-
PDF
- Split View
-
Views
-
Cite
Cite
Basam Z. Barkho, Ari E. Munoz, Xuekun Li, Lu Li, Lee Anna Cunningham, Xinyu Zhao, Endogenous Matrix Metalloproteinase (MMP)-3 and MMP-9 Promote the Differentiation and Migration of Adult Neural Progenitor Cells in Response to Chemokines, Stem Cells, Volume 26, Issue 12, December 2008, Pages 3139–3149, https://doi.org/10.1634/stemcells.2008-0519
Close - Share Icon Share
Abstract
Adult neurogenesis is regulated by both intrinsic programs and extrinsic stimuli. The enhanced proliferation of adult neural stem/progenitor cells (aNPCs) in the subventricular zone and the migration of neuroblasts toward the ischemic region in adult brains present a unique challenge as well as an opportunity to understand the molecular mechanisms underlying the extrinsic cue-induced neurogenic responses. Matrix metalloproteinases (MMPs) are a family of proteinases known to play a role in extracellular matrix remodeling and cell migration. However, their presence in aNPCs and their potential function in injury-induced aNPC migration remain largely unexplored. Here we demonstrate that in response to two injury-induced chemokines, stromal cell-derived factor 1 (SDF-1) and vascular endothelial growth factor, aNPCs differentiated into migratory cells that expressed increased levels of MMP-3 and MMP-9. Whereas differentiated neuroblasts and a subpopulation of astrocytes migrated toward the chemokines, undifferentiated progenitors did not migrate. Blocking the expression of MMP-3 or MMP-9 in aNPCs interfered with both the differentiation of aNPCs and chemokine-induced cell migration. Thus, endogenous MMPs expressed by aNPCs are important for mediating their neurogenic response to extrinsic signals.
Disclosure of potential conflicts of interest is found at the end of this article.
Introduction
Adult neural stem/progenitor cells (aNPCs) participate in active neurogenesis in two locations of the adult brain: the subgranular zone of the dentate gyrus in the hippocampus and the subventricular zone (SVZ) bordering the lateral ventricles. In response to focal ischemic injury, the proliferation of aNPCs residing in the SVZ increases. A subpopulation of these newborn aNPCs differentiate into neuroblasts and migrate away from the SVZ and the rostral migratory stream into the damaged region [1]. Several injury-induced factors produced by reactive cells, such as astrocytes, endothelial cells, and immune cells, are known to modify the niche for aNPCs [2, 3]. Among these factors, stromal cell-derived factor 1 (SDF-1) and vascular endothelial growth factor (VEGF), acting through their cell surface receptors chemokine (C-X-C motif) receptor 4 (CXCR4) and vascular endothelial growth factor receptor 2 (VEGF-R2), respectively, are found to be chemoattractive to new cells generated in the SVZ [4, 5–6]. Nevertheless, the exact mechanism by which these chemokines promote the ischemia-induced migration of aNPCs is unclear.
Chemokine-induced cell migration requires the remodeling of the extracellular matrix (ECM). The chemotaxic functions of SDF-1 and VEGF are known to be mediated by the activation of matrix metalloproteinases (MMPs) [7, 8]. MMPs are a family of enzymes that collectively are able to degrade all the components of the ECM [9, 10]. MMPs participate in a host of important physiological processes, including CNS development, embryological remodeling, wound healing, and angiogenesis, and their role in cancer cell metastasis has been studied extensively [11, 12]. Although MMPs have been investigated for their involvement in ischemic brain injuries, such as neuronal death and blood-brain barrier breakdown [13], their role in the neurogenic response of aNPCs after ischemic insults has only recently been considered. Neuroblast migration is known to require ECM remodeling [14], and MMP-9 immunoreactivity is colocalized with migrating neuroblasts [15]. Furthermore, MMP-2 and MMP-9 expressed by endothelial cells promote neuroblast migration [16]. However, whether aNPCs express MMPs and what the potential functions of these endogenous MMPs are in aNPC differentiation, proliferation, survival, and migration remain unknown.
In this study we demonstrate that MMP activity promotes the aNPC neurogenic response to stroke-induced chemokines. In response to SDF-1 and VEGF, aNPCs differentiate into migratory cells that express higher levels of MMP-3 and MMP-9 compared with nonmigratory cells. Blocking the expression of endogenous MMP-3 and MMP-9 in aNPCs using specific small interfering RNAs (siRNAs) inhibited both aNPC differentiation into the neuronal lineage and subsequent cell migration in response to SDF-1 and VEGF. Furthermore, we show that migrating neuroblasts in a rodent focal ischemia model express endogenous MMP-3 and MMP-9 mRNAs. This study presents the first evidence for the function of endogenous MMPs expressed by aNPCs in the regulation of their neurogenic response to ischemic insult.
Materials and Methods
Neural Stem/Progenitor Cell Culture
Isolation procedure and culture conditions for multipotent mouse aNPCs were as described [17]. Briefly, aNPCs were kept under proliferating conditions using N2 medium (Dulbecco's modified Eagle's medium/Ham's F-12 medium [1:1] [DM-25; Omega Scientific, Tarzana, CA, http://www.omegascientific.com] supplemented with N2 [Life Technologies, Gaithersburg, MD, http://www.lifetech.com], l-glutamine [25030; Invitrogen, Carlsbad, CA, http://www.invitrogen.com], and 1× Antibiotic-Antimycotic [15240; Invitrogen]), 20 ng/ml fibroblast growth factor-2 (FGF-2; Peprotech, Rocky Hill, NJ, http://www.peprotech.com), and 20 ng/ml epidermal growth factor (EGF; 100-15; Peprotech) and incubated at 5% CO2 at 37°C.
Recombinant Proteins and Reagents
Human recombinant SDF-1α/PBSF (350-NS/CF; R&D Systems Inc., Minneapolis, http://www.rndsystems.com) and human recombinant VEGF (293-VE/CF; R&D Systems) were used at 100 ng/ml. GM6001 MMP inhibitor (CC1000; Chemicon, Temecula, CA, http://www.chemicon.com) was used at a final concentration of 10 μM.
Cell Migration Assay
Cell migration assays were performed on the basis of published methods with modifications [18]. Briefly, Transwells with 8.0-μm Pore Polycarbonate Membrane Inserts (353097; BD Biosciences, San Jose, CA, http://www.bdbiosciences.com) were coated with polyornithine and laminin as described [19]. Approximately 1 × 105 mouse aNPCs were plated onto the upper chamber of an insert that fit into 1 well of a 24-well plate in N2 medium without growth factors or chemokines. After 60 minutes to allow the cells to attach to the surface of the membrane, SDF-1 or VEGF was added at 100 ng/ml into the lower chamber of the Transwell. For the MMP inhibitor studies, GM6001 (dissolved in dimethyl sulfoxide [DMSO]) was diluted in phosphate-buffered saline and administered to both top and bottom chambers at a final concentration of 10 μM, whereas the controls received an equal volume of DMSO in both chambers. The cells were then incubated for 16 hours in a cell culture incubator. For quantification of migrated cells, the lower chamber was fixed with 4% paraformaldehyde (PFA) and stained with 4,6-diamidino-2-phenylindole, and the cells remaining in the upper chamber were scraped off with a cotton swab. The membrane was cut out from the insert and mounted onto a glass slide with DAVCO-PVA and coverslipped. For lentivirus-infected cells, the membrane was immunostained using an anti-green fluorescent protein (anti-GFP) antibody (described below) to amplify the GFP signal. To determine whether lentivirus itself has deleterious effect on aNPCs, cells infected by either control lentiviruses or siRNA lentiviruses, as well as uninfected cells, were plated onto the Transwell membranes for 16 hours without inducing migration, followed by GFP signal amplification as described above. Cell numbers were estimated using unbiased stereology (MicroBrightField Inc., Williston, VT, http://www.mbfbioscience.com) and a ×20 objective lens on an Olympus BX51 epifluorescent microscope (Olympus, Tokyo, http://www.olympus-global.com). The results were statistically analyzed using a two-tailed, unpaired Student's t test.
Immunohistochemistry
Cells were fixed using 4% PFA, followed by immunocytochemical staining and stereological quantification as described [17, 19]. Details are given in supplemental online data.
Cell Proliferation Assay
Cell proliferation was performed as described [19], with modifications. Details are given in supplemental online data.
Real-Time Quantitative Polymerase Chain Reaction
Real-time polymerase chain reaction (PCR) was performed using established methods [17, 19]. Briefly, total RNA was isolated from cells using either TRIzol (Gibco-BRL, Gaithersburg, MD, http://www.gibcobrl.com) for cells grown on culture dishes or the Ambion RNAqueous Kit (AM1912; Ambion, Austin, TX, http://www.ambion.com) for cells on Transwells. The cDNA was synthesized using a Superscript II kit (Invitrogen). A detailed description and sequences of the primers are provided in supplemental online data.
Western Blotting
Western blotting was performed based on a standard protocol [17]. Details are given in supplemental online data.
Recombinant Lentiviruses and In Vitro Gene Knockdown
The vectors expressing MMP-siRNA driven by a U1 promoter were purchased from SuperArray (SureSilencing siRNA plasmid: MMP-3, KM03673G; MMP-9, KM03661G; SuperArray Bioscience Corp., Frederick, MD, http://www.superarray.com). The U1-siRNA cassettes were subsequently cloned into a third-generation lentiviral vector [20]. Lentivirus production was performed as described previously [19]. A detailed description is provided in supplemental online data. All recombinant DNA research was performed on the basis of NIH guidelines.
Mouse Middle Cerebral Artery Occlusion
All animal procedures used in this study were approved by institutional animal care and use committees. Middle cerebral artery occlusion (MCAO) followed by reperfusion was conducted using the intraluminal method as described [21]. Adult male C57BL/6 mice (3 months of age; weight, ∼25 g) were used for a 60-minute MCAO followed by reperfusion. At 14 days post-MCAO, mice were sacrificed, and brains were rapidly removed, quick-frozen in isopentane equilibrated in dry ice-ethanol slurry, and stored at −80°C until further processing. Coronal brain sections (20 μm) were prepared using a cryostat and arranged on slides (Superfrost Plus; VWR, West Chester, PA, http://www.vwr.com). A detailed description of MCAO is provided in supplemental online data.
Fluorescence In Situ Hybridization
Fluorescence in situ hybridization (FISH) was performed as described [22]. Briefly, the DNA templates for riboprobe synthesis were cloned using PCR on the basis of GenBank sequences (supplemental online data). PCR products were ligated to TOPO II vector (45-0640; Invitrogen) and sequenced before they were linearized to generate templates for riboprobe synthesis. Digoxigenin-labeled antisense and sense cRNA riboprobes were made using MAXIscript T7/SP6 (1322 or 1326; Ambion) and digoxigenin RNA labeling mix (11277073; Roche Diagnostics, Basel, Switzerland, http://www.roche-applied-science.com). For FISH, the riboprobes were hybridized on fixed brain sections at 56°C overnight and detected with the use of a commercial tyramide signal amplification cyanine-3 or fluorescein tyramide signal amplification kit (SAT704A001EA or SAT701001EA; PerkinElmer Life and Analytical Sciences, Boston, http://www.perkinelmer.com). z-Stack images were captured using a Zeiss laser scanning confocal microscope (LSM 510-META; ×40 oil; 1.2 numerical aperture) at 1-μm intervals. For quantification of DCX and MMP double-labeled cells, we took three confocal images spanning from the SVZ to the border of infarct area on the ipsilateral side (Fig. 5B, red squares). In each confocal image, we first selected 10–15 DCX+ cells and determined whether they were also MMP-3- or MMP-9-positive. Then we selected 10–15 MMP-positive cells and determined whether they were DCX. Three independent MCAO animals with confirmed stroke lesions were used in this quantification.
Results
SDF-1 and VEGF Induce the Differentiation of Multipotent aNPCs
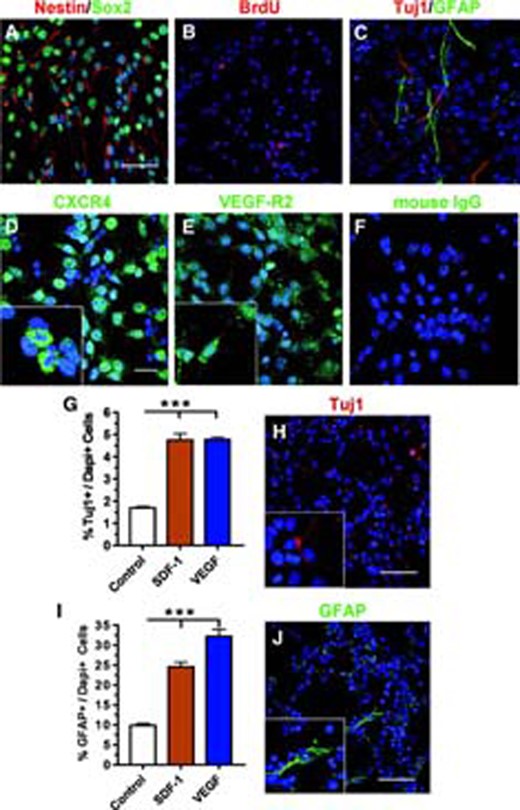
To define the molecular mechanism by which aNPCs migrate in response to chemokines, we first isolated aNPCs from adult mouse brains and determined the effects of SDF-1 and VEGF on these cells. These primary aNPCs cultured under proliferating conditions express both immature progenitor cell markers, sex-determining region Y-box 2 (Sox2) and Nestin (Fig. 1A), indicating relative phenotypic homogeneity. When cultured under proliferating conditions, aNPCs incorporated the thymidine analog bromodeoxyuridine (BrdU; Fig. 1B). Upon growth factor withdrawal, aNPCs differentiated into all major brain cell types, including neurons (neuronal class III β-tubulin [Tuj1]), astrocytes (glial fibrillary acidic protein [GFAP]; Fig. 1C), and oligodendrocytes (detected by NG2, a chondroitin sulfate proteoglycan expressed by oligodendrocyte progenitor cells, supplemental online Fig. 1E).
Multipotent adult neural stem/progenitor cells (aNPCs) differentiate in response to SDF-1 and VEGF. (A): Cultured aNPCs expressed immature stem cell markers Sox2 (green) and Nestin (red). (B): Under proliferating conditions, cultured aNPCs incorporated BrdU (red). Blue, Dapi. (C): In the absence of growth factors, aNPCs differentiated into neurons (Tuj1, red) and astrocytes (GFAP, green). (D–F): Proliferating aNPCs expressed the receptors for SDF-1 and VEGF. (D): CXCR4 (green). (E): VEGF-R2 (green). (F): Control immunostaining using mouse IgG. Scale bar = 50 μm. Blue, Dapi. Insets in (D, E) show higher-magnification images. (G): Both SDF-1 and VEGF promoted neuronal differentiation of aNPCs after 16 hours of treatment (***, p < .001; n = 3). (H): Example of Tuj1+ neurons quantified in (G). Scale bar = 50 μm. (I): Both SDF-1 and VEGF led to increased astrocyte differentiation of aNPCs after 16 hours of treatment (***, p < .001; n = 3). (J): Example of GFAP+ astrocytes quantified in (I). Scale bar = 50 μm. Abbreviations: BrdU, bromodeoxyuridine; CXCR4, chemokine (C-X-C motif) receptor 4; Dapi, 4,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein; SDF-1, stromal cell-derived factor 1; Tuj1, β-tubulin; VEGF, vascular endothelial growth factor; VEGF-R2, vascular endothelial growth factor receptor 2.
To examine aNPC response to SDF-1 and VEGF, we used immunocytochemistry and reverse transcriptase-PCR and confirmed that these aNPCs indeed expressed both CXCR4, the receptor for SDF-1 (Fig. 1D; supplemental online Fig. 1C), and VEGF-R2, the receptor that mediates VEGF-induced cell migration (Fig. 1E; supplemental online Fig. 1C). Using real-time quantitative polymerase chain reaction (qPCR), we further demonstrated that the mRNA levels of CXCR4 and VEGF-R2 in aNPCs did not change either after growth factor withdrawal or upon stimulation by SDF-1 or VEGF (supplemental online Fig. 1A, 1B). Thus, cultured aNPCs express both receptors, CXCR4 and VEGF-R2, indicating the potential responsiveness of these cells to exogenous SDF-1 and VEGF.
We and other laboratories have previously shown that chemokines and cytokines promote the proliferation and differentiation of aNPCs [19, 23, 24]. Therefore, we first examined how SDF-1 and VEGF affect stem cell properties of primary aNPCs in the absence of the mitogens FGF-2 and EGF. Compared with control conditions (growth factor withdrawal), allowing for spontaneous differentiation, both SDF-1 and VEGF treatment led to a 51.6% increase in neuronal differentiation (Tuj1+ cells; p < .001; Fig. 1G, 1H) and 60.0% and 69.2% increases, respectively, in astrocyte differentiation (GFAP+ cells; p < .001; Fig. 1I, 1J). The number of oligodendrocytes (NG2+) showed no significant difference after treatment with SDF-1 or VEGF compared with the control (supplemental online Fig. 1D, 1E). We used 8-hour BrdU pulse labeling to assess the effects of SDF-1 and VEGF on aNPC proliferation in the absence of mitogen. There was no evident difference in the number of dividing cells under either the SDF-1- or VEGF-treated conditions compared with control cultures (supplemental online Fig. 1F). Therefore, neither SDF-1 nor VEGF functions as a mitogen for aNPCs, but both promote the differentiation of aNPCs. Finally, to ensure that the effect of these chemokines on aNPC differentiation was not due to their effects on cell survival, we performed a propidium iodide permeability assay and found that neither SDF-1 nor VEGF altered the cell death of aNPCs compared with control growth factor withdrawal conditions (supplemental online Fig. 1G). Hence, cultured aNPCs express the receptors for SDF-1 and VEGF, and these chemokines promote both neuronal and astrocyte differentiation of aNPCs without affecting proliferation or survival.
SDF-1 and VEGF Induce the Migration of aNPC-Differentiated Neurons and Astrocytes
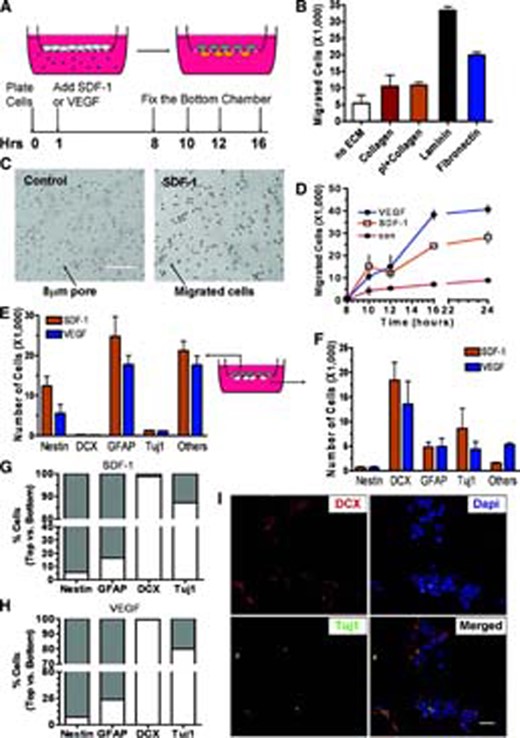
To dissect the molecular mechanisms underlying aNPC migration in response to SDF-1 and VEGF, we established an in vitro migration assay based on a modified Boyden chamber assay [2, 6]. aNPCs were plated in the top chamber of the cell culture insert (Transwell), and then either SDF-1 or VEGF was added to the bottom chamber. At different time points, we fixed, stained, and quantified the cells that migrated to the bottom chamber (Fig. 2A). We discovered that most cells plated onto noncoated cell culture inserts failed to migrate toward either chemokine. Since the ECM is known to be important for cell mobility [25, 26], we coated tissue culture inserts with several different ECM components native to the brain and compared their ability to promote cell migration in response to SDF-1 and VEGF. Consistent with previous reports [25, 26], we found that laminin is the most effective ECM coating for mediating cell migration (approximately 1.75-fold more effective than fibronectin; Fig. 2B). We therefore decided to use laminin for further migration studies. An example of cells that migrated to the bottom chamber on a laminin-coated insert is shown (Fig. 2C, control vs. SDF-1). Next we determined the time course of migration and found that migrating cells began to appear in the bottom chamber at 10 hours and reached a plateau at 16 hours post-chemokine addition, with approximately one-third of the total plated cells having migrated (Fig. 2D). When fresh SDF-1 or VEGF was administered to the bottom chamber at 16 hours, significantly more cells migrated, suggesting that the plateau at 16 hours did not represent a limited potential of plated aNPCs but rather equilibrium of the chemokine gradient (supplemental online Fig. 2A). These data indicate that isolated aNPCs migrated through a laminin-coated matrix toward SDF-1 and VEGF, which allows us to distinguish the molecular mechanisms underlying chemokine-induced aNPC migration using this assay.
Adult neural stem/progenitor cells (aNPCs) differentiate and migrate in response to SDF-1 and VEGF. (A): Experimental schematics showing in vitro migration assay. Tissue culture inserts with an 8.0-μm pore were used to separate the top and bottom chambers. aNPCs were plated onto the membrane of the top chamber, and either SDF-1 or VEGF was administered to the bottom chamber. At specific time points, the migratory and stationary cells were analyzed. (B): Quantification of cell migration on different ECM substrates indicated that laminin was the most effective ECM at promoting cell migration (n = 4). (C): Bright-field image of cells migrated to the bottom of chambers on laminin-coated inserts. con: no SDF-1 was added. Scale bar = 100 μm. (D): Time course of adult aNPC migration toward SDF-1 and VEGF. The cell migration was initially observed at 10 Hrs and plateaus at 16 Hrs (n = 3). (E): The majority of stationary cells on the top chamber were Nestin+ immature cells and GFAP+ astrocytes (n = 3). (F): The majority of migratory cells at the bottom were DCX+ and Tuj1+ neuroblasts and some GFAP+ astrocytes (n = 3). (G): The percentage distribution of different lineages of cells between the top chamber and the bottom chamber after SDF-1 treatment. (H): The percentage distribution of different lineages of cells between the top chamber and the bottom chamber after VEGF treatment. (I): Example of migratory DCX+ (red) and Tuj1+ (green) cells in the bottom chamber. Merged image shows the colocalization of DCX- and Tuj1-expressing cells. Blue, Dapi. Scale = 20 μm. Abbreviations: con, control; Dapi, 4,6-diamidino-2-phenylindole; ECM, extracellular matrix; GFAP, glial fibrillary acidic protein; Hrs, hours; pI, polylysine; SDF-1, stromal cell-derived factor 1; Tuj1, β-tubulin; VEGF, vascular endothelial growth factor.
There is ample evidence demonstrating the migration of neuroblasts to ischemic regions [1, 27, 28–29]. However, few studies have characterized other migratory cell types derived from proliferating aNPCs residing in the SVZ. Since we observed an effect of SDF-1 and VEGF on the differentiation of aNPCs (Fig. 1), we next characterized the phenotypes of those cells derived from aNPCs that either migrated (in the bottom chamber) or remained stationary (in the top chamber) after 16 hours of chemokine-induced migration. We observed that Nestin+ immature cells and GFAP+ astrocytes constituted the majority of the stationary cells, along with some other cells of unknown identity (Fig. 2E). The majority of migratory cells were DCX+ and Tuj1+ neuroblasts, plus some GFAP+ astrocytes (Fig. 2F). Thus, upon SDF-1 or VEGF stimulation, most of the Nestin+ cells (92%–94%) and the majority of GFAP+ astrocytes (76%–83%) failed to migrate (Fig. 2G), whereas ∼98% of the DCX+ and 80%–87% of the Tuj1+ neuroblasts migrated (Fig. 2H). Not all of the DCX+ cells were positive for Tuj1+ in either the migration assay (Fig. 2I) or the differentiation assay (supplemental online Fig. 2C–2E), possibly because these two markers label different stages of neuronal differentiation. These data suggest that the differentiation and migration of aNPCs are integral components of the SDF-1- and VEGF-induced phenotypic response. Since the expression levels of the chemokine receptors did not change upon chemokine treatment (Fig. 1), this suggests that cellular differentiation is likely a required process for the migration of neuronal and astrocyte cell lineages.
Endogenous MMP-3 and MMP-9 Are Involved in Chemokine-Induced aNPC Migration
With the establishment of an effective in vitro migration assay, we next determined whether endogenous MMPs play a role in the migration of aNPCs. We found that a broad-spectrum MMP inhibitor, GM6001, significantly reduced aNPC migration in response to either SDF-1 or VEGF (Fig. 3A), suggesting that MMPs sensitive to GM6001 are involved in the migration of aNPCs. To identify specific MMPs involved in aNPC migration, we selected candidate MMPs already known to be involved in other migratory processes, such as cancer metastasis, angiogenesis, and stem cell homing [30]. We first compared the mRNA expression levels of the candidate MMPs in migratory cells collected from the bottom chamber versus stationary cells from the top chamber after exposure to SDF-1 and VEGF. Using qPCR, we found that MMP-3 mRNA levels were 43.8% and 37.5% higher in migratory cells compared with stationary cells in SDF-1- and VEGF-induced migration, respectively (p < .05; Fig. 3B). Moreover, MMP-9 mRNA levels were 53.5% and 75.0% higher in migratory cells compared with stationary cells in SDF-1 and VEGF treatment, respectively (p < .05; Fig. 3C). The expression of MMP-2, -7, and -10 mRNA transcripts, however, did not differ between migratory and stationary cells (supplemental online Fig. 3A–3C). We then confirmed that MMP-3 and MMP-9 protein levels were also higher in both the medium (Fig. 3D; quantification is shown in supplemental online Fig. 3F, 3G) and cell lysate collected from the top or bottom chambers (Fig. 3E; quantification is shown in supplemental online Fig. 3H, 3I). Furthermore, we confirmed that the DCX+ neurons that migrated to the bottom chambers of Transwells are positive for MMP-3 and MMP-9 immunoreactivity (Fig. 3F–3H). Since MMP-2 is known to be regulated at the protein-expression and activity levels, rather than the transcriptional level, we also analyzed the protein-expression levels of MMP-2, but we did not detect differential expression of this MMP in the migratory cells (supplemental online Fig. 3D). Therefore, migrated cells express higher levels of MMP-3 and MMP-9 at both the mRNA and protein levels.
MMP-3 and MMP-9 expressed by adult neural stem/progenitor cells (aNPCs) are important for their migration response to SDF-1 and VEGF. (A): A broad-spectrum MMP inhibitor, GM6001, inhibited SDF-1- or VEGF-induced cell migration (**, p < .01; n = 4). (B, C): Quantitative polymerase chain reaction analysis of migratory and stationary cells demonstrated that mRNA levels of MMP-3 (B) and MMP-9 (C) were significantly higher in migratory cells (Bt) compared with stationary cells (top chamber) (n = 4). (D, E): The protein levels of MMP-3 and MMP-9 in the culture medium (D) and cell lysate (E) of the Bt were higher than those in the top chamber (Cyclophillin A antibody used as a loading Con). (F–H): Migrated DCX+ neuroblasts (red) expressed MMP-3 ([F], green) and MMP-9 ([G], green). Mouse IgG, instead of MMP antibodies, was used as negative Con ([H], green). Scale bar = 20 μm. (I): Western blot analysis showing that lentivirus-MMP-3-siRNA could efficiently knockdown endogenous MMP-3 (54 kDa) in aNPCs compared with the Con lentivirus (lentivirus-NC-siRNA and lentivirus-GFP)-infected aNPCs and uninfected aNPCs (β-actin antibody used as a loading Con). (J): Western blot analysis showing that lentivirus-MMP-9-siRNA could efficiently knock down endogenous MMP-9 (98 kDa) in aNPCs compared with Con lentivirus-infected aNPCs and uninfected aNPCs. (K, L): The knockdown of MMP-3 and MMP-9 led to reduced cell migration in response to either SDF-1 (K) or VEGF (L) (**, p < .01; n = 3). Abbreviations: Bt, bottom chamber; Con, control; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFP, green fluorescent protein; MMP, matrix metalloproteinase; NC, control nonsilencing; SDF-1, stromal cell-derived factor 1; siRNA, small interfering RNA; VEGF, vascular endothelial growth factor.
Although pharmacological MMP inhibitors have been widely used to study the roles of MMPs in cellular functions, these inhibitors lack specificity, which confounds data interpretation. We therefore decided to use siRNAs that specifically knockdown endogenous MMP-3 and MMP-9 expression to investigate whether increased expression of MMP-3 and MMP-9 in migrating cells is critical for the migration of these cells. To this end we used lentiviral vectors that expressed MMP-3-siRNA, MMP-9-siRNA, or control nonsilencing (NC)-siRNAs under a U1 RNA polymerase III promoter and enhanced green fluorescent protein under a cytomegalovirus promoter, so that the cells that underwent acute knockdown of MMPs could be tracked by GFP fluorescence (supplemental online Fig. 3E). To determine whether lentivirus itself had a deleterious effect on aNPCs, we plated cells infected by control viruses (lenti-GFP and lenti-NC-siRNA), as well as by MMP-3- and MMP-9-siRNA lentiviruses, onto the Transwell membranes for 16 hours without inducing migration (no chemokine added). We found that all virus-infected cells had a similar percentage of GFP+ cells (32.8% ± 2.4% infection efficiency), suggesting that neither lentivirus nor MMP-siRNAs had a negative effect on cell survival. To assess the nonspecific effect of the virus on cell migration, we then determined that the number of migrated cells in control lentivirus-infected conditions was comparable to that of uninfected cells (supplemental online Fig. 3L), suggesting that the lentivirus itself had no significant negative effect on our in vitro assay. We then confirmed that these siRNAs could indeed reduce the protein-expression levels of endogenous MMP-3 (Fig. 3I) and MMP-9 (Fig. 3J) compared with the NC-siRNA, lenti-GFP, and uninfected control cells (quantification is given in supplemental online Fig. 3J, 3K). To determine the effect of MMP-3 and MMP-9 knockdown on cell migration, we infected proliferating aNPCs with siRNA-lentivirus for 24 hours, allowing the siRNA sequences to be incorporated into the genome. Then the cells were plated onto migration chambers and subjected to cell migration assays. We found that acute knockdown of either MMP-3 or MMP-9 led to a significant reduction in cell migration in response to either SDF-1 (Fig. 3K) or VEGF (Fig. 3L) compared with the lentivirus-NC-siRNA-infected or lentivirus-GFP-infected cells. Specifically, we observed that MMP-3-siRNA-infected cells had a 33.8% reduction in SDF-1-stimulated migration (p < .05) and a 38.6% reduction in VEGF-stimulated migration (p < .01; Fig. 3K) compared with the NC-siRNA-infected cells. Knockdown of endogenous MMP-9 led to a 42.3% reduction in SDF-1-stimulated cell migration (p < .05) and a 53.3% reduction in VEGF-stimulated cell migration compared with the NC-siRNA-infected cells (p < .01; Fig. 3L). Therefore, both endogenous MMP-3 and MMP-9 expressed by aNPCs are involved in their migration toward stroke-induced chemokines. Together these data indicate that in response to SDF-1 and VEGF, aNPCs differentiate and express higher levels of MMP-3 and MMP-9, which are required for their migratory response.
MMP-3 and MMP-9 Are Involved in aNPC Proliferation and Differentiation
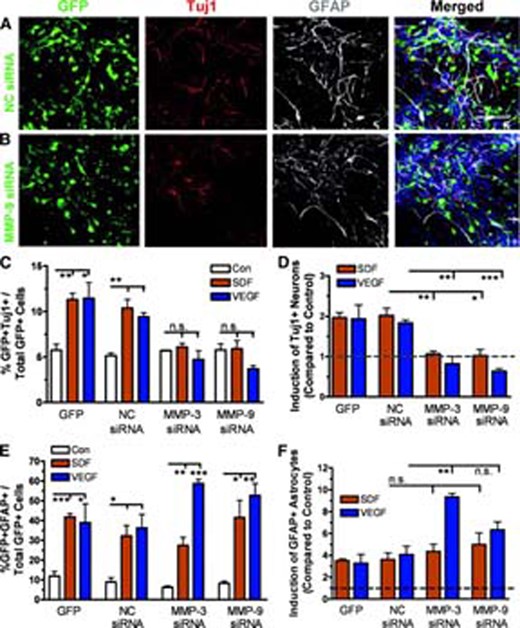
Since we demonstrated that SDF-1 and VEGF led to both the differentiation of aNPCs and increased MMP-3 and MMP-9 expression in migrating cells, we next investigated whether MMP-3 and MMP-9 were involved in SDF-1- and VEGF-induced aNPC differentiation. MMP-3- or MMP-9-siRNA lentivirus-infected aNPCs were treated with SDF-1 or VEGF in the absence of stem cell mitogens, and we used Tuj1 expression as an index of neuronal differentiation and GFAP expression for glial differentiation (NC-siRNA vs. MMP-9-siRNA; Fig. 4A, 4B). Consistent with what we found in uninfected cells (Fig. 1G, 1J; supplemental online Fig. 4A, 4B), SDF-1 and VEGF treatment led to increased neuronal and astrocyte differentiation in both NC-siRNA-infected and lentivirus-GFP-infected aNPCs (Fig. 4C, 4E). However, acute knockdown of either MMP-3 or MMP-9 abolished SDF-1- or VEGF-induced neuronal differentiation (Fig. 4C). Such inhibitory effects became more apparent when we compared the fold induction of neuronal differentiation by SDF-1 and VEGF in siRNA-infected aNPCs with that of controls (Fig. 4D, dotted line). Interestingly, acute knockdown of MMP-9 inhibited the neuronal differentiation induced by VEGF more than that induced by SDF-1 (Fig. 4D). On the other hand, acute knockdown of MMP-3, but not MMP-9, significantly potentiated VEGF-induced astrocyte differentiation (Fig. 4E, 4F).
Knockdown of MMP-3 and MMP-9 interferes with SDF-1- and VEGF-induced adult neural stem/progenitor cell (aNPC) differentiation. (A, B): Sample images showing that lentivirus-NC-siRNA-infected (A) and lentivirus-MMP-9-siRNA-infected (B) cells differentiated into Tuj1+ neurons (red) or GFAP+ astrocytes (white) in the presence of SDF-1. Blue, 4,6-diamidino-2-phenylindole. Green, GFP. Scale bar = 50 μm. (C): Acute knockdown of MMP-3 or MMP-9 using lentivirus-siRNA abolished SDF-1- and VEGF-induced neuronal differentiation compared with lentivirus-NC-siRNA- and lentivirus-GFP-infected aNPCs. (D): The neuronal induction by SDF-1 or VEGF was abolished by acute knockdown of MMP-3 or MMP-9. The data shown in (E) was normalized to a no-chemokine control condition (shown in dotted line). (E): Acute knockdown of MMP-3 (**, p < .01) using lentivirus-siRNA potentiated VEGF- but not SDF-1-induced astrocyte differentiation compared with controls. MMP-9-siRNA did not have a similar effect (p = .1) (F): The data shown in (F) was normalized to a no-chemokine condition (shown in dotted line). *, p < .05; p < .01; ***, p < .001. Abbreviations: GFAP, glial fibrillary acidic protein; GFP, green fluorescent protein; MMP, matrix metalloproteinase; NC, control nonsilencing; n.s., nonsignificant; SDF, stromal cell-derived factor; siRNA, small interfering RNA; Tuj1, β-tubulin; VEGF, vascular endothelial growth factor.
Using BrdU pulse labeling, we determined that MMP-3-siRNA and MMP-9-siRNA had no effect on the proliferation of SDF-1- or VEGF-treated aNPCs in the absence of mitogens (supplemental online Fig. 4C–4E), which is consistent with our observation that neither SDF-1 nor VEGF affected aNPC proliferation in the absence of mitogens (supplemental online Fig. 1G). On the other hand, under proliferating conditions (in the presence of FGF-2 and EGF), cell division was reduced in MMP-3-siRNA- or MMP-9-siRNA-infected cells compared with NC-siRNA-infected cells (supplemental online Fig. 4F), suggesting that intrinsic MMP-3 and MMP-9 are required for mitogen-dependent maintenance of aNPCs. Together, these data indicate that both MMP-3 and MMP-9 play important roles in the fate choice of aNPCs into the neuronal and astrocyte lineages in response to SDF-1 or VEGF.
Neuroblasts in a Rodent Stroke Model Express Endogenous MMP-3 and MMP-9
To verify that migrating neuroblasts in stroke brains indeed express endogenous MMP-3 and MMP-9, we decided to use a mouse model of MCAO. Recent studies have shown that in this rodent stroke model, DCX+ migrating neuroblasts are double-labeled for MMP-9 immunoreactivity [31]; however, since most MMPs are secreted into the ECM, it is difficult to assess the origin of this metalloproteinase on the basis of the immunoreactivity of its antibody. We therefore decided to use multicolor FISH to examine the colocalization of MMP mRNA and DCX mRNA expressed by migrating neuroblast at 2 weeks post-MCAO (Fig. 5A). Mice received a 60-minute MCAO to one hemisphere (ipsilateral; Fig. 5B) and were analyzed at 2 weeks postreperfusion, when ischemia-induced cell migration was at its peak [1]. We observed that there was an increased FISH-positive hybridization signal for both MMP-3 and MMP-9 mRNAs in the ipsilateral striatum compared with the contralateral side (Fig. 5C–5N); notably, normal adult brain tissues express undetectable levels of MMP-3 or MMP-9 [9, 10]. In addition, high-resolution confocal analysis indicated that both MMP-3 (Fig. 5C, red) and MMP-9 (Fig. 5I, red) riboprobes were colocalized with DCX riboprobe (migrating neuroblast; Fig. 5D, 5J, green). Neither antisense probes on the contralateral side (Figs. 5H, 5N; supplemental online Fig. 5A–5C, 5G–5H) nor sense riboprobes on the ipsilateral side (supplemental online Fig. 5D–5F, 5J–5L) showed positive signal. To determine the extent to which DCX+ cells in the ischemic region expressed MMP-3 and MMP-9, we selected consecutive regions between the SVZ and the infarct core and quantified the colocalization of DCX+ to MMP+ cells (Fig. 5B, red boxes). We found that all of the DCX+ cells expressed mRNA of MMP-3 and MMP-9 and that ∼90% of MMP-3- or MMP-9-positive cells were also DCX+. Therefore, migratory neuroblasts in ischemic brains express endogenous MMP-3 and MMP-9, supporting a role for these MMPs in stroke-induce neurogenesis.
Migrating DCX+ cells in an MCAO mouse model express MMP-3 and MMP-9 mRNA. (A): Mice were analyzed at 14 days post-MCAO, corresponding to the peak of neuroblast migration (based on literature [1]). (B): Schematic diagram showing mouse brains subjected to unilateral MCAO. Both ipsilateral and contralateral sides were analyzed. The gray boxes indicate the brain regions where images were taken (shown in [C–M]). The red boxes indicate the regions subjected to quantification of DCX+ and MMP+ cells. (C–G): Riboprobes detected increased MMP-3 mRNA ([C], red) in DCX mRNA expressing neuroblast (D) in the ipsilateral side compared with the contralateral side (H). Scale bar = 50 μm. (G): Image of a single cell from (F) (white box) showing that MMP-3 and DCX mRNA are expressed in the same cells. Scale bar = 20 μm. (I–M): Riboprobes detected increased MMP-9 mRNA ([I], red) in DCX mRNA expressing neuroblast (J) in the ipsilateral side but not the contralateral side (N). Scale bar = 50 μm. (N): Image of a single cell from (L) (white box) showing that MMP-9 and DCX mRNA are expressed in the same cells. Scale bar = 20 μm. Abbreviations: DAPI, 4,6-diamidino-2-phenylindole; MCAO, middle cerebral artery occlusion; MMP, matrix metalloproteinase.
Discussion
The discovery of a neurogenic response subsequent to an ischemic injury has generated vast interest, largely because of the desire to understand more about the functions and plasticity of the adult human brain. However, despite the extensive efforts and substantial progress made to date, the molecular mechanisms underlying aNPC differentiation, proliferation, survival, and migration remain unclear [28]. One obstacle has been the complexity of the in vivo environment that adult progenitors encounter after a brain injury [1, 32]. Injured brain tissue and surrounding regions contain many dynamically changing cell types, ranging from astrocytes to endothelial and immune cells [2, 3]. The power behind our study is a well-defined in vitro assay that allowed us to identify the molecular characteristics of migrating cells in response to two well-studied stroke-induced chemokines, namely SDF-1 and VEGF. By using these two distinct chemokines that act via independent receptors, we can also derive a relatively common mechanism for injury-induced aNPC migration. Using this assay system, we found that aNPCs differentiated and that differentiated cells, as opposed to undifferentiated cells, migrated in response to chemokines. Our most intriguing finding is that such differentiation and migration are mediated, at least partially, by endogenous MMP-3 and MMP-9 expression by aNPCs. This represents the first evidence to account for the way extrinsic cues trigger the endogenous MMP expression that leads to downstream aNPC phenotypic changes and cell migration.
SDF-1 and VEGF Direct aNPC Differentiation and Migration
Enormous efforts have been devoted to the identification of chemokines that attract aNPCs and newborn cells in injured brains; among these, the most studied are SDF-1 and VEGF [2, 3, 5, 33, 34]. It has been shown that in response to ischemic injuries, SVZ aNPCs differentiate into DCX+ neuroblasts that migrate into the injured regions [1, 35, 36]; however, the repertoire of the migratory cell types derived from SVZ aNPCs has not been fully characterized. We found that in response to both of these chemokines, the majority of DCX+ and Tuj1+ neuroblasts migrate, whereas nearly all Nestin+ immature cells remain stationary, despite the fact that these cells may express comparable levels of receptors for these chemokines. Nevertheless, some researchers have found that Nestin+ undifferentiated cells did migrate in response to ischemia [6, 36]. It is possible, however, that the migration of these cells is triggered by other chemokines, such as stem cell factor [37] and monocyte chemoattractant protein-1 [38] in stroke brains. Such possibilities await further investigation. We also found that approximately 20%–25% of GFAP+ astrocytes migrated toward SDF-1 and VEGF, but we could not distinguish the morphological differences between migratory and stationary astrocytes. Whether these astrocytes belong to a distinct type of astrocyte (e.g., reactive astrocytes) is unclear. Because of the complex and dynamic nature of the in vivo post-ischemia environment, more research using a well-defined assay system is needed to determine the functional effect of other extrinsic cues on aNPCs.
Endogenous MMPs Promote aNPC Migration in Response to Injury
A collection of cancer studies has linked chemokine-induced MMP expression to the migration of the cells expressing them (reviewed by [12]). However, the roles of MMPs expressed by aNPCs in cell migration have gone largely unexplained. Moreover, earlier studies into the roles MMPs play in cell migration were carried out using MMP inhibitors that generally lack specificity. Our study made use of MMP-3- and MMP-9-specific siRNAs, providing clear evidence for the involvement of endogenous MMP-3 and MMP-9 expressed by aNPCs in chemokine-induced migration. A major challenge to identifying the sources of MMPs is that most MMPs are secreted proteins; therefore their immunoreactivity is localized on both target and source cells, making it difficult to discern which exact cells express these MMPs. For example, MMP-9 immunoreactivity is colocalized with DCX+ cells [31], but we also know that endothelial cell-secreted MMP-2 and MMP-9 are important for neuroblast migration [16]. To demonstrate the presence of endogenous MMPs in aNPC-derived neuroblasts and support our in vitro finding, we used multicolor FISH and confocal microscopy to colocalize the MMP mRNAs with DCX mRNAs in the same cells, which yielded definitive evidence for the expression of MMPs by migrating neuroblasts. We anticipate that the methodology we developed in this manuscript (i.e., qPCR of migrated cells, siRNAs for MMPs, and multicolor FISH) will advance the current standard for the study of MMP functions in the brain.
By using both SDF-1 and VEGF, we have found that MMP-3 and MMP-9 are required for the migration that occurs in response to chemokines, suggesting that the signaling pathways initiated by these two chemokines converge at MMP-3 and MMP-9 expression in mediating cell migration. Both SDF-1 and VEGF are known to activate MMP transcription, which leads to cell migration [7, 39]. Cell migration has been studied extensively in other cell systems, and the process is appreciated as a balanced action involving MMPs and their natural inhibitors, TIMPs, and a dynamic interaction between the cells and the ECM. These ECM factors include laminin [14], integrins [40], cadherins [41], and growth factor receptors [2]. The activation of MMPs and subsequent cleavage of these factors may trigger intracellular signaling that leads to changes in neural stem/progenitor cell (NPC) functional properties. Our data provide the first evidence that MMP-3 and MMP-9 expressed by aNPCs contribute to the balance shift that is important for cell migration.
Endogenously Expressed MMP-3 and MMP-9 Promote aNPC Differentiation
Despite the intense interest in the role of MMPs in cancer cell migration and in brain development and tissue injuries, their functions in aNPC proliferation and differentiation are poorly understood [11, 12]. We demonstrated that the acute reduction of endogenous MMP-3 and MMP-9 in aNPCs abolished the neuronal differentiation induced by SDF-1 and VEGF but promoted the astrocyte differentiation induced by VEGF. In addition, we showed that MMP-3 and MMP-9 expressed by proliferating aNPCs are critical for their proliferation in the presence of mitogen. These data provide an intriguing model in which MMPs, although conventionally thought of as extracellular protease, could modulate the basic properties of aNPCs: namely, proliferation and fate determination. Although MMP-3- and MMP-9-null mice have been generated by other laboratories [42, 43], the advantage of using MMP-specific siRNAs is that we were able to avoid compensatory effects from other mechanisms during mouse development, which can confound data interpretation. Future studies using NPC-specific inducible Cre mice [44] together with floxed MMP-3 and MMP-9 condition knockout mutant mice (these mice are yet to be made) will make a valuable in vivo extension of our current study.
Recent studies have suggested that ECM remodeling and changes in cell-cell interactions, such as the breakdown of laminin and integrins, have an effect on the differentiation of embryonic and adult stem cells [45, 46]. Therefore, the breakdown of MMP substrates may activate secondary mechanisms that stimulate the differentiation of aNPCs. To our surprise, as chemokines promote the differentiation of aNPCs, we also observed a distinctive migratory response on the part of differentiated cells compared with immature undifferentiated cells, suggesting that differentiation and migration are integral parts of the aNPC response to chemokines. Since a lack of MMP-3 or MMP-9 hindered the capability of aNPCs to differentiate into the neuronal lineage, our data suggest that chemokines may promote a neurogenic response, at least in part, by inducing the expression of endogenous MMPs in aNPCs.
Conclusion
In this study, we provide extensive evidence of roles for MMP-3 and MMP-9 endogenously expressed in aNPC differentiation and migration in response to chemokines. Increased MMP expression likely represents a general mechanism by which aNPCs adjust their gene expression program and interact with the extrinsic niche. Hence an understanding of this response would help to establish the intrinsic potential of adult brains and reveal how we might use these mechanisms for tissue restoration.
Acknowledgements
We thank Michael Wilson, Paul McGuire, Brian Kaspar, and David Schaffer for helpful discussions and critical reading of the manuscript, as well as Erin Finn-Flesher for technical assistance. We thank Fred H. Gage for providing the lentiviral vector used to engineer the expression vector for siRNAs. This work was supported by NIH Grants MH080434, MH078972, and P20RR15636 (to X.Z.), American Heart Association Predoctoral Fellowship 0810123Z (to B.Z.B.), an Autism Speaks Postdoctoral Fellowship (to X.L.), and the NIH/Institutional Minority Student Development program (IMSD GM060201) (to A.E.M.).
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
Author notes
Author contributions: B.B.: concept and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; A.E.M.: collection of data; X.L., L.L., and L.A.C.: provision of study material; X.Z.: concept and design, data analysis and interpretation, financial support, manuscript writing, final approval of manuscript.


![MMP-3 and MMP-9 expressed by adult neural stem/progenitor cells (aNPCs) are important for their migration response to SDF-1 and VEGF. (A): A broad-spectrum MMP inhibitor, GM6001, inhibited SDF-1- or VEGF-induced cell migration (**, p < .01; n = 4). (B, C): Quantitative polymerase chain reaction analysis of migratory and stationary cells demonstrated that mRNA levels of MMP-3 (B) and MMP-9 (C) were significantly higher in migratory cells (Bt) compared with stationary cells (top chamber) (n = 4). (D, E): The protein levels of MMP-3 and MMP-9 in the culture medium (D) and cell lysate (E) of the Bt were higher than those in the top chamber (Cyclophillin A antibody used as a loading Con). (F–H): Migrated DCX+ neuroblasts (red) expressed MMP-3 ([F], green) and MMP-9 ([G], green). Mouse IgG, instead of MMP antibodies, was used as negative Con ([H], green). Scale bar = 20 μm. (I): Western blot analysis showing that lentivirus-MMP-3-siRNA could efficiently knockdown endogenous MMP-3 (54 kDa) in aNPCs compared with the Con lentivirus (lentivirus-NC-siRNA and lentivirus-GFP)-infected aNPCs and uninfected aNPCs (β-actin antibody used as a loading Con). (J): Western blot analysis showing that lentivirus-MMP-9-siRNA could efficiently knock down endogenous MMP-9 (98 kDa) in aNPCs compared with Con lentivirus-infected aNPCs and uninfected aNPCs. (K, L): The knockdown of MMP-3 and MMP-9 led to reduced cell migration in response to either SDF-1 (K) or VEGF (L) (**, p < .01; n = 3). Abbreviations: Bt, bottom chamber; Con, control; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFP, green fluorescent protein; MMP, matrix metalloproteinase; NC, control nonsilencing; SDF-1, stromal cell-derived factor 1; siRNA, small interfering RNA; VEGF, vascular endothelial growth factor.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/stmcls/26/12/10.1634_stemcells.2008-0519/2/m_stmcls_26_12_3139_nfig003.jpeg?Expires=1716516765&Signature=QhUGRn8yvc8WOdXQiouJyhPy4tiru23pFUzvPRmsVnAM89ssLEvdVbBKBJ~K8pl4DxsMSsF9bc1FJqyn5DpFY8qyniblq75hCCKoSMPXfKncsB9jdJf~QhWheBJdSunSXcf7l5BH2mKweHmYPBfxmWNlAnn9jNzooE8RpSzdR3YMZ9-TKc0L5kIirKupPvTwrar3ymZANz-Ru6CzF~9E8ZW47LlMftFz4~kmElZYmhoU-yUZ5GeYoanwdZ2FZlCpHSzju7gItYzVu5AumipD0KkoITKpiSYzh~C1LyupzOiP~QB1wolvNU~Hl96Hgl~g7YB-ZdNNOCHUvnApA0cuHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Migrating DCX+ cells in an MCAO mouse model express MMP-3 and MMP-9 mRNA. (A): Mice were analyzed at 14 days post-MCAO, corresponding to the peak of neuroblast migration (based on literature [1]). (B): Schematic diagram showing mouse brains subjected to unilateral MCAO. Both ipsilateral and contralateral sides were analyzed. The gray boxes indicate the brain regions where images were taken (shown in [C–M]). The red boxes indicate the regions subjected to quantification of DCX+ and MMP+ cells. (C–G): Riboprobes detected increased MMP-3 mRNA ([C], red) in DCX mRNA expressing neuroblast (D) in the ipsilateral side compared with the contralateral side (H). Scale bar = 50 μm. (G): Image of a single cell from (F) (white box) showing that MMP-3 and DCX mRNA are expressed in the same cells. Scale bar = 20 μm. (I–M): Riboprobes detected increased MMP-9 mRNA ([I], red) in DCX mRNA expressing neuroblast (J) in the ipsilateral side but not the contralateral side (N). Scale bar = 50 μm. (N): Image of a single cell from (L) (white box) showing that MMP-9 and DCX mRNA are expressed in the same cells. Scale bar = 20 μm. Abbreviations: DAPI, 4,6-diamidino-2-phenylindole; MCAO, middle cerebral artery occlusion; MMP, matrix metalloproteinase.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/stmcls/26/12/10.1634_stemcells.2008-0519/2/m_stmcls_26_12_3139_nfig005.jpeg?Expires=1716516765&Signature=xRxRRnpjrnms9tvJw1bGyap~dKXJ4EYkrvjUJdsW5n6S78IF9R7a0sSw9Di~vGtHJ-p-7BA4o~BtMa776DiUoClAl-LjQgS5EejrUzT8NalinaEJs-LkYIq8NblBk3W1nZzQsYdZA6yfh6mGCPfdznGncDyDjd3zGbyXfVxRgTHxpdGWCzyGwgFjef34Ihlq6mzpCm9Ex8HQLyo-Ziss53--Q~KQ-GDcXn-3rFDX7fghkzN-DMPy0wO4NIxRLyzQY8wXEdZQpZoj2djKguxyPPDq9xOuYFOWM3BRZn9gvHEF59jVJnCOKDxjDGiWsmj0OxgHH6udYa4XSdeGF~Ukdg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
