Abstract
Background
mTOR signaling has been suggested to be an important factor involved in tumorigenesis, but its role in human colorectal cancer (CRC) has not been completely elucidated. Herein, the purpose of this study was to analyze the distribution pattern of mTOR signaling components in CRC and adenoma and to determine whether targeted inhibition of mTOR could be a potential therapeutic strategy for CRC.
Methods
Immunohistochemical analysis was performed on human CRC and adenoma for mTOR signaling components, including mTOR, p70s6 K, and 4EBP1. HCT116 and SW480 human CRC cell lines were treated with siRNA directed against mTOR, and cell viability, cell cycle, and apoptosis were assessed. HCT116 and SW480 cells were injected into athymic nude mice to establish a CRC xenograft model. Mice were randomly transfected with either nontargeting control or mTOR siRNA, and tumor volume, mTOR signaling activity, and apoptosis were evaluated.
Results
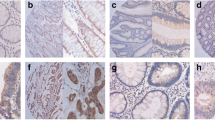
mTOR signaling components, including mTOR, p70s6 K, and 4EBP1, were highly activated in glandular elements of CRC and colorectal adenomas with high-grade intraepithelial neoplasia (HIN), with a correlation between staining intensity and depth of infiltration in CRC. Inhibition of mTOR expression using a specific mTOR siRNA resulted in considerably decreased in vitro and in vivo cell growth.
Conclusions
mTOR signaling is associated with the clinical pathological parameters of human CRC. siRNA-mediated gene silencing of mTOR may be a novel therapeutic strategy for CRC.




Similar content being viewed by others
References
Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84.
Tsang CK, Zheng XF. TOR-in(g) the nucleus. Cell Cycle. 2007;6:25–9.
Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–75.
Jacinto E, Loewith R, Schmidt A, Lin S, Rüegg MA, Hall A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–8.
Graff JR, Konicek BW, Carter JH, Marcusson EG. Targeting the eukaryotic translation initiation factor 4E for cancer therapy. Cancer Res. 2008;68:631–4.
Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007;13:252–9.
Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22.
Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–22.
Chiang GG, Abraham RT. Targeting the mTOR signaling network in cancer. Trends Mol Med. 2007;13:433–42.
Dancey JE. Therapeutic targets: MTOR and related pathways. Cancer Biol Ther. 2006;5:1065–73.
Tsang CK, Qi H, Liu LF, Zheng XF. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov Today. 2007;12:112–24.
Zhang YJ, Tian XQ, Sun DF, Zhao SL, Xiong H, Fang JY. Combined inhibition of MEK and mTOR signaling inhibits initiation and progression of colorectal cancer. Cancer Invest. 2009;27:273–85.
Foster DA, Toschi A. Targeting mTOR with rapamycin: One dose does not fit all. Cell Cycle. 2009; 8:1026–9.
Haney SA. Expanding the repertoire of RNA interference screens for developing new anticancer drug targets. Expert Opin Ther Targets. 2007;11:1429–41.
Micklem DR, Lorens JB. RNAi screening for therapeutic targets in human malignancies. Curr Pharm Biotechnol. 2007;8:337–43.
Hamilton SR, Vogelstein B, Kudo S, Riboli E, Nakamura S, Hainaut P, et al. In: Hamilton SR, Aaltonen L, editors. Pathology and genetics of tumours of the digestive system. World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2001. p. 104–19.
Rojo F, Najera L, Lirola J, Jiménez J. Guzmán M, Sabadell MD, et al. 4E-binding protein 1, a cell signaling hallmark in breast cancer that correlates with pathologic grade and prognosis. Clin Cancer Res. 2007;13:81–9.
Zhang YJ, Dai Q, Wu SM, Zhu HY, Shen GF, Li EL, et al. Susceptibility for NSAIDs-induced apoptosis correlates to p53 gene status in gastric cancer cells. Cancer Invest. 2008;26:868–77.
Niola F, Evangelisti C, Campagnolo L, Massalini S, Bué MC, Mangiola A, et al. A plasmid-encoded VEGF siRNA reduces glioblastoma angiogenesis and its combination with interleukin-4 blocks tumor growth in a xenograft mouse model. Cancer Biol Ther. 2006;5:174–9.
Nozawa H, Watanabe T, Nagawa H. Phosphorylation of ribosomal p70 S6 kinase and rapamycin sensitivity in human colorectal cancer. Cancer Lett. 2007;251:105–13.
Faried LS, Faried A, Kanuma T, Aoki H, Sano T, Nakazato T, et al. Expression of an activated mammalian target of rapamycin in adenocarcinoma of the cervix: a potential biomarker and molecular target therapy. Mol Carcinog. 2008;47:446–57.
Darb-Esfahani S, Faggad A, Noske A, Weichert W, Buckendahl AC, Müller B, et al. Phospho-mTOR and phospho-4EBP1 in endometrial adenocarcinoma: association with stage and grade in vivo and link with response to rapamycin treatment in vitro. J Cancer Res Clin Oncol. 2009;135:933–41.
Khaleghpour K, Li Y, Banville D, Yu Z, Shen SH. Involvement of the PI 3-kinase signaling pathway in progression of colon adenocarcinoma. Carcinogenesis. 2004;25:241–8.
Bjornsti MA, Houghton PJ. Lost in translation: dysregulation of cap-dependent translation and cancer. Cancer Cell. 2004;5:519–23.
Armengol G, Rojo F, Castellví J, Iglesias C, Cuatrecasas M, Pons B, et al. 4E-binding protein 1: a key molecular “funnel factor” in human cancer with clinical implications. Cancer Res. 2007;67:7551–5.
Castellvi J, Garcia A, Rojo F, Ruiz-Marcellan C, Gil A, Baselga J, et al. Phosphorylated 4E binding protein 1: a hallmark of cell signaling that correlates with survival in ovarian cancer. Cancer. 2006;107:1801–11.
Seeliger H, Guba M, Kleespies A, Jauch KW, Bruns CJ. Role of mTOR in solid tumor systems: a therapeutical target against primary tumor growth, metastases, and angiogenesis. Cancer Metastasis Rev. 2007;26: 611–21.
Easton JB, Houghton PJ. mTOR and cancer therapy. Oncogene. 2006;25:6436–46.
Georgakis GV, Younes A. From Rapa Nui to rapamycin: targeting PI3 K/Akt/mTOR for cancer therapy. Expert Rev Anticancer Ther. 2006;6:131–40.
Peponi E, Drakos E, Reyes G, Leventaki V, Rassidakis GZ, Medeiros LJ. Activation of mammalian target of rapamycin signaling promotes cell cycle progression and protects cells from apoptosis in mantle cell lymphoma. Am J Pathol. 2006;169:2171–80.
Hou G, Xue L, Lu Z, Fan T, Tian F, Xue Y. An activated mTOR/p70S6 K signaling pathway in esophageal squamous cell carcinoma cell lines and inhibition of the pathway by rapamycin and siRNA against mTOR. Cancer Lett. 2007;253:236–48.
Ke N, Zhou D, Chatterton JE, Liu G, Chionis J, Zhang J, et al. A new inducible RNAi xenograft model for assessing the staged tumor response to mTOR silencing. Exp Cell Res. 2006;312:2726–34.
George J, Tsutsumi M. siRNA-mediated knockdown of connective tissue growth factor prevents N-nitrosodimethylamine-induced hepatic fibrosis in rats. Gene Ther. 2007;14:790–803.
Paranjpe S, Bowen WC, Bell AW, Nejak-Bowen K, Luo JH, Michalopoulos GK. Cell cycle effects resulting from inhibition of hepatocyte growth factor and its receptor c-Met in regenerating rat livers by RNA interference. Hepatology. 2007;45:1471–7.
Grzelinski M, Urban-Klein B, Martens T, Lamszus K, Bakowsky U, Höbel S, et al. RNA interference-mediated gene silencing of pleiotrophin through polyethylenimine-complexed small interfering RNAs in vivo exerts antitumoral effects in glioblastoma xenografts. Hum Gene Ther. 2006;17:751–66.
Corey DR. Chemical modification: the key to clinical application of RNA interference? J Clin Invest. 2007;117:3615–22.
Prakash TP, Bhat B. 2′-Modified oligonucleotides for antisense therapeutics. Curr Top Med Chem. 2007;7:641–9.
Acknowledgment
This work was supported by grants from the National Science Fund for Distinguished Young Scholars (30625034), the National Basic Research of China 973 program (2005CB522400), the Shanghai Leading Academic Discipline Project (Y0205), and the Changjiang Plan in China to Fang JY. This work was also supported by grants from the Science and Technology Commission of Shanghai Municipality (08ZR1413100), the Shanghai Baoshan District Science Fund (07-E-2), and the Shanghai Jiaotong University School of Medicine (BXJ0721) to Zhang YJ.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, YJ., Dai, Q., Sun, DF. et al. mTOR Signaling Pathway Is a Target for the Treatment of Colorectal Cancer. Ann Surg Oncol 16, 2617–2628 (2009). https://doi.org/10.1245/s10434-009-0555-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-009-0555-9