Abstract
Background
Activation-induced cytidine deaminase (AID) is expressed in B lymphocytes and triggers antibody diversification. Recent reports have indicated that the constitutive expression of AID in mice causes not only lymphomas, but also cancers of some organs including the lung, prompting us to investigate the expression and effect of AID on human lung cancer.
Materials and Methods
We examined AID mRNA expression in 17 lung cancer cell lines and 51 primary lung cancers using a quantitative RT-PCR analysis. Next, we established H1299 lung cancer cells stably overexpressing AID and performed a supF forward mutation assay. We then examined AID protein expression and p53 mutation in 129 primary lung cancers by an immunohistochemical analysis and PCR-SSCP and sequencing analyses, respectively.
Results
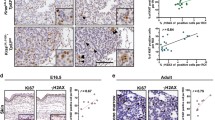
Aberrant mRNA expression of AID was detected in 29% (5 of 17) of the lung cancer cell lines and 31% (16 of 51) of the primary lung cancers. AID-overexpressing H1299 clones showed a 5.0- to 6.1-fold higher mutation frequency than an empty vector-transfected H1299 clone, and about half of the AID-induced mutations were base substitutions, indicating that AID induces gene mutations in lung cancer cells. Furthermore, an association was found between the AID protein expression level and the p53 mutation status in an analysis of 129 primary lung cancers. A further expression analysis revealed that a portion of AID is localized at the centrosomes.
Conclusion
Our current findings suggest that the aberrant expression of AID may be involved in a subset of human lung cancers as a result of its mutation-inducing activity.




Similar content being viewed by others
References
Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–75.
Quadrelli S, Lyons G, Colt H, Chimondeguy D, Silva C. Lung cancer as a second primary malignancy: increasing prevalence and its influence on survival. Ann Surg Oncol. 2009;16:1033–8.
Mitsudomi T, Yatabe Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 2010;277:301–8.
Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–80.
Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers—a different disease. Nat Rev Cancer. 2007;7:778–90.
Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–63.
Delker RK, Fugmann SD, Papavasiliou FN. A coming-of-age story: activation-induced cytidine deaminase turns 10. Nat Immunol. 2009;10:1147–53.
Liu M, Duke JL, Richter DJ, Vinuesa CG, Goodnow CC, Kleinstein SH, et al. Two levels of protection for the B cell genome during somatic hypermutation. Nature. 2008;451:841–5.
Yoshikawa K, Okazaki IM, Eto T, Kinoshita K, Muramatsu M, Nagaoka H, et al. AID enzyme-induced hypermutation in an actively transcribed gene in fibroblasts. Science. 2002;296:2033–6.
Okazaki IM, Hiai H, Kakazu N, Yamada S, Muramatsu M, Kinoshita K, et al. Constitutive expression of AID leads to tumorigenesis. J Exp Med. 2003;197:1173–81.
Morisawa T, Marusawa H, Ueda Y, Iwai A, Okazaki IM, Honjo T, et al. Organ-specific profiles of genetic changes in cancers caused by activation-induced cytidine deaminase expression. Int J Cancer. 2008;123:2735–40.
Matsumoto Y, Marusawa H, Kinoshita K, Endo Y, Kou T, Morisawa T, et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13:470–6.
Klemm L, Duy C, Iacobucci I, Kuchen S, von Levetzow G, Feldhahn N, et al. The B cell mutator AID promotes B lymphoid blast crisis and drug resistance in chronic myeloid leukemia. Cancer Cell. 2009;16:232–45.
Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, et al. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10:38–47.
Tsuboi M, Mori H, Bunai T, Kageyama S, Suzuki M, Okudela K, et al. Secreted form of EphA7 in lung cancer. Int J Oncol. 2010;36:635–40.
Shinmura K, Tao H, Nagura K, Goto M, Matsuura S, Mochizuki T, et al. Suppression of hydroxyurea-induced centrosome amplification by NORE1A and down-regulation of NORE1A mRNA expression in non-small cell lung carcinoma. Lung Cancer. 2011;71:19–27.
Shinmura K, Bennett RA, Tarapore P, Fukasawa K. Direct evidence for the role of centrosomally localized p53 in the regulation of centrosome duplication. Oncogene. 2007;26:2939–44.
Matsuda T, Yagi T, Kawanishi M, Matsui S, Takebe H. Molecular analysis of mutations induced by 2-chloroacetaldehyde, the ultimate carcinogenic form of vinyl chloride, in human cells using shuttle vectors. Carcinogenesis. 1995;16:2389–94.
Yamane A, Shinmura K, Sunaga N, Saitoh T, Yamaguchi S, Shinmura Y, et al. Suppressive activities of OGG1 and MYH proteins against G:C to T:A mutations caused by 8-hydroxyguanine but not by benzo[a]pyrene diol epoxide in human cells in vivo. Carcinogenesis. 2003;24:1031–7.
Yamada H, Shinmura K, Yamamura Y, Kurachi K, Nakamura T, Tsuneyoshi T, et al. Identification and characterization of a novel germline p53 mutation in a patient with glioblastoma and colon cancer. Int J Cancer. 2009;125:973–6.
Goto M, Shinmura K, Igarashi H, Kobayashi M, Konno H, Yamada H, et al. Altered expression of the human base excision repair gene NTH1 in gastric cancer. Carcinogenesis. 2009;30:1345–52.
Shinmura K, Iwaizumi M, Igarashi H, Nagura K, Yamada H, Suzuki M, et al. Induction of centrosome amplification and chromosome instability in p53-deficient lung cancer cells exposed to benzo[a]pyrene diol epoxide (B[a]PDE). J Pathol. 2008;216:365–74.
Moudjou M, Bornens M. Method of centrosome isolation from cultured animal cells. In: Celis JE, editor. Cell biology: a laboratory handbook. New York: Academic Press; 1998. p. 111–9.
Suzuki H, Takahashi T, Kuroishi T, Suyama M, Ariyoshi Y, Takahashi T, et al. p53 mutations in non-small cell lung cancer in Japan: association between mutations and smoking. Cancer Res. 1992;52:734–6.
Greiner A, Tobollik S, Buettner M, Jungnickel B, Herrmann K, Kremmer E, et al. Differential expression of activation-induced cytidine deaminase (AID) in nodular lymphocyte-predominant and classical Hodgkin lymphoma. J Pathol. 2005;205:541–7.
Fukasawa K. Oncogenes and tumour suppressors take on centrosomes. Nat Rev Cancer. 2007;7:911–24.
Pfeifer GP, Besaratinia A. Mutational spectra of human cancer. Hum Genet. 2009;125:493–506.
Dorsett Y, Robbiani DF, Jankovic M, Reina-San-Martin B, Eisenreich TR, Nussenzweig MC. A role for AID in chromosome translocations between c-myc and the IgH variable region. J Exp Med. 2007;204:2225–32.
Robbiani DF, Bothmer A, Callen E, Reina-San-Martin B, Dorsett Y, Difilippantonio S, et al. AID is required for the chromosomal breaks in c-myc that lead to c-myc/IgH translocations. Cell. 2008;135:1028–38.
Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6.
Shinmura K, Kageyama S, Tao H, Bunai T, Suzuki M, Kamo T, et al. EML4-ALK fusion transcripts, but no NPM-, TPM3-, CLTC-, ATIC-, or TFG-ALK fusion transcripts, in non-small cell lung carcinomas. Lung Cancer. 2008;61:163–9.
Takahashi T, Sonobe M, Kobayashi M, Yoshizawa A, Menju T, Nakayama E, et al. Clinicopathologic features of non-small-cell lung cancer with EML4-ALK fusion gene. Ann Surg Oncol. 2010;17:889–97.
Olivier M, Hussain SP, Caron de Fromentel C, Hainaut P, Harris CC. TP53 mutation spectra and load: a tool for generating hypotheses on the etiology of cancer. Lyon: IARC Sci Publ.; 2004. p. 247–70.
Lingle WL, Barrett SL, Negron VC, D’Assoro AB, Boeneman K, Liu W, et al. Centrosome amplification drives chromosomal instability in breast tumor development. Proc Natl Acad Sci USA. 2002;99:1978–83.
Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460:278–82.
Acknowledgment
We are grateful to Dr. D.C. Gruenert (California Pacific Medical Center Research Institute, USA), Dr. T. Kaneko (Yokohama City University, School of Medicine, Japan), Dr. T. Niki (Jichi Medical University, Japan), and Dr. Y. Dobashi (Omiya Medical Center, Japan) for providing us with the cell lines. We acknowledge Ms. K. Nagura, Mr. K. Mizuno, and Ms. N. Sakamoto (Hamamatsu University School of Medicine) for their technical assistance. This work was supported by grants from the MHLW (21-1), the JSPS (22590356), the MEXT (20014007 and 221S0001), and the Smoking Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shinmura, K., Igarashi, H., Goto, M. et al. Aberrant Expression and Mutation-Inducing Activity of AID in Human Lung Cancer. Ann Surg Oncol 18, 2084–2092 (2011). https://doi.org/10.1245/s10434-011-1568-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-011-1568-8