ligands and members of the EGFR family are overexpressed in tumor cells.52 More research needs to be done on biologic mechanisms of symptoms to better understand the connection between symptoms and tumor factors and to develop better targeted interventions.
Symptom Management
Despite the significant burden of symptoms that brain tumor patients face, few QOL interventions have been tested in brain tumor patients. Of all symptoms, interventions for fatigue and cognitive dysfunction have been the most widely studied. A phase II study of donepezil in 24 irradiated brain tumor patients showed statistically significant improvements in cognitive functioning, mood, and health-related QOL.57 Methylphenidate has also been used to improve cognitive dysfunction and fatigue. In a pilot study, methylphenidate improved cognitive function and fatigue in patients with brain tumors.58 A randomized trial of methylphenidate in a heterogeneous population of brain tumor patients receiving radiation therapy was closed early due to lack of accrual, high dropout rates, an interim analysis showing no effect, and withdrawal of drug company support.59 Modafinil is another wake-promoting agent used for fatigue, although no large-scale studies have been performed in brain tumor patients. A pilot study of modafinil in brain tumor patients showed some improvement in cognitive, mood, and fatigue outcome measures, although the study randomized patients to two different doses of modafinil rather than to placebo.60
Nonpharmacologic interventions such as exercise are thought to potentially improve QOL outcomes, as well, and will likely be tested in brain tumor patients in the near future.61 Complementary alternative medicine (CAM) is also commonly used, although studies to date have failed to show a clear correlation between use of CAM and improved QOL.62,63 In addition, cognitive training modules have been developed and tested in non-cancer patients64 and will likely be tested in brain tumor patients in the future.
A Model for Future QOL Research
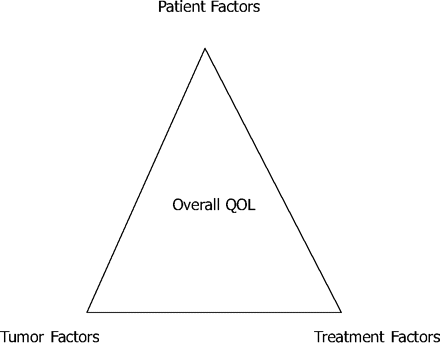
Because of the difficulty of studying symptoms that may have many potential contributing etiologies, better research models are needed to understand what contributes to decreased QOL and symptom burden in order to develop targeted interventions to improve patients' QOL. Figure 1 describes one way of examining the individual contributions to a patient's overall QOL. Patient factors that can contribute to QOL include demographic characteristics and comorbidities that may affect a patient's perception and symptom experience. Tumor factors include tumor laterality, size, and location, which may, in brain tumor patients, affect the specific neurologic symptoms they experience. Finally, treatment factors include surgery, radiation, chemotherapies, and concomitant medications that can cause or relieve symptoms that affect QOL.
Patient Factors
Studies focusing on brain tumor patient comorbidities and demographics and their effects on QOL are largely lacking. Gender differences in brain tumor patients' reported QOL have been the most studied patient factor, although results have been mixed. A correlation between lower QOL scores and being female has been shown in several studies,29,65 although not in others.6,12 Higher levels of mood disturbance have been found in women with brain tumors than in men,66,67 but not in all studies.25 The etiology of this proposed difference remains unexplained, but reporting bias could certainly contribute.
Tumor Factors
Tumor location and laterality has been shown to be correlated to several specific symptoms in some studies but not in others. Because patients with tumors in the left hemisphere may have greater problems with communication even before treatment is initiated,35 the reliability of conclusions about laterality based on self-reported symptoms and QOL is sometimes in question.
Mood changes may be affected by lesion location. For example, depression may arise from left brain injury,67 and anxious states may arise from right brain injury.68 Depression may also be more frequent in malignant glioma patients with left-hemisphere tumors.7 Another study showed that patients with a right-hemisphere primary brain tumor had statistically significantly higher mean anxiety scores, which improved 3 months and 1 year after surgical resection.67 Low-grade glioma patients with lesions in the ventral frontal cortex or lesions in the temporoparietal cortex were reported to have statistically significantly worse mood states after surgery than those patients with lesions in other regions of the brain.69
Cognitive function may also be related to tumor laterality. For example, left-hemisphere tumors have in general been associated with lower scores on verbal tests,7 while right-hemisphere lesions have been related to lower scores on facial recognition tests.70 In another study, cognitive functioning in patients referred for neuropsychiatric evaluation was poor but was better in younger patients and in those with frontal brain tumors.71 In low-grade glioma patients, greater cognitive disability has been noted in those with tumors in the dominant hemisphere.11,15 Patients with tumors in the left hemisphere report more difficulty concentrating than do patients with right-hemisphere lesions,2,11 although patients with right-hemisphere lesions report more tension.11 The effect of tumor laterality may differ between brain tumor patients and other populations. For example, one small study compared 17 brain tumor patients to 17 stroke patients with similar laterality and found that these two populations differed tremendously in their neuropsychologic profiles. In this study, brain tumor patients tended to have more global cognitive deficits compared to the site-specific deficits apparent in stroke patients.72 Tumor progression may have more of an impact on declining cognitive function in patients than do treatment factors such as radiation,38 but the difficulty in separating these effects often makes it difficult to attribute symptomatic progression to a single cause.
Model to evaluate effects of different factors on brain tumor patients' overall quality of life (QOL).
Treatment Factors
Treatment factors that can affect brain tumor patients' overall QOL include surgery, radiation, chemotherapy, and concomitant medications. Relationships between QOL and these treatments have been studied, although they suffer from lack of adequate power and control populations.
In surgical series of high-grade glioma patients, the extent of resection may correlate with QOL outcomes, with biopsy patients having worse QOL than those who have undergone gross total resection.5,10,15,26 Unfortunately, selection bias of patients who undergo either a gross total resection or a biopsy based on factors such as tumor size, multifocality, location, and functionality may confound these findings.
Radiation therapy may adversely affect QOL in brain tumor patients by leading to increased fatigue in the short term and by contributing to cognitive dysfunction in the long term. Both radiation-induced fatigue and cognitive dysfunction have been reviewed in the literature.73,74 One extensive review of clinical studies published on the neurobehavioral sequelae of therapeutic cranial irradiation in adults found significant cognitive deterioration in at least 92 of 748 patients reviewed.73 In long-term survivors who received radiation therapy, cognitive functioning was more significantly impaired in those that had received whole-brain irradiation compared to focused radiotherapy.74 Although these studies attribute cognitive decline to treatment effect, it is often difficult to differentiate tumor factors from treatment factors. For example, some studies show that patients with tumors have poor QOL regardless of whether radiation has been administered.11,15 Furthermore, modern radiotherapy techniques may not cause the same types of long-term cognitive effects as whole-brain radiotherapy.14,15,75–77
The few studies that have evaluated chemotherapy-related QOL suffer from an inability to differentiate between the effects of chemotherapy and the effects of other treatments or the tumor itself. The QOL in newly diagnosed glioblastoma patients receiving either radiotherapy alone or radiotherapy with concomitant and adjuvant temozolomide (TMZ) has been reported.20 Both groups were substantially impaired compared to historical controls, but no significant decrease in overall QOL was noted throughout treatment. Patients receiving TMZ had more vomiting, anorexia, constipation, and decreased social functioning. Fatigue during radiation therapy was more frequent in those assigned to TMZ. Not surprisingly, patients who responded to TMZ reported improvement in multiple domains of QOL.78
Concurrent medication use may confound the study of therapy-related symptoms. Medications commonly given to brain tumor patients include seizure medications and steroids. Both these medications can adversely affect physical, emotional, and cognitive functioning.79 Anti-epileptics, in particular, have been linked to cognitive dysfunction.5,13,15 Analysis of this link is confounded by whether symptoms are caused by antiepileptics or by seizure activity. Corticosteroids have been linked to depression in high-grade glioma patients19,26 and to lower survival in recurrent malignant glioma patients.80
While treatment-related effects on QOL have been examined, existing studies are often retrospective and not controlled. In addition, it has not been clear which concurrent or sequential treatments are affecting QOL and whether patient factors or tumor factors are contributing to loss of QOL. Further research that accounts for confounding factors is necessary in order to define specific treatment-related symptoms and their contribution to decreased QOL.
QOL Measurement
Another challenge in QOL research is finding validated instruments to use. Early QOL reports in brain tumor patients evaluated only the functional domain of QOL using KPS scores.81,82 A review of studies up to 1998 in brain tumor patients81 suggests that functional status as measured by KPS remains high until near the time of death. KPS generally correlates with overall QOL83,5 and appears to have prognostic value.80 However, using the KPS to measure QOL is problematic because it is only a single measurement of functional ability, and its reliability and validity depend on the observer. Methods of obtaining more information about a patient's QOL include considering the patient's level of independence, training observers in KPS measurement,84 and developing adjunctive questionnaires such as the independent-living score to corroborate KPS scores.85
More recent studies have assessed the multidimensional aspect of QOL. However, conclusions are still difficult to make given that the questionnaires are often not validated in the brain tumor population and heterogeneous groups of brain tumor patients at all stages in their disease course are assessed. Attempts to validate questionnaires in the brain tumor population have been made, but large, well-run studies that employ these questionnaires remain lacking.86,87
In order to address these questions, new instruments that can measure the multidimensional nature of QOL in brain tumor patients have been developed. The two multidimensional QOL instruments that have been employed most extensively in brain tumor patients are the Functional Assessment of Cancer Therapy–Brain (FACT-Br) and the Brain Cancer Module–20 (BCM-20).
The Functional Assessment of Cancer Therapy–General (FACT-G) is an instrument that has been used to measure QOL and has been modified in brain tumor patients in the FACT-Br.87 In contrast, the BCM-20 is designed as a supplement to other general cancer-specific questionnaires.86 It contains questions targeted at four different areas: future uncertainty, visual disorder, communication deficit, and motor dysfunction. In addition, it has seven single-item questions that address physical symptoms regarding headache, seizure, drowsiness, hair loss, itching, weakness, and loss of bladder function. Whereas the BCM-20 groups items into four subscales with symptom domains and additional individual items, FACT-Br groups all 19 of its brain tumor–specific questions into a single subscale. The two questionnaires have not been compared to each other.
Other questionnaires are also being developed. Recently, a single-item linear analog scale assessment of QOL in neurooncology patients has been validated. This simple instrument may allow a quick assessment of the different dimensions of QOL in brain tumor patients, especially when further detail into specific areas of QOL is not the primary objective.88 In addition, the M. D. Anderson Symptom Inventory Brain Tumor Module is a validated 22-item questionnaire that identifies specific symptoms that brain tumor patients face.89 More research is needed to better utilize existing instruments and to validate new ones in brain tumor patients designed to answer specific questions.
Other QOL Research Challenges
Additional challenges face investigators interested in pursuing QOL research in brain tumor patients. Foremost among these challenges is finding the right instruments to measure QOL. There must be a balance between collecting detailed information with well-validated questionnaires and minimizing questionnaire burden. QOL studies in brain tumor patients that are prospective, randomized, and hypothesis driven will allow investigators to pick appropriate instruments that will answer their specific questions without running into difficulties with compliance and the hazards of multiple unintended comparisons.
Questionnaire burden and resulting noncompliance have been a major problem with existing instruments. While semistructured interviews may elicit more information from patients and caregivers, the interview format can be quite demanding on the brain tumor population.90 Many patients with recurrent malignant glioma who filled out a baseline FACT-Br questionnaire were unable to complete it at their first follow-up assessment.36,91 Another study examining longitudinal questionnaire compliance among newly diagnosed malignant glioma patients showed that compliance dropped to less than 50% at 6 months, with administrative failure being by far the largest reason for failure of compliance.92
One way of improving compliance is by simplifying the questionnaires; another is by providing more time for administration. For example, a 10-point Likert scale with brain tumor-specific questions was investigated in a preliminary study and found to be feasible in an unselected brain tumor population.65 Another strategy to improve compliance has been to use proxy raters. The reliability of proxy raters has been examined, and results have been mixed.7 Further study into proxy raters is needed, and documentation of whether or not questionnaires were obtained from proxy raters should be incorporated into QOL studies.
Another challenge with QOL research in brain tumor patients is related to the potential lack of correlation between self-reported measures and objective measurements. For example, poor correlation exists between self-report and objective measures of cognitive functioning in patients with low-grade tumors15 and in an unselected population of brain tumor patients prior to treatment.35 The problem of self-reporting cognitive dysfunction is exacerbated in patients with frontal tumors, who may underreport cognitive dysfunction due to impaired judgment, or in patients with anxiety, depression, or fatigue who may overreport cognitive dysfunction.34 Unfortunately, physicians may not be better at determining the severity of symptoms, and in low-grade glioma patients may in fact be underestimating the severity of most symptoms.76
Missing data is another major challenge that faces a QOL researcher. It is critical to document the reason for missing data and to prospectively design a trial to account for missing data. One of the dangers of not adequately considering missing data is that the sickest patients with the highest symptom burden do not fill out questionnaires and are underrepresented in studies. This is a particular challenge with longitudinal QOL studies in which patients who have the highest symptom burden are often the ones that may find it most difficult to complete questionnaires. Again, the careful selection of instruments that have minimal item burden and instruments that still capture the needed data are crucial to the success of such a study.
Once data are collected in brain tumor patients, they should be analyzed with consideration for response shifts. Response shifts are natural changes in a patient's perception of their QOL over time in response to changing internal standards when they are faced with a life-threatening illness. If these natural changes and adaptations over time are not considered, data from longitudinal QOL studies may incorrectly attribute changes in QOL to other external factors. Research on accounting for this phenomenon is still ongoing, and models to account for response shift have been proposed.93
Finally, because the symptoms and realms of QOL can be interrelated (e.g., insomnia can cause fatigue, which leads to decreased social interaction), and because an individual patient is subject to different environmental and demographic factors that can independently affect individual symptoms and realms of QOL, the study of QOL can become quite complex. The large number of interdependent variables may explain why many QOL studies have produced mixed results. The best way to account for these interactions in a trial is to have a focused question that is prospectively measured and ideally compared between adequately powered randomized groups in order to reduce the number of confounders.
Future Directions
Several exciting avenues of research remain in the vastly unexplored area of QOL in brain tumor patients. Research needs to be performed on validation of easy-to-use questionnaires and cognitive tests with incorporation of these instruments into ongoing clinical trials. Moreover, a better description of longitudinal QOL and exploration into specific causes of symptoms and survivorship is necessary. In addition, the significance of other nontraditional patient factors that contribute to QOL, such as caregiver, spiritual, and financial aspects of a patient's life, need to be better explored. Finally, once QOL in brain tumor patients has been adequately described, interventions—both pharmacologic and nonpharmacologic—are necessary in order to improve QOL. Ultimately, the exploration of imaging, serum, genetic, or other biomarkers that can more objectively quantify symptoms not only will allow the field of QOL to be more consistently described, but also will likely have significant diagnostic, prognostic, and treatment value. While funding may be limited to pursue these primary QOL research goals, efforts should be made to incorporate QOL research as secondary end points into ongoing therapy trials. Few trials have done this to date,94 although the potential impact of QOL on survival and prognosis makes incorporation of QOL end points especially important.95–97
Conclusions
QOL in brain tumor patients is complex and multidimensional in nature, with symptoms having interrelationships with each other as well as patient, tumor, and treatment factors. Assessing QOL is challenging given the scarcity of well-validated instruments, difficulty with compliance especially in longitudinal measurements over time, and the lack of well-designed trials. Assessing QOL in brain tumor patients is additionally complicated by the relative rarity of the disease compared to other malignancies, functional limitations to self-reporting, and concurrent medications such as steroids and antiepileptics.
Despite these limitations, some conclusions can be made regarding QOL studied to date on brain tumor patients. Specifically, the burden of symptoms that affect QOL is significant, but understudied. There are limited therapeutic options to improve QOL. The incorporation of QOL as both primary and secondary end points is crucial because QOL can have prognostic value, and improvement of QOL may in turn increase overall survival. As such, QOL should be included as a secondary outcome measure and also be tested as a primary outcome measure in intervention studies designed to improve the lives of brain tumor patients.
Clearly, more work is needed in the field of QOL in brain tumor patients. Adequately powered, high-quality studies from descriptive to diagnostic to interventional in nature are needed in order to increase both the quality and quantity of these patients' lives.
We are grateful to Ilona Garner, Department of Neurological Surgery, University of California, San Francisco, for editorial support.
References
Cella D, Chang CH, Lai JS, Webster K. Advances in quality of life measurements in oncology patients.
Heimans JJ, Taphoorn MJ. Impact of brain tumour treatment on quality of life.
Osoba D, Brada M, Prados MD, Yung WK. Effect of disease burden on health-related quality of life in patients with malignant gliomas.
Davies E, Clarke C, Hopkins A. Malignant cerebral glioma—I: survival, disability, and morbidity after radiotherapy.
Klein M, Taphoorn MJ, Heimans JJ, et al. Neurobehavioral status and health-related quality of life in newly diagnosed high-grade glioma patients.
Brown PD, Ballman KV, Rummans TA, et al. Prospective study of quality of life in adults with newly diagnosed high-grade gliomas.
Hahn CA, Dunn RH, Logue PE, King JH, Edwards CL, Halperin EC. Prospective study of neuropsychologic testing and quality-of-life assessment of adults with primary malignant brain tumors.
Giovagnoli AR, Tamburini M, Boiardi A. Quality of life in brain tumor patients.
Giovagnoli AR, Silvani A, Colombo E, Boiardi A. Facets and determinants of quality of life in patients with recurrent high grade glioma.
Brown PD, Maurer MJ, Rummans TA, et al. A prospective study of quality of life in adults with newly diagnosed high-grade gliomas: the impact of the extent of resection on quality of life and survival.
Taphoorn MJ, Schiphorst AK, Snoek FJ, et al. Cognitive functions and quality of life in patients with low-grade gliomas: the impact of radiotherapy.
Gustafsson M, Edvardsson T, Ahlstrom G. The relationship between function, quality of life and coping in patients with low-grade gliomas.
Klein M, Engelberts NH, van der Ploeg HM, et al. Epilepsy in low-grade gliomas: the impact on cognitive function and quality of life.
Laack NN, Brown PD, Ivnik RJ, et al. Cognitive function after radiotherapy for supratentorial low-grade glioma: a North Central Cancer Treatment Group prospective study.
Klein M, Heimans JJ, Aaronson NK, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study.
Pignatti F, van den Bent M, Curran D, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma.
Lote K, Egeland T, Hager B, et al. Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: a retrospective study in 379 patients.
Salo J, Niemela A, Joukamaa M, Koivukangas J. Effect of brain tumour laterality on patients' perceived quality of life.
Wellisch DK, Kaleita TA, Freeman D, Cloughesy T, Goldman J. Predicting major depression in brain tumor patients.
Taphoorn MJ, Stupp R, Coens C, et al. Health-related quality of life in patients with glioblastoma: a randomised controlled trial.
Whitton AC, Rhydderch H, Furlong W, Feeny D, Barr RD. Self-reported comprehensive health status of adult brain tumor patients using the Health Utilities Index.
Leighton C, Fisher B, Bauman G, et al. Supratentorial low-grade glioma in adults: an analysis of prognostic factors and timing of radiation.
van Veelen ML, Avezaat CJ, Kros JM, van Putten W, Vecht C. Supratentorial low grade astrocytoma: prognostic factors, dedifferentiation, and the issue of early versus late surgery.
Mainio A, Hakko H, Niemela A, Koivukangas J, Rasanen P. Depression and functional outcome in patients with brain tumors: a population-based 1-year follow-up study.
Kaplan CP, Miner ME. Relationships: importance for patients with cerebral tumours.
Litofsky NS, Farace E, Anderson F Jr, Meyers CA, Huang W, Laws ER Jr. Depression in patients with high-grade glioma: results of the Glioma Outcomes Project.
Arnold SD, Forman LM, Brigidi BD, et al. Evaluation and characterization of generalized anxiety and depression in patients with primary brain tumors.
Pelletier G, Verhoef MJ, Khatri N, Hagen N. Quality of life in brain tumor patients: the relative contributions of depression, fatigue, emotional distress, and existential issues.
Mainio A, Hakko H, Niemela A, Koivukangas J, Rasanen P. Gender difference in relation to depression and quality of life among patients with a primary brain tumor.
Mainio A, Hakko H, Timonen M, Niemela A, Koivukangas J, Rasanen P. Depression in relation to survival among neurosurgical patients with a primary brain tumor: a 5-year follow-up study.
Mainio A, Tuunanen S, Hakko H, Niemela A, Koivukangas J, Rasanen P. Decreased quality of life and depression as predictors for shorter survival among patients with low-grade gliomas: a follow-up from 1990 to 2003.
Whittle IR, Kelly PA. Mechanisms of peritumoural brain dysfunction: metabolic and neuroreceptor findings in striatal C6 glioma.
Merzak A, Koochekpour S, Fillion MP, Fillion G, Pilkington GJ. Expression of serotonin receptors in human fetal astrocytes and glioma cell lines: a possible role in glioma cell proliferation and migration.
Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours.
Tucha O, Smely C, Preier M, Lange KW. Cognitive deficits before treatment among patients with brain tumors.
Meyers CA, Hess KR. Multifaceted end points in brain tumor clinical trials: cognitive deterioration precedes MRI progression.
Klein M, Postma TJ, Taphoorn MJ, et al. The prognostic value of cognitive functioning in the survival of patients with high-grade glioma.
Brown PD, Jensen AW, Felten SJ, et al. Detrimental effects of tumor progression on cognitive function of patients with high-grade glioma.
Gorlia T, Stupp R, Eisenhauer EA, et al. Clinical prognostic factors affecting survival in patients with newly diagnosed glioblastoma multiforme (GBM) [abstract].
Brown PD, Buckner JC, O'Fallon JR, et al. Importance of baseline Mini-Mental State Examination as a prognostic factor for patients with low-grade glioma.
Meyers CA, Wefel JS. The use of the Mini-Mental State Examination to assess cognitive functioning in cancer trials: no ifs, ands, buts, or sensitivity.
Archibald YM, Lunn D, Ruttan LA, et al. Cognitive functioning in long-term survivors of high-grade glioma.
Imperato JP, Paleologos NA, Vick NA. Effects of treatment on long-term survivors with malignant astrocytomas.
Maire JP, Coudin B, Guerin J, Caudry M. Neuropsychologic impairment in adults with brain tumors.
Giovagnoli AR. Quality of life in patients with stable disease after surgery, radiotherapy, and chemotherapy for malignant brain tumour.
Schmidinger M, Linzmayer L, Becherer A, et al. Psychometric- and quality-of-life assessment in long-term glioblastoma survivors.
Leibel SA, Gutin PH, Wara WM, et al. Survival and quality of life after interstitial implantation of removable high-activity iodine-125 sources for the treatment of patients with recurrent malignant gliomas.
Scott JN, Rewcastle NB, Brasher PM, et al. Which glioblastoma multiforme patient will become a long-term survivor? A population-based study.
Awwad S, Cull A, Gregor A. Long-term survival in adult hemispheric glioma: prognostic factors and quality of outcome.
Steinbach JP, Blaicher HP, Herrlinger U, et al. Surviving glioblastoma for more than 5 years: the patient's perspective.
Giovagnoli AR, Boiardi A. Cognitive impairment and quality of life in long-term survivors of malignant brain tumors.
Rich TA. Symptom clusters in cancer patients and their relation to EGFR ligand modulation of the circadian axis.
Armstrong TS, Cohen MZ, Eriksen LR, Hickey JV. Symptom clusters in oncology patients and implications for symptom research in people with primary brain tumors.
Fox SW, Lyon D, Farace E. Symptom clusters in patients with high-grade glioma.
Taylor BV, Buckner JC, Cascino TL, et al. Effects of radiation and chemotherapy on cognitive function in patients with high-grade glioma.
Lovely MP, Miaskowski C, Dodd M. Relationship between fatigue and quality of life in patients with glioblastoma multiformae.
Shaw EG, Rosdhal R, D'Agostino RB Jr, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life.
Meyers CA, Weitzner MA, Valentine AD, Levin VA. Methylphenidate therapy improves cognition, mood, and function of brain tumor patients.
Butler JM Jr, Case LD, Atkins J, et al. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threomethylphenidate HCl in brain tumor patients receiving radiation therapy.
Kaleita TA, Wellisch DK, Graham CA, et al. Pilot study of modafinil for treatment of neurobehavioral dysfunction and fatigue in adult patients with brain tumors.
Jones LW, Guill B, Keir ST, et al. Using the theory of planned behavior to understand the determinants of exercise intention in patients diagnosed with primary brain cancer.
Fox S, Laws ER Jr, Anderson F Jr, Farace E. Complementary therapy use and quality of life in persons with high-grade gliomas.
Armstrong T, Cohen MZ, Hess KR, et al. Complementary and alternative medicine use and quality of life in patients with primary brain tumors.
Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults.
Rogers MP, Orav J, Black PM. The use of a simple Likert scale to measure quality of life in brain tumor patients.
Pringle AM, Taylor R, Whittle IR. Anxiety and depression in patients with an intracranial neoplasm before and after tumour surgery.
Mainio A, Hakko H, Niemela A, Tuurinkoski T, Koivukangas J, Rasanen P. The effect of brain tumour laterality on anxiety levels among neurosurgical patients.
Cummings JL. Neuropsychiatric manifestations of right hemisphere lesions.
Irle E, Peper M, Wowra B, Kunze S. Mood changes after surgery for tumors of the cerebral cortex.
Scheibel RS, Meyers CA, Levin VA. Cognitive dysfunction following surgery for intracerebral glioma: influence of histopathology, lesion location, and treatment.
Kaleita TA, Wellisch DK, Cloughesy TF, et al. Prediction of neurocognitive outcome in adult brain tumor patients.
Anderson SW, Damasio H, Tranel D. Neuropsychological impairments associated with lesions caused by tumor or stroke.
Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy.
Gregor A, Cull A, Traynor E, Stewart M, Lander F, Love S. Neuropsychometric evaluation of long-term survivors of adult brain tumours: relationship with tumour and treatment parameters.
Brown PD, Buckner JC, O'Fallon JR, et al. Effects of radiotherapy on cognitive function in patients with low-grade glioma measured by the Folstein Mini-Mental State Examination.
Kiebert GM, Curran D, Aaronson NK, et al. Quality of life after radiation therapy of cerebral low-grade gliomas of the adult: results of a randomised phase III trial on dose response (EORTC trial 22844). EORTC Radiotherapy Co-operative Group.
Surma-aho O, Niemela M, Vilkki J, et al. Adverse long-term effects of brain radiotherapy in adult low-grade glioma patients.
Osoba D, Brada M, Yung WK, Prados MD. Health-related quality of life in patients with anaplastic astrocytoma during treatment with temozolomide.
Carson KA, Grossman SA, Fisher JD, Shaw EG. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials.
Sachsenheimer W, Piotrowski W, Bimmler T. Quality of life in patients with intracranial tumors on the basis of Karnofsky's Performance Status.
Mackworth N, Fobair P, Prados MD. Quality of life self-reports from 200 brain tumor patients: comparisons with Karnofsky performance scores.
Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky Performance Status scale. An examination of its reliability and validity in a research setting.
Recht L, Glantz M, Chamberlain M, Hsieh CC. Quantitative measurement of quality outcome in malignant glioma patients using an independent living score (ILS). Assessment of a retrospective cohort.
Osoba D, Aaronson NK, Muller M, et al. The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires.
Weitzner MA, Meyers CA, Gelke CK, Byrne KS, Cella DF, Levin VA. The Functional Assessment of Cancer Therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors.
Locke DE, Decker PA, Sloan JA, et al. Validation of single-item linear analog scale assessment of quality of life in neuro-oncology patients.
Armstrong TS, Mendoza T, Gning I, et al. Validation of the M.D. Anderson Symptom Inventory Brain Tumor Module (MDASI-BT).
Lyons GJ. The “PRESTON Profile”—the first disease-specific tool for assessing quality of life in patients with malignant glioma.
Meyers CA, Hess KR, Yung WK, Levin VA. Cognitive function as a predictor of survival in patients with recurrent malignant glioma.
Walker M, Brown J, Brown K, Gregor A, Whittle IR, Grant R. Practical problems with the collection and interpretation of serial quality of life assessments in patients with malignant glioma.
Schwartz CE, Bode R, Repucci N, Becker J, Sprangers MA, Fayers PM. The clinical significance of adaptation to changing health: a meta-analysis of response shift.
Efficace F, Bottomley A. Health related quality of life assessment methodology and reported outcomes in randomised controlled trials of primary brain cancer patients.
Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials.
Mauer ME, Taphoorn MJ, Bottomley A, et al. Prognostic value of health-related quality-of-life data in predicting survival in patients with anaplastic oligodendrogliomas, from a phase III EORTC brain cancer group study.