Abstract
Background
Sentinel lymph node biopsy (SLNB) in melanoma is currently performed using the standard dual technique (radioisotope and blue dye). The magnetic technique is non-radioactive and provides a brown color change in the sentinel lymph node (SLN) through an intradermal injection of a magnetic tracer, and utilizes a handheld magnetometer. The MELAMAG Trial compared the magnetic technique with the standard technique for SLNB in melanoma.
Methods
Clinically node-negative patients with primary cutaneous melanoma were recruited from four centers. SLNB was undertaken after intradermal administration of both the standard (blue dye and radioisotope) and magnetic tracers. The SLN identification rate per patient, with the two techniques, was compared.
Results
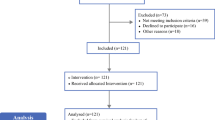
A total of 133 patients were recruited, 129 of which were available for final analysis. The sentinel node identification rate was 97.7 % (126/129) with the standard technique and 95.3 % (123/129) with the magnetic technique [2.3 % difference; 95 % upper confidence limit (CL) 6.4; 5.4 % discordance]. With radioisotope alone, the SLN identification rate was 95.3 % (123/129), as with the magnetic technique (0 % difference; 95 % upper CL 4.5; 7.8 % discordance). The lymph node retrieval rate was 1.99 nodes per patient overall, 1.78 with the standard technique and 1.87 with the magnetic technique.
Conclusions
The magnetic technique is feasible for SLNB in melanoma with a high SLN identification rate, but is associated with skin staining. When compared with the standard dual technique, it did not reach our predefined non-inferiority margin.
Similar content being viewed by others
References
Wong SL, Balch CM, Hurley P, et al. Sentinel lymph node biopsy for melanoma: American Society of Clinical Oncology and Society of Surgical Oncology joint clinical practice guideline. J Clin Oncol. 2012;30(23):2912–18.
Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370(7):599–609.
Valsecchi ME, Silbermins D, de Rosa N, Wong SL, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in patients with melanoma: a meta-analysis. J Clin Oncol. 2011;29(11):1479–87.
Morton DL, Thompson JF, Essner R, et al. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: a multicenter trial. Multicenter Selective Lymphadenectomy Trial Group. Ann Surg. 1999;230(4):453–63 (Discussion 463–455).
Neves RI, Reynolds BQ, Hazard SW, Saunders B, Mackay DR. Increased post-operative complications with methylene blue versus lymphazurin in sentinel lymph node biopsies for skin cancers. J Surg Oncol. 2011;103(5):421–25.
Morton DL, Cochran AJ, Thompson JF, et al. Sentinel node biopsy for early-stage melanoma: accuracy and morbidity in MSLT-I, an international multicenter trial. Ann Surg. 2005;242(3):302–11 (Discussion 311–303).
Barthelmes L, Goyal A, Newcombe RG, et al. Adverse reactions to patent blue V dye: the NEW START and ALMANAC experience. Eur J Surg Oncol. 2010;36(4):399–403.
Leong SP, Donegan E, Heffernon W, Dean S, Katz JA. Adverse reactions to isosulfan blue during selective sentinel lymph node dissection in melanoma. Ann Surg Oncol. 2000;7(5):361–66.
Stratmann SL, McCarty TM, Kuhn JA. Radiation safety with breast sentinel node biopsy. Am J Surg. 1999;178(6):454–57.
Ruth TJ. The medical isotope crisis: how we got here and where we are going. J Nucl Med Technol. 2014;42(4):245–48.
Ahmed M, Purushotham AD, Douek M. Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. Lancet Oncol. 2014;15(8):e351–62.
Joshi T, Pankhurst QA, S. H, Douek M. Magnetic nanoparticles for detecting cancer spread. Breast Cancer Res Treat. 2007;1006(1):S129.
Johnson L, Douek M. Magnetic sentinel lymph node detection for breast cancer. Cancer Res. 2010;70:140s.
Douek M, Klaase J, Monypenny I, et al. Sentinel node biopsy using a magnetic tracer versus standard technique: the SentiMAG Multicentre Trial. Ann Surg Oncol. 2014;21(4):1237–45.
Piñero-Madrona A, Torró-Richart JA, de León-Carrillo JM, et al. Superparamagnetic iron oxide as a tracer for sentinel node biopsy in breast cancer: a comparative non-inferiority study. Eur J Surg Oncol. 2015;41(8):991–97.
Rubio IT, Diaz-Botero S, Esgueva A, et al. The superparamagnetic iron oxide is equivalent to the Tc99 radiotracer method for identifying the sentinel lymph node in breast cancer. Eur J Surg Oncol. 2015;41(1):46–51.
Thill M, Kurylcio A, Welter R, et al. The Central-European SentiMag study: sentinel lymph node biopsy with superparamagnetic iron oxide (SPIO) vs. radioisotope. Breast. 2014;23(2):175–79.
Winter A, Woenkhaus J, Wawroschek F. A novel method for intraoperative sentinel lymph node detection in prostate cancer patients using superparamagnetic iron oxide nanoparticles and a handheld magnetometer: the initial clinical experience. Ann Surg Oncol. 2014;21(13):4390–96.
Anninga B, Ahmed M, Douek M. Magnetic guidance for cancer surgery. Br J Surg. 2015;102(2):e12–14.
Newcombe RG. Improved confidence intervals for the difference between binomial proportions based on paired data. Stat Med. 1998;17(22):2635–50.
Ahmed M, de Rosales RT, Douek M. Preclinical studies of the role of iron oxide magnetic nanoparticles for nonpalpable lesion localization in breast cancer. J Surg Res. 2013;185(1):27–35.
Acknowledgments
The authors thank the patients and their relatives for participating in this trial, and also thank the research nurses and the National Institute for Health Research (NIHR) for helping recruiting patients into the trial, members of the Data Management Committee for their advice, and Endomagnetics Ltd (UK) for providing devices and maintaining them during the recruitment phase of this trial. The sponsors of this trial were King’s College London (UK), and Guy’s and St. Thomas’ NHS Foundation Trust (UK).
Author Contributions
Bauke Anninga, Samantha White, Marc Moncrieff, Peter Dziewulski, Jenny Geh, Joost Klaase, Hans Garmo, Fernanda Castro, Sarah Pinder, Quentin Pankhurst, Margaret Hall-Craggs, and Michael Douek participated in the design and concept of the study; Bauke Anninga, Samantha White, Marc Moncrieff, Peter Dziewulski, Jenny Geh, Joost Klaase, Fernanda Castro, and Michael Douek participated in the data collection and enrolment of participants in this study; Bauke Anninga, Hans Garmo, Fernanda Castro, and Michael Douek participated in the data analysis; and Bauke Anninga and Michael Douek drafted the manuscript. All authors interpreted the data and approved the final manuscript.
Conflicts of interest
Although one of the authors (Quentin Pankhurst) fulfills a part-time paid advisory role as Chief Scientist (Physics) for the magnetic SLNB company Endomagnetics Ltd, his role in this work has been purely academic. All other authors have no disclosures to make regarding financial and personal relationships with other people or organizations that could inappropriately influence their work.
Disclosures
None.
Funding
This work was supported by a grant from the Technology Strategy Board (now known as Innovate UK). The funder (Technology Strategy Board), as well as the manufacturer of the investigated devices (Endomagnetics Ltd, UK), had no role in the trial design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the trial and had final responsibility for the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
The members of MELAMAG Multicentre Trialists Group are given in Appendix.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix: MELAMAG Trialists Group (Trial Collaborators)
Prof. Michael Douek, CI, Chair of TMG (King’s College London, London, UK)
Prof. Peter Dziewulski, PI, Co-Chair of TMG (Broomfield Hospital, Chelmsford, UK)
Mr Muneer Ahmed (King’s College London, London, UK)
Mr Bauke Anninga, TMG (King’s College London, London, UK)
Dr Eduardo Calonje, Lead Pathologist (Guy’s and St. Thomas’ Hospitals, London, UK)
Dr Fernanda Castro, CTC, TMG (Guy’s and St. Thomas’ Hospitals, London, UK)
Dr Hans Garmo, STAT (King’s College London, London, UK)
Ms Jenny Geh, PI (Guy’s and St. Thomas’ Hospitals, London, UK)
Ms Samantha H. White, TMG (Norfolk and Norwich University Hospital, Norwich, UK)
Dr Bennie ten Haken, TMG (University of Twente, Enschede, The Netherlands)
Prof. Margaret A. Hall-Craggs, Lead Radiologist (University College Hospital, London, UK)
Dr Mark Harries (Guy’s and St. Thomas’ Hospitals, London, UK)
Mr Joost Klaase, PI, TMG (Medisch Spectrum Twente, Enschede, The Netherlands)
Dr Katie Lacy (Guy’s and St. Thomas’ Hospitals, London, UK)
Mr Dan Marsh (Broomfield Hospital, Chelmsford, UK)
Ms April Matthews, PA (Independent Cancer Patients’ Voice, London, UK)
Mr Marc Moncrieff, PI, TMG (Norfolk and Norwich University Hospital, Norwich, UK)
Mr Tom Oxenham (University College Hospital, London, UK)
Prof. Quentin A Pankhurst, TMG (University College London, London, UK)
Prof. Sarah Pinder (King’s College London, London, UK)
Mr Joost Pouw (University of Twente, Enschede, The Netherlands)
Ms Vernie Ramalingam, CTM, TMG (Guy’s and St. Thomas’ Hospitals, London, UK)
Prof. Tobias Scheaffter (King’s College London, London, UK)
Dr Rafael Torres De Rosales (King’s College London, London, UK)
Ms Susan Vreemann (University of Twente, Enschede, The Netherlands)
Key: CI Chief Investigator, TMG Trial Management Group, PI Principal Investigator, CTC Clinical Trial Coordinator, STAT Statistician, PA Patient Advocate, CTM Clinical Trial Manager
Surgical Collaborators
Mr Naguib El-Muttardi (Broomfield Hospital, Chelmsford, UK)
Mr Quentin Frew (Broomfield Hospital, Chelmsford, UK)
Mr Martin Heaton (Norfolk and Norwich University Hospital, Norwich, UK)
Mr Kenneth Kok (Norfolk and Norwich University Hospital, Norwich, UK)
Mr Alastair MacKenzie-Ross (Guy’s and St. Thomas’ Hospitals, London, UK)
Mr Daniel Marsh (Broomfield Hospital, Chelmsford, UK)
Ms Sankhya Sen (Broomfield Hospital, Chelmsford, UK)
Mr Nicholas Sheppard (Norfolk and Norwich University Hospital, Norwich, UK)
Mr Mobinulla Syed (Broomfield Hospital, Chelmsford, UK)
Mr Ewan Wilson (Norfolk and Norwich University Hospital, Norwich, UK)
Research Nurses and Trial Coordinators
Ms Karen Collins (Broomfield Hospital, Chelmsford, UK)
Ms Fiona Mc Neela (Broomfield Hospital, Chelmsford, UK)
Ms Vernie Ramalingam (Guy’s and St. Thomas’ Hospitals, London, UK)
Ms Sweta Sethi (Guy’s and St. Thomas’ Hospitals, London, UK)
Ms Anja Stam (Medisch Spectrum Twente, Enschede, The Netherlands)
Ms Beverly Underwood (Norfolk and Norwich University Hospital, Norwich, UK)
Ms Sara Wilkinson (Norfolk and Norwich University Hospital, Norwich, UK)
Data Monitoring Committee (Independent)
Dr Jurgen Fütterer, Interventional Radiologist (University Medical Centre Nijmegen, Nijmegen, The Netherlands)
Mr Antonio Orlando, Consultant Plastic Surgeon (North Bristol NHS Trust, Bristol, UK)
Ms Jane Warwick, Statistician (University of London, London, UK)
Rights and permissions
About this article
Cite this article
Anninga, B., White, S.H., Moncrieff, M. et al. Magnetic Technique for Sentinel Lymph Node Biopsy in Melanoma: The MELAMAG Trial. Ann Surg Oncol 23, 2070–2078 (2016). https://doi.org/10.1245/s10434-016-5113-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5113-7