Abstract
Sodium oxybate, also known as gamma-hydroxybutyric acid (GHB), was discovered in 1960 and has been described both as a therapeutic agent with high medical value and, more recently, a substance of abuse. The naturally occurring form of this drug is found in various body tissues but has been studied most extensively in the CNS where its possible function as a neurotransmitter continues to be studied. Sodium oxybate has been approved in different countries for such varied uses as general anaesthesia, the treatment of alcohol withdrawal and addiction, and, most recently, cataplexy associated with narcolepsy.
During the 1980s, easy access to GHB-containing products led to various unapproved uses, including weight loss, bodybuilding and the treatment of sleeplessness, sometimes with serious long-term effects. The availability of these unapproved and unregulated forms of the drug led to GHB and its analogues1 being popularised as substances of abuse and subsequent notoriety as agents used in drug-facilitated sexual assault, or ‘date rape’, eventually leading to the prohibition of GHB sales in the US.
Legal efforts to control the sale and distribution of GHB and its analogues nearly prevented the clinical development of sodium oxybate for narcolepsy in the US. However, following extensive discussions with a variety of interested parties, a satisfactory solution was devised, including legislative action and the development of the Xyrem® Risk Management Program.
Amendments to the US Controlled Substances Act made GHB a schedule I drug, but also contained provisions that allow US FDA-approved products to be placed under schedule III. This unique, bifurcated schedule for sodium oxybate/GHB allowed the clinical development of sodium oxybate to proceed and, in July 2002, it was approved by the FDA as an orphan drug for the treatment of cataplexy in patients with narcolepsy as Xyrem® (sodium oxybate) oral solution.
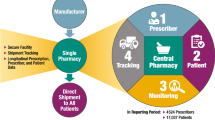
To promote the safe use of sodium oxybate, as well as alleviate concerns over possible diversion and abuse following product approval, a proprietary restricted drug distribution system was created, called the Xyrem® Success ProgramSM. Components of the programme include a centralised distribution and dispensing system, a physician and patient registry, compulsory educational materials for patients and physicians, a specially trained pharmacy staff, a method for tracking prescription shipments, and an initial post-marketing surveillance programme. The system has created a unique opportunity to provide both physician and patient education and ongoing patient counselling, promoting greater drug safety and enhanced patient compliance.




Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
In the US, only federal agencies may impose such restrictions.
Off-label use refers to the use of a drug for a purpose other than the approved indication.
References
Laborit H. Sodium 4-hydroxybutyrate. Int J Neuropharmacol 1964; 3: 433–52
Bessman SP, Fishbein WN. Gamma hydroxybutyrate: a new metabolite in brain. FASEB J 1963; 22: 334
Maitre M. The γ-hydroxybutyrate signalling system in brain: organisation and functional implications. Prog Neurobiol 1997; 51: 337–61
Van Cauter E, Plat L, Scharf MB, et al. Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young men. J Clin Invest 1997; 100: 745–53
Lapierre O, Montplaisir M, Lamarre M, et al. The effect of gamma-hydroxybutyrate on nocturnal and diurnal sleep of normal subjects: further considerations on REM sleep-triggering mechanisms. Sleep 1990; 13: 24–30
Mamelak M, Caruso VJ, Stewart K. Narcolepsy: a family study. Biol Psychiatry 1979; 14: 821–34
Entholzner E, Mielke L, Pichlmeier R, et al. EEG-veränderungen unter sedierung mit γ-hydroxybutteräure (GHB). Anaethesist 1995; 44: 345–50
Broughton R, Mamelak M. Gamma-hydroxybutyrate in the treatment of narcolepsy: a preliminary report. In: Guilleminault C, Dement WC, Passousant P, editors. Advances in sleep research. New York: Spectrum, 1976: 659–67
Scharf M, Brown D, Woods M, et al. The effects and effectiveness of γ-hydroxybutyrate in patients with narcolepsy. J Clin Psychiatry 1985; 46: 222–5
Lammers GJ, Arends J, Declerck AC, et al. Gamma-hydroxybutyrate and narcolepsy: a double-blind placebo-controlled study. Sleep 1993; 6: 216–20
Agabio R, Gessa GL. The therapeutic uses of γ-hydroxy-butyrate. In: Tunnicliff G, Cash CD, editors. Gamma-hydroxybutyrate. New York: Taylor & Francis, 2002: 169–87
Addolorato G, Balducci G, Capristo E, et al. Gamma-hydroxybutyric acid (GHB) in the treatment of alcohol withdrawal syndrome: a randomized comparative study versus benzodiazepine. Alcohol Clin Exp Res 1999; 23: 1596–604
Gallimberti L, Spella MR, Soncini CA, et al. Gamma-hydroxybutyric acid in the treatment of alcohol and heroin dependence. Alcohol 2000; 20: 257–62
Overeem S, Mignot E, van Dijk JG, et al. Narcolepsy: clinical features, new pathological insights, and future perspectives. J Clin Neurophysiol 2001; 18: 78–105
Dauvilliers Y, Bazin M, Ondze B, et al. Severity of narcolepsy among French of different ethnic origins (South of France and Martinique). Sleep 2002; 25: 50–5
Anic-Labat S, Guilleminault C, Kraemer HC, et al. Validation of a cataplexy questionnaire in 983 sleep-disorders patients. Sleep 1999; 22: 77–87
Broughton R, Mamelak M. The treatment of narcolepsy-cataplexy with nocturnal gamma-hydroxybutyrate. Can J Neurol Sci 1979; 6: 1–6
US Xyrem® Multicenter Study Group. A randomized, double blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep 2002; 25: 42–9
US Xyrem® Multicenter Study Group. A 12-month, open-label, multi-center extension trial of orally administered sodium oxybate for the treatment of narcolepsy. Sleep, 2003; 26: 31–5
Xyrem®. Package insert. Orphan Medical Inc, 2002 Jul
Centers for Disease Control. Multistate outbreak of poisonings associated with illicit use of gamma hydroxy butyrate. JAMA 1991; 256: 447–8
Ferrara SD, Frison G, Tedeschi L, et al. Gamma-hydroxybutyrate (GHB) and related products. In: LeBeau MA, Mozayani A, editors. Drug-facilitated sexual assault: a forensic handbook. New York: Academic Press, 2001: 107–26
Vree TB, Damsma J, Van den Bogert AG, et al. Pharmacokinetics of 4-hydroxybutyric acid in man, rhesus monkey and dog. Anasthesiol Intensivmed Prax 1978; 110: 21–39
Koesters SC, Rogers PD, Rajasingham CR. MDMA (‘ecstasy’) and other club drugs: the new epidemic. Pediatr Clin North Am 2002; 49: 415–33
McCabe ER, Layne EC, Sayler DF, et al. Synergy of ethanol and a natural soporific gamma hydroxybutyrate. Science 1971; 171: 404–6
Li J, Arnaud Stokes S, Woeckener A. A tale of novel intoxication: seven cases of γ-hydroxybutyric acid overdose. Ann Emerg Med 1998; 31: 723–8
ElSohly MA, Salamone SJ. Prevalence of drugs used in cases of alleged sexual assault. J Anal Toxicol 1999; 23: 141–6
Zvosec DL, Smith SW, McCutcheon JR, et al. Adverse events, including death, associated with the use of 1,4-butandiol. N Engl J Med 2001; 344: 87–94
Craig K, Gomez HF, McManus JL, et al. Severe gamma-hydroxybutyrate withdrawal: a case report and literature review. J Emerg Med 2000; 18: 76–0
Dyer JE, Roth B, Hyma BA. Gamma-hydroxybutyrate withdrawal syndrome. Ann Emerg Med 2001; 37: 147–53
Sivilotti MLA, Burns MJ, Aaron CK, et al. Pentobarbital for severe gamma-butyrolactone withdrawal. Ann Emerg Med 2001; 38: 66–665
US Xyrem® Multicenter Study Group. The abrupt cessation of therapeutically administered sodium oxybate (GHB) does not cause withdrawal symptoms. J Toxicol Clin Toxicol 2003; 41: 131–5
US Code of Federal Regulations. Title 21. Food and Drugs Chapter 13. Drug abuse Prevention and Control Subchapter I - Control and Enforcement Part B -Authority To Control; Standards and schedules, Section 812. Schedules of controlled substances
Task Force on Risk Management. Managing the risks from medical product use. Food and Drug Administration, 1999 May
US Code of Federal Regulations. Title 21. Food and Drugs, Chapter I. FDA, Department of Health and Human Services, Part 314.510 Applications for FDA approval to market a new drug: approval based on a surrogate endpoint or on an effect on a clinical endpoint other than survival or irreversible morbidity
Federal Register. Schedules of controlled substances: addition of gamma hydroxybutyric acid to schedule I. Fed Regist 2000 Mar 13; 65 (49): 13235–8
Food and Drug Administration. Addressing OxyContin abuse. FDA Consum 2001; 35: 3
Acknowledgements
The authors of this manuscript are all employees of Orphan Medical Inc.
The authors wish to acknowledge Patti Engel, President and CEO of Engage Health and Bob Gagne, Public Affairs Counsel of Colle & McVoy and the many, many stakeholders involved in this project who together helped make the Xyrem® Risk Management Program possible. The authors have no conflicts of interest that are directly relevant to the contents of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
In this article, the term analogue refers to substances that are either structurally related to, or have pharmacological activity similar to GHB.
Rights and permissions
About this article
Cite this article
Fuller, D.E., Hornfeldt, C.S., Kelloway, J.S. et al. The Xyrem® Risk Management Program. Drug-Safety 27, 293–306 (2004). https://doi.org/10.2165/00002018-200427050-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200427050-00002