Abstract
Background
This study attempts to understand the association of candidate tumour suppressor genes SH3GL2, CDKN2A (p16–p14) and CDKN2B (p15) in development of early-onset (group A) and late-onset (group B) breast carcinoma (BC).
Methods
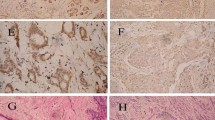
Deletion, methylation, and mutation of the candidate tumour suppressor genes (TSGs) were analysed in 47 group A and 59 group B samples. Immunohistochemical analysis was used to identify the expression status of SH3GL2 and p16. Clinicopathological correlation of the alterations was analysed by the chi-square and log-rank tests.
Results
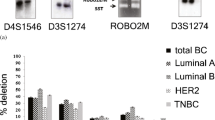
Higher frequency of overall alterations (46–62%) in SH3GL2 and p16-p14 than p15 (22–26%) indicated their importance in BC. Deletion frequencies were in the following order: group A: p14 (43%) > p16 (42%) > SH3GL2 (38%) > p15 (33%) and group B: p14 (36%) > p16 (33%) > SH3GL2 (31%) > p15 (14%) while, methylation frequencies were: group A: SH3GL2 (34%) > p16 (28%) > p14 (26%) > p15 (15%) and group B: SH3GL2 (36%) > p16 (31%) > p14 (29%) > p15 (15%). Infrequent mutation was observed only in CDKN2A common exon-2. Immunohistochemical analysis showed significant association between expression of SH3GL2 and p16 with their deletion (P = 0.01 and 0.02, respectively) and methylation status (P = 0.007 and 0.01, respectively). In group A, overall alterations of SH3GL2 showed significant association with CDKN2A locus with significant prognostic implications, whereas CDKN2A and CDKN2B loci were associated in both groups.
Conclusions
The molecular mechanisms involving CDKN2A inactivation seem to follow similar pathway in the pathogenesis of both age groups of BC while significant association of SH3GL2 with CDKN2A might play a synergistic role in the development of group A.





Similar content being viewed by others
References
Sen U, Sankaranarayanan R, Mandal S, et al. Cancer patterns in eastern India: The first report of the Kolkata Cancer Registry. Int J Cancer. 2002;100:86–91.
Chunder N, Mandal S, Basu D, et al. Deletion mapping of chromosome 1 in early onset and late onset breast tumors–a comparative study in eastern India. Pathol Res Pract. 2003;199:313–21.
Chunder N, Mandal S, Roy A, et al. Analysis of different deleted regions in chromosome 11 and their interrelations in early- and late-onset breast tumors: association with cyclin D1 amplification and survival. Diagn Mol Pathol. 2004;13:172–82.
Foo CS, Su D, Chong CK, et al. Breast cancer in young Asian women: study on survival. ANZ J Surg. 2005;75:566–72.
Nessling M, Richter K, Schwaenen C, et al. Candidate genes in breast cancer revealed by microarray based comparative genomic hybridization of archived tissue. Cancer Res. 2005;65:439–47.
Marsk KL, Varley JM. Loss of heterozygosity at chromosome 9p in ductal carcinoma in situ and invasive carcinoma of the breast. Br J Cancer. 1998;77:1439–47.
Eiriksdottir G, Sigurdsson A, Jonasson JG, et al. Loss of heterozygosity on chromosome 9 in human breast cancer: association with clinical variables and genetic changes at other chromosome regions. Int J Cancer. 1995;64:378–82.
An HX, Claas A, Savelyeva L, et al. Two regions of deletion in 9p23–24 in sporadic breast cancer. Cancer Res. 1999;59:3941–3.
Sharpless NE, DePinho RA. The INK4A/ARF locus and its two gene products. Curr Opin Genet Dev. 1999;9:22–30.
Dikic I. Mechanisms controlling EGF receptor endocytosis and degradation. Biochem Soc Trans. 2003;31:1178–81.
Xu L, Sgroi D, Sterner CJ, et al. Mutational analysis of CDKN2 (MTS1/p16ink4) in human breast carcinomas. Cancer Res. 1994;54:5262–4.
Brenner AJ, Aldaz CM. Chromosome 9p allelic loss and p16/CDKN2 in breast cancer and evidence of p16 inactivation in immortal breast epithelial cells. Cancer Res. 1995;55:2892–5.
An HX, Niederacher D, Picard F, et al. Frequent allele loss on 9p21–22 defines a smallest common region in the vicinity of the CDKN2 gene in sporadic breast cancer. Gene Chromosomes Can. 1996;17:14–20.
Quesnel B, Fenaux P, Philippe N, et al. Analysis of p16 gene deletion and point mutation in breast carcinoma. Br J Cancer. 1995;72:351–3.
Herman JG, Merlo A, Mao L, et al. Inactivation of the CDKN2/p16/ MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995;55:4525–30.
Roa JC, Anabalon L, Tapia O, et al. Promoter methylation profile in breast cancer. Rev Med Chil. 2004;132:1069–77.
Silva J, Silva JM, Dominguez G, et al. Concomitant expression of p16INK4a and p14ARF in primary breast cancer and analysis of inactivation mechanisms. J Pathol. 2003;199:289–97.
Brenner AJ, Paladugu A, Wang H, et al. Preferential loss of expression of p16(INK4a) rather than p19(ARF) in breast cancer. Clin Cancer Res. 1996;2:1993–8.
Herman JG, Jen J, Merlo A, et al. Hypermethylation-associated inactivation indicates a tumor suppressor role for p15INK4B. Cancer Res. 1996;56:722–7.
Pollock PM, Welch J, Hayward NK. Evidence for three tumor suppressor loci on chromosome 9p involved in melanoma development. Cancer Res. 2001;61:1154–61.
Giordani L, Iolascon A, Servedio V, et al. Two regions of deletion in 9p22–p24 in neuroblastoma are frequently observed in favorable tumors. Cancer Genet Cytogenet. 2002;135:42–7.
Sobin LH, Wittekind C. (eds) TNM Classification of Malignant Tumors, 6th ed. New York: Wiley; 2002
Pandey M, Singh SP, Behere PB, et al. Quality of life in patients with early and advanced carcinoma of the breast. Eur J Surg Oncol. 2000;26:20–4.
Dasgupta S, Mukherjee N, Roy S, et al. Mapping of candidate tumor suppressor genes’ loci on human chromosome 3 in head and neck squamous cell carcinoma of Indian patient population. Oral Oncol. 2002;38:6–15.
Tripathi Bhar A, Banerjee S, Chunder N, et al. Differential alterations of the genes in the CDKN2A-CCND1-CDK4-RB1 pathway are associated with the development of head and neck squamous cell carcinoma in Indian patients. J Can Res Clin Oncol. 2003;129:642–50.
Ichimura K, Bolin MB, Goike HM, et al. Deregulation of the p14ARF/MDM2/p53 pathway is a prerequisite for human astrocytic gliomas with G1-S transition control gene abnormalities. Cancer Res. 2000;60:417–24.
Singh RK, Indra D, Mitra S, et al. Deletions in chromosome 4 differentially associated with the development of cervical cancer: evidence of slit2 as a candidate tumor suppressor gene. Hum Genet. 2007;122:71–81.
Boynton RF, Blount PL, Yin J, et al. Loss of heterozygosity involving the APC and MCC genetic loci occurs in the majority of human esophageal cancers. Proc Natl Acad Sci USA. 1992;89:3385–8.
Ivanova T, Petrenko A, Gritsko T, et al. Methylation and silencing of the retinoic acid receptor-β2 gene in cervical cancer. BMC Cancer. 2002;2:4.
Heyman M, Rasool O, Brandter LB, et al. Prognostic importance of p15INK4B and p16INK4 gene inactivation in childhood acute lymphocytic leukemia. J Clin Oncol. 1996;14:1512–20.
Tannapfel A, Sommerer F, Benicke M, et al. Genetic and epigenetic alterations of the INK4a-ARF pathway in cholangiocarcinoma. J Pathol. 2002;197:624–31.
Milde-Langosch K, Bamberger AM, Rieck G, et al. Overexpression of the p16 cell cycle inhibitor in breast cancer associated with a more malignant phenotype. Breast Cancer Res Treat. 2001;67:61–70.
Berggren de Verdier PJ, Kumar R, Adolfsson J, et al. Prognostic significance of homozygous deletions and multiple duplications at the CDKN2A (p16INK4a)/ARF (p14ARF) locus in urinary bladder cancer. Scand J Urol Nephrol. 2006;40:363–9.
Geisler SA, Olshan AF, Weissler MC, et al. p16 and p53 protein expression as prognostic indicators of survival and disease recurrence from head and neck cancer. Clin Cancer Res. 2002;8:3445–53.
Shang C, Fu WN, Guo Y, et al. Study of the SH3-domain GRB2-like 2 gene expression in laryngeal carcinoma. Chin Med. J 2007;120:385–8.
Silva J, Dominguez G, Silva JM, et al. Analysis of genetic and epigenetic processes that influence p14ARF expression in breast cancer. Oncogene. 2001;20:4586–90.
Bisogna M, Calvano JE, Ho GH, et al. Molecular analysis of the INK4A and INK4B gene loci in human breast cancer cell lines and primary carcinomas. Cancer Genet Cytogenet. 2001;125:131–8.
Hewitt C, Lee Wu C, Evans G, et al. Germline mutation of ARF in a melanoma kindred. Hum Mol Genet. 2002;11:1273–9.
Acknowledgements
We are thankful to the Director, Chittaranjan National Cancer Institute, Kolkata-700026, India. Financial support was provided by CSIR grant no. 60(0077)/06/EMR-II to Dr. C. K. Panda and CSIR–JRF/NET Fellowship grant no. 9/30(031)/2002- EMR-I to Mr. S. Sinha.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sinha, S., Chunder, N., Mukherjee, N. et al. Frequent Deletion and Methylation in SH3GL2 and CDKN2A Loci are Associated with Early- and Late-onset Breast Carcinoma. Ann Surg Oncol 15, 1070–1080 (2008). https://doi.org/10.1245/s10434-007-9790-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-007-9790-0