Abstract
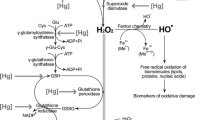
In order to explore the observed association among mercury, atherosclerosis, and coronary heart disease, the effects of mercury, copper, and iron on the peroxidation of low-density lipoprotein (LDL) and on the enzymatic activities of glutathione peroxidase and myeloperoxidase were investigated in vitro. On the basis of our nuclear magnetic resonance (NMR) experiments, we conclude that mercury does not promote the direct nonenzymatic peroxidation of LDL, like copper and iron. In our enzyme measurements, mercury inhibited slightly myeloperoxidase, although not significantly in presence of LDL. Instead, inorganic mercury, but not methylmercury chloride, inhibited glutathione peroxidase effectively and copper event at 10 μmol/L, below physiological concentrations, doubled the inhibition rate. Copper and iron had no direct effect on glutathione peroxidase, but they both seem to activate production of HOCl by myeloperoxidase. We conclude here that, first, mercury and methylmercury do not promote direct lipid peroxidation, but that, second, a simultaneous exposure to high inorganic mercury, copper, and iron and low selenium concentrations can lead to a condition in which mercury promotes lipid peroxidations. This mechanism provides a plausible molecular-level explanation for the observed association between high body mercury content and atherosclerosis.
Similar content being viewed by others
References
I. Pinchuk and D. Lichtenberg, The mechanism of action of antioxidants against lipoprotein peroxidation, evaluation based on kinetic experiments, Prog. Lipid Res. 41, 279–314 (2002).
H. Kuhn and L. Chan, The role of 15-lipoxygenase in atherogenesis: pro- and antiatherogenic actions, Curr. Opin. Lipidol. 8, 111–117 (1997).
A. Lass, J. Belkner, H. Esterbauer, and H. Kuhn, Lipoxygenase treatment renders low density lipoprotein susceptible to Cu2+-catalysed oxidation, Biochem. J. 314, 577–585 (1996).
V. J. O’Leary, V. M. Darley-Usmar, L. J. Russell, and D. Stone, Pro-oxidant effects of lipoxygenase-derived peroxides on the copper-initiated oxidation of low density lipoprotein, Biochem. J. 282, 631–634 (1992).
G. A. Francis, High density lipoprotein oxidation: in vitro susceptibility and potential in vivo consequences, BBA-Mol. Cell Biol. Lipids 1483, 217–235 (2000).
P. Libby, Inflammation in atherosclerosis, Nature 420, 868–874 (2002).
H. Esterbauer, J. Gebicki, H. Puhl, and G. Jurgens, The role of lipid peroxidation and antioxidants in oxidative modification of LDL, Free Radical Biol. Med. 13, 341–390 (1992).
Y. Yoshida, J. Tsuchiya, and E. Niki, Interaction of alpha-tocopherol with copper and its effect on lipid peroxidation, Biochim. Biophys. Acta-Gen. Subjects 1200, 85–92 (1994).
A. Kontush, S. Meyer, B. Finckh, A. Kohlschutter, and U. Beisiegel, Alpha-tocopherol a reductant for Cu(II) in human lipoproteins: triggering role in the initiation of lipoprotein oxidation, J. Biol. Chem. 271, 11,106–11,112 (1996).
O. Ziouzenkova, A. Sevanian, P. M. Abuja, P. Ramos, and H. Esterbauer, Copper can promote oxidation of LDL by markedly different mechanisms, Free Radical Biol. Med. 24, 607–623 (1998).
J. T. Salonen, K. Seppänen, K. Nyyssönen, et al., Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in Eastern Finnish men, Circulation 91, 645–655 (1995).
E. Guallar, M. I. Sanz-Gallardo, P. Van’t Veer, et al., Mercury, fish oils, and myocardial infarction, N. Engl. J. Med. 347, 1747–1754 (2002).
J. T. Salonen, K. Seppänen, T. A. Lakka, R. Salonen, and G. A. Kaplan, Mercury accumulation and accelerated progression of carotid atherosclerosis: a population-based prospective 4-year follow-up study in men in Eastern Finland, Atherosclerosis 148, 265–273 (2000).
Y. L. Huang, S. L. Cheng, and T. H. Lin, Lipid peroxidation in rats administrated with mercuric chloride, Biol. Trace Element Res. 52, 193–206 (1996).
T. H. Lin, Y. L. Huang, and S. F. Huang, Lipid peroxidation in liver of rats administrated with methyl mercuric chloride, Biol. Trace Element Res. 54, 33–41 (1996).
K. Takahashi, N. Avissar, J. Whitin, and H. Cohen, Purification and characterization of human plasma glutathione peroxidase: a selenoglycoprotein distinct from the known cellular enzyme, Arch. Biochem. Biophys. 256, 677–686 (1987).
J. Gailer, G. N. George, I. J. Pickering, et al., Structural basis of the antagonism between inorganic mercury and selenium in mammals, Chem. Res. Toxicol. 13, 1135–1142 (2000).
H. J. Raderecht, Molecular biology in the interpretation of metabolic toxic mechanisms and possibilities for estimating the potential toxicity of metals, illustrated by the example of mercury and iron, Clin. Lab. 44, 33–50 (1998).
S. J. Klebanoff, Myeloperoxidase, Proc. Assoc. Am. Physician 111, 383–389 (1999).
R. Laatikainen, M. Niemitz, W. J. Malaisse, M. Biesemans, and R. Willem, A computational strategy for the deconvolution of NMR spectra with multiplet structures and constraints: analysis of overlapping C-13-H-2 multiplets of C-13 enriched metabolites from cell suspensions incubated in deuterated media, Magn. Reson. Med. 36, 359–365 (1996).
S. Blankenberg, H. J. Rupprecht, C. Bickel, et al., Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease, N. Engl. J. Med. 349, 1605–1613 (2003).
M. L. Brennan, M. S. Penn, F. Van Lente, et al., Prognostic value of myeloperoxidase in patients with chest pain, N. Engl. J. Med. 349, 1595–1604 (2003).
S. Baldus, C. Heeschen, T. Meinertz, et al., Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes, Circulation 108, 1440–1445 (2003).
G. V. Iyengar, Reevaluation of the trace element content in reference man, Radiat. Phys. Chem. 51, 545–560 (1998).
D. Metodiewa and H. B. Dunford, The reactions of horseradish peroxidase, lactoperoxidase, and myeloperoxidase with enzymatically generated superoxide, Arch. Biochem. Biophys. 272, 245–253 (1989).
Y. Z. Fang, S. Yang, and G. Wu, Free radicals, antioxidants, and nutrition, Nutrition 18, 872–879 (2002).
A. G. Splittgerber and A. L. Tappel, Inhibition of glutathione peroxidase by cadmium and other metal ions, Arch. Biochem. Biophys. 197, 534–542 (1979).
E. M. Bem, K. Mailer, and C. M. Elson, Influence of mercury(II), cadmium(II), methylmercury, and phenylmercury on the kinetic properties of rat liver glutathione peroxidase, Can. J. Biochem. Cell Biol. 63, 1212–1216 (1985).
Y. Hirota, Effect of methylmercury on the activity of glutathione peroxidase in rat liver, Am. Ind. Hyg. Assoc. J. 47, 556–558 (1986).
J. A. Berliner and J. W. Heinecke, The role of oxidized lipoproteins in atherogenesis, Free Radical Biol. Med. 20, 707–727 (1996).
J. W. Heinecke, W. Li, G. A. Francis, and J. A. Goldstein, Tyrosyl radical generated by myeloperoxidase catalyzes the oxidative cross-linking of proteins, J. Clin. Invest. 91, 2866–2872 (1993).
J. W. Heinecke, W. Li, H. L. Daehnke, and J. A. Goldstein, Dityrosine, a specific marker of oxidation, is synthesized by the myeloperoxidase-hydrogen peroxide system of human neutrophils and macrophages, J. Biol. Chem. 268, 4069–4077 (1993).
M. I. Savenkova, D. M. Mueller, and J. W. Heinecke, Tyrosyl radical generated by myeloperoxidase is a physiological catalyst for the initiation of lipid peroxidation in low density lipoprotein, J. Biol. Chem. 269, 20,394–20,400 (1994).
A. Jerlich, J. S. Fabjan, S. Tschabuschnig, et al., Human low density lipoprotein as a target of hypochlorite generated by myeloperoxidase, Free Radical Biol. Med. 24, 1139–1148 (1998).
G. M. Chisolm, S. L. Hazen, P. L. Fox, and M. K. Cathcart, The oxidation of lipoproteins by monocytes-macrophages, J. Biol. Chem. 274, 25,959–25,962 (1999).
C. C. Winterbourn, J. J. M. Vandenberg, E. Roitman, and F. A. Kuypers, Chlorohydrin formation from unsaturated fatty acids reacted with hypochlorous acid, Arch. Biochem. Biophys. 296, 547–555 (1992).
J. W. Heinecke, Oxidants and antioxidants in the pathogenesis of atherosclerosis: implications for the oxidiized low density lipoprotein hypothesis, Atherosclerosis 141, 1–15 (1998).
M. O. Pentikäinen, K. Oorni, and P. T. Kovanen, Myeloperoxidase and hypochlorite, but not copper ions, oxidize heparin-bound LDL particles and release them from heparin, Arteriosclosis Thromb. Vasc. 21, 1902–1908 (2001).
S. L. Hazen, J. P. Gaut, J. R. Crowley, F. F. Hsu, and J. W. Heinecke, Elevated levels of protein-bound p-hydroxyphenylacetaldehyde, an amino acid derived aldehyde generated by myeloperoxidase, are present in human fatty streaks, intermediate lesions and advanced atherosclerotic lesions, Biochem. J. 352, 693–699 (2000).
P. Leppänen, K. Seppänen, J. T. Salonen, and S. Ylä-Herttuala, Effects of mercury, iron and antioxidants on atherosclerosis in LDLR-deficient mice, in XIIth International Symposium on Atherosclerosis, 2000.
J. Parizek and I. Ostadalova, The protective effect of small amounts of selenite in sublimate intoxication, Experientia 23, 142–143 (1967).
K. Seppänen, R. Laatikainen, J. T. Salonen, et al., Mercury-binding capacity of organic and inorganic selenium in rat blood and liver, Biol. Trace Element Res. 65, 197–210 (1998).
K. Seppänen, M. Kantola, R. Laatikainen, et al., Effect of supplementation with organic selenium on mercury status as measured by mercury in pubic hair, J. Trace Elements Med. Bio. 14, 84–87 (2000).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Seppänen, K., Soininen, P., Salonen, J.T. et al. Does mercury promote lipid peroxidation?. Biol Trace Elem Res 101, 117–132 (2004). https://doi.org/10.1385/BTER:101:2:117
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/BTER:101:2:117